Abstract
Low serum high density lipoprotein cholesterol level (HDL-C) < 40 mg/dL in men and < 50 mg/dL in women are a significant independent risk factor for cardiovascular disease (CVD), and are often observed in patients with hypertriglyceridemia, obesity, insulin resistance, and diabetes. Patients with marked deficiency of HDL-C (< 20 mg/dL) in the absence of secondary causes are much less common (< 1% of the population). These patients may have homozygous, compound heterozygous, or heterozygous defects involving the apolipoprotein (APO)AI, ABCA1, or lecithin:cholesterol acyl transferase genes, associated with Apo A-I Deficiency, ApoA-I Variants, Tangier Disease, Familial Lecithin:Cholesteryl Ester Acyltransferase Deficiency, and Fish Eye Disease. There is marked variability in laboratory and clinical presentation, and DNA analysis is necessary for diagnosis. These patients can develop premature CVD, neuropathy, kidney failure, neuropathy, hepatosplenomegaly and anemia. Treatment should be directed at optimizing all non-HDL risk factors.
Keywords: Lipoproteins, HDL, high-density lipoproteins, HDL-C, genetic dyslipidemias
High density lipoproteins (HDL) are generally spherical lipoprotein particles with a diameter of about 6–11 nm, and usually have α mobility on electrophoresis. HDL have been defined as having a density of 1.063 g/mL–1.21 g/mL in plasma. Normal HDL particles contain (weight %) about 50% protein, 25% phospholipid, 20% cholesterol, and 5% triglyceride (TG). About 70% of the cholesterol is in the esterified form. In HDL the protein, phospholipid, and free cholesterol is on the surface, with cholesteryl ester (CE) and TG in the core. The major proteins of HDL are apolipoprotein (apo) A-I and apoA-II, present in a molar ratio of about 3:1, with many other proteins including apoA-IV, apoA-V, apoC-I, apoC-II, apoC-III, and apoE being found on HDL particles in much smaller amounts. The production and plasma residence time of HDL apoA-I in normal subjects is about 12 mg/kg/day and 4.0 days, respectively, while for apoA-II these values are about 3.0 mg/kg/day and 4.5 days, respectively. Other apolipoproteins within HDL have much lower plasma residence with these values of about 0.5–2.0 days. HDL has been further divided into HDL2 of density 1.063–1.125 g/mL and HDL3 of density 1.125–1.21 g/mL. In patients with low HDL, there is a much greater decrease in HDL2 than in HDL3.
HDL is quantified in the general laboratory by measuring its cholesterol content using automated enzymatic analyses after removing all other lipoproteins by precipitation. An HDL-C level of < 40 mg/dL in men and < 50 mg/dL in women has been defined as decreased, and has been associated with an increased risk of cardiovascular disease (CVD). HDL-C is one of the parameters used in the risk calculator for CVD risk (1). It has been recommended by the guidelines that subjects with CVD, diabetes mellitus (DM), an elevated low density lipoprotein cholesterol (LDL-C >190 mg/dL) and an estimated 10 year CVD risk of > 7.5% be considered for statin therapy in addition to lifestyle modification (1,2). Recent expert opinion guidelines indicate that non statin agents such as ezetimibe, anion exchange resins, and proprotein convertase subtilisin kexin 9 inhibitors can be used along with statins to get at least a 50% decrease in LDL-C with an option to get LDL-C levels to < 70 mg/dL in CVD patients and to < 100 mg/dL in high risk patients (3).
A common observation in the statin intervention trials was that patients with dyslipidemia (elevated TGs > 200 mg/dL and decreased HDL-C < 35 mg/dL) had the highest residual CVD risk. Moreover the addition of fenofibrate to statin therapy in DM subjects was not shown to provide significant benefit in CVD risk reduction, except in the dyslipidemic subgroup (4). The same was true for the addition of niacin to statin therapy in CVD patients selected for having an HDL-C level < 40 mg/dL (5). Only those subjects with TG values > 200 mg/dL and HDL-C values < 32 mg/dL got benefit (5). Interestingly, in the Japan Eicosapentaenoic Acid (EPA) Lipid Intervention Trial, the addition of EPA to statin therapy in hypercholesterolemic patients did lower CVD risk by 19%; however this risk reduction was most striking (−53%) in patients with dyslipidemia, despite no clinically significant effects on lipid levels (6).
