Abstract
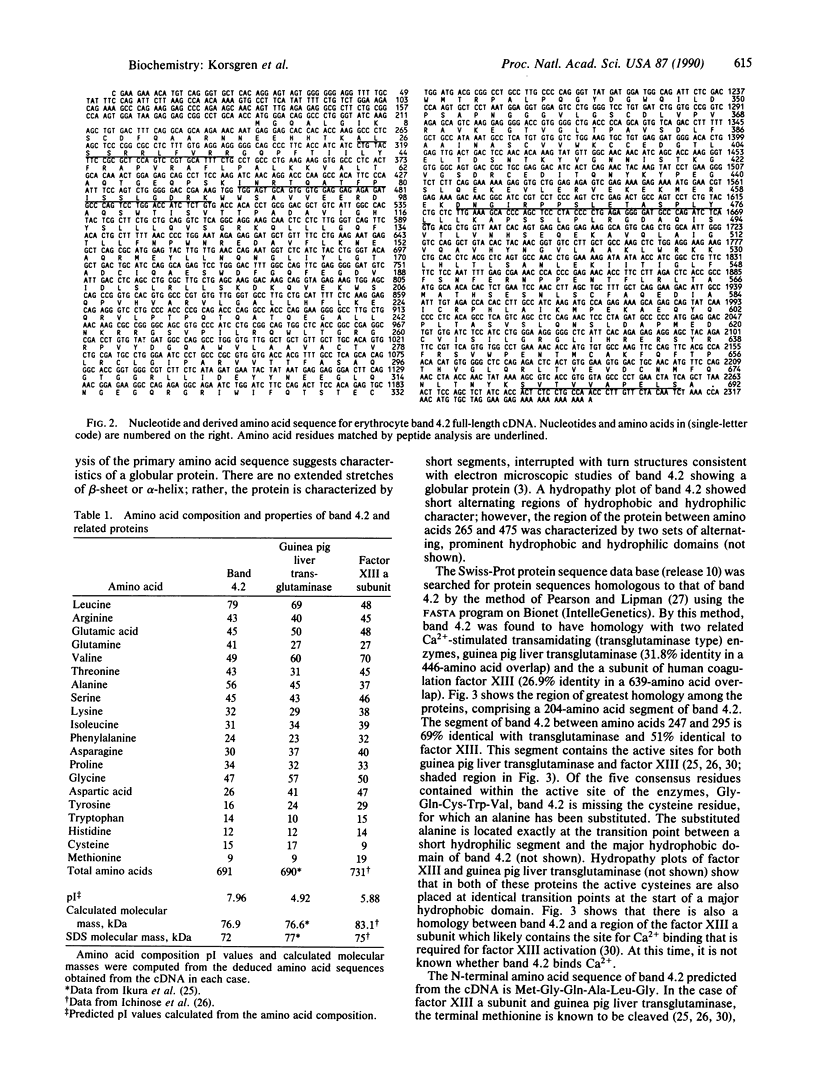
The complete amino acid sequence for human erythrocyte band 4.2 has been derived from the nucleotide sequence of a full-length 2.35-kilobase (kb) cDNA. The 2.35-kb cDNA was isolated from a human reticulocyte cDNA library made in the expression vector lambda gt11. Of the 2348 base pairs (bp), 2073 bp encode 691 amino acids representing 76.9 kDa (the SDS/PAGE molecular mass is 72 kDa). RNA blot analysis of human reticulocyte total RNA gives a message size for band 4.2 of 2.4 kb. The amino acid sequence of band 4.2 has homology with two closely related Ca2(+)-dependent cross-linking proteins, guinea pig liver transglutaminase (protein-glutamine gamma-glutamyltransferase; protein-glutamine: amine gamma-glutamyltransferase, EC 2.3.2.13) (32% identity in a 446-amino acid overlap) and the a subunit of human coagulation factor XIII (27% identity in a 639-amino acid overlap), a transglutaminase that forms intermolecular gamma-glutamyl-epsilon-lysine bonds between fibrin molecules. The region of greatest identity includes a 49-amino acid stretch of band 4.2, which is 69% and 51% identical with guinea pig liver transglutaminase and the a subunit of factor XIII, respectively, within the regions that contain the active sites of these enzymes. Significantly, within the five contiguous consensus residues of the transglutaminase active site, Gly-Gln-Cys-Trp-Val, band 4.2 has an alanine substituted for cysteine (which is apparently essential for activity). Consistent with this active site substitution, erythrocyte membranes or inside-out vesicles, which contain band 4.2, show no evidence of transglutaminase activity by two types of in vitro assay.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Singer S. J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2568–2571. doi: 10.1073/pnas.72.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus L., Thomazy V. Searching for the function of tissue transglutaminase: its possible involvement in the biochemical pathway of programmed cell death. Adv Exp Med Biol. 1988;231:119–134. doi: 10.1007/978-1-4684-9042-8_10. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Friedrichs B., Koob R., Kraemer D., Drenckhahn D. Demonstration of immunoreactive forms of erythrocyte protein 4.2 in nonerythroid cells and tissues. Eur J Cell Biol. 1989 Feb;48(1):121–127. [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Hayashi S., Koomoto R., Yano A., Ishigami S., Tsujino G. Abnormality in a specific protein of the erythrocyte membrane in hereditary spherocytosis. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1038–1044. doi: 10.1016/0006-291x(74)90801-8. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Iida H., Hasegawa I., Nozawa Y. Biochemical studies on abnormal erythrocyte membranes. Protein abnormality of erythrocyte membrane in biliary obstruction. Biochim Biophys Acta. 1976 Sep 7;443(3):394–401. doi: 10.1016/0005-2736(76)90459-4. [DOI] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. Calcium effects on human erythrocyte membrane proteins. Biochim Biophys Acta. 1977 Nov 15;471(1):162–168. doi: 10.1016/0005-2736(77)90404-7. [DOI] [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem. 1988 Jul 25;263(21):10212–10218. [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J Biol Chem. 1986 Apr 25;261(12):5536–5543. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Dailey J. E., Turner P. M. Fibronectin as a carrier for the transglutaminase from human erythrocytes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1057–1059. doi: 10.1073/pnas.85.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Shishido R., Parameswaran K. N., Steck T. L. Modification of human erythrocyte ghosts with transglutaminase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1158–1166. doi: 10.1016/0006-291x(75)90795-0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Formation of gamma-glutamyl-epsilon-lysine bridges between membrane proteins by a Ca2+-regulated enzyme in intact erythrocytes. J Supramol Struct. 1978;9(3):427–440. doi: 10.1002/jss.400090313. [DOI] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki A. C., Heath R., Wolf J. L., Lubin B., Schwartz R. S. Deficiency of protein 4.2 in erythrocytes from a patient with a Coombs negative hemolytic anemia. Evidence for a role of protein 4.2 in stabilizing ankyrin on the membrane. J Clin Invest. 1988 Mar;81(3):893–901. doi: 10.1172/JCI113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L. A., Chien S., Chang L. S., Lambert K., Bliss S. A., Bouhassira E. E., Nagel R. L., Schwartz R. S., Rybicki A. C. Molecular cloning of human protein 4.2: a major component of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1990 Feb;87(3):955–959. doi: 10.1073/pnas.87.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B. C. DNA in heritable disease. Lancet. 1983 Oct 1;2(8353):787–788. doi: 10.1016/s0140-6736(83)92314-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8019–8023. doi: 10.1073/pnas.83.21.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]