In studies of families with premature CVD it was originally documented that about 15% of these families had familial combined hyperlipidemia with elevations of both total plasma cholesterol and TG levels, and about 1% had familial hypercholesterolemia(FH) (7). In our own studies of families with premature CVD (< age 60 years), we measured plasma concentrations of total cholesterol, TG, LDL-C, HDL-C, apoB, and lipoprotein (a) or Lp(a). We noted that 19% of families had Lp(a) excess, 15% had familial dyslipidemia (high TGs and low HDL-C), 14% had familial combined hyperlipidemia (mostly also with low HDL), 5% had isolated apoB elevation, 4% had isolated low HDL (hypoalphalipoproteinemia), and 1% had FH (8,9). Cutpoints utilized were 10th and 90th percentile values for normal age and gender matched controls from the Framingham Offspring Study. These data indicate that low HDL-C in families with premature CVD is frequently associated with either elevated TGs or elevations of both TGs and LDL-C.
Our own early studies indicated that patients with very high TG levels (> 500 mg/dL) had very low HDL-C values (10). These patients were subsequently shown to have markedly enhanced clearance of HDL apoA-I (11). Moreover in the Framingham Offspring Study low HDL-C levels were significantly associated with hypertriglyceridemia, obesity, DM, male gender, sedentary lifestyle, and cigarette smoking (12). In men selected for CVD, low HDL-C (< 40 mg/dL), LDL-C < 140 mg/dL, and TG levels < 150 mg/dL in the Veterans Affairs HDL Intervention Trial (VA-HIT), almost all subjects were overweight or obese, many were insulin-resistant, and 25% were diabetic (13). Moreover in this trial the occurrence of new CVD and the benefit of fibrate therapy was much less dependent on levels of HDL-C or TGs than on the presence or absence of insulin resistance (13). The overall data indicate that HDL-C levels in the general population are more strongly affected by TGs, body mass index and insulin resistance than genetic factors.
HDL Particle Analysis
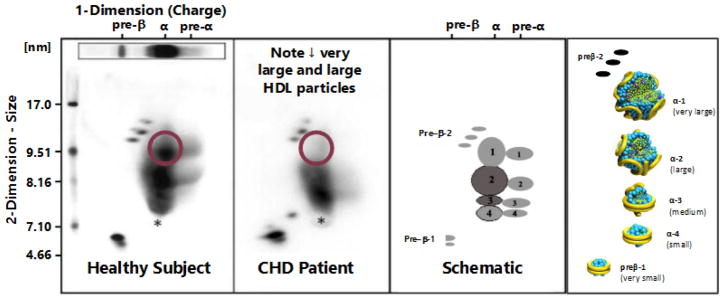
HDL particles have been characterized by ultracentrifugation, nuclear magnetic resonance, one dimensional gel electrophoresis, high performance liquid chromatography, and ion mobility. These methods in our view lack the resolution and specificity provided by two dimensional gel electrophoresis of whole plasma followed by immunoblotting with apoA-I specific antibodies, developed by Asztalos and Roheim (14). In this analysis there are five major HDL particles which are separated by size and charge. There are two major discoidal HDL particles: 1) very small preβ-1 migrating HDL of about 5.6 nm in diameter containing only apoA-I and phospholipid (about 6% of total apoA-I), and 2) small α-4 HDL of about 7.4 nm in diameter containing apoA-I, phospholipid, and free cholesterol (about 14% of total apoA-I). Then there are three larger spherical HDL particles all of which have α mobility: 1) medium α-3 HDL of about 8.1 nm in diameter containing both apoA-I and apoA-II, as well as phospholipid, free and esterified cholesterol, and TG (about 16% of total apoA-I), 2) large α-2 HDL of about 9.2 nm in diameter containing the same constituents as α-3 HDL (about 40% of the total apoA-I), and 3) very large α-1 HDL of about 11.0 nm in diameter containing the same constituents as α-3 and α-2 HDL except without apoA-II (about 16% of total apoA-I). About 8% of apoA-I is found in pre α migrating HDL adjacent to α migrating HDL particles, with small amounts in large preβ-2 migrating HDL, as well as in TG-rich lipoproteins (see Figure 1).
Figure 1.
Two dimensional gel electrophoresis patterns of HDL particles from a normal subject (far left) and a patient with premature coronary heart disease (CHD) (second from left) along with two depictions of the position (middle right) and the potential structure (far right) of apoA-I containing HDL particles are shown. On the gel patterns the particle size in nm (diameter) is plotted on the vertical axis and the electrophoretic mobility (preβ, α, and preα) is plotted on the horizontal axis. The CHD patient clearly has a marked reduction in the apoA-I concentration in very large α-1 HDL.
Therefore the most abundant apoA-I containing HDL particle in the plasma of normal subjects is α-2 HDL. Patients with CVD have significantly lower levels of apoA-I in very large α-1 HDL and large α-2 HDL, and significantly more apoA-I in very small preβ-1 HDL than do control subjects (14) (see figure 1). HDL particle analysis in our view provides significantly more information about CVD risk than does HDL-C. A marked increase of these large HDL particles with the simvastatin/niacin combination was associated with regression of coronary atherosclerosis (14). Other modalities that significantly raise very large α-1 HDL include substantial weight loss, diets low in sugar, and rosuvastatin therapy (14).
Functional studies indicate that very small discoidal preβ-1 HDL picks up free cholesterol from cells via the ATP binding cassette A1 (ABCA1) transporter and in the process is converted to small discoidal α-4 HDL. These latter particles are then converted to larger spherical α HDL by the action of lecithin:cholesterol acyl transferase (LCAT) which converts free cholesterol to CE. Lipoprotein lipase (LPL) is also necessary for this conversion. Larger α HDL can then donate their cholesteryl ester to TG-rich lipoproteins (TRL) in exchange for TG via the action of CE transfer protein (CETP) or donate their CE and free cholesterol to the liver via scavenger receptor-B1 (SR-B1) (14). During this process apoA-I in our view can recycle back to form very small and small HDL discoidal particles, or be catabolized from plasma by the kidney via the action of cubilin. Both hepatic lipase and endothelial lipase play a role as phospholipases in removing phospholipid from large HDL and converting them to smaller HDL (14). Studies indicate that serum cholesterol efflux capacity serves as a better marker of CVD risk than does HDL-C levels (15,16).
Disorders Characterized by Marked HDL Deficiency
Patients with marked deficiency of high density lipoproteins (HDL cholesterol < 20 mg/dL) in the absence of marked hypertriglyceridemia (> 500 mg/dL), uncontrolled DM, liver disease, or the use of anabolic steroids, are uncommon (< 1% of the population). These patients may have homozygous, compound heterozygous, or heterozygous defects involving the APOAI, ABCA1, or LCAT genes. Such patients can be divided into five groups: 1) ApoA-I Deficiency, 2) ApoA-I Variants, 3) Tangier Disease (TD), 4) Familial Lecithin:Cholesteryl Ester Acyltrasferase Deficiency (FLD), and 5) Fish Eye Disease (FED). There are a number of forms of familial apolipoprotein A-I deficiency: those characterized by a lack of plasma apoA-I, apoC-III, and apoA-IV, those characterized by a lack of apoA-I and apoC-III, and those characterized by a lack of apoA-I only (17,18). These states are briefly reviewed below.
Apolipoprotein A-I Deficiency States
We described a kindred in 1982 in which the female proband had marked HDL deficiency, undetectable plasma apoA-I levels, low-TG levels, normal LDL-C levels, corneal arcus, and premature CVD (17,18). The patient had severe CVD and planar xanthomas and died during coronary artery bypass surgery at age 43 years. She had no other known CVD risk factors, and on autopsy was found to have very severe diffuse coronary and peripheral atherosclerosis. Her plasma LCAT activity was normal, and her defect was found to be due to a homozygous deletion of the entire APOA1/C3/A4 gene complex. Heterozygotes had plasma HDL-C, apoA-I, apoA-IV, and apoC-III levels that were about 50% of normal. This disorder is known as familial apolipoprotein A-I/C-III/A-IV deficiency (17,18).
Norum and colleagues in 1982 described two sisters with marked HDL deficiency, undetectable plasma apoA-I and apoC-III, planar xanthomas, and premature CVD requiring bypass surgery at ages 29 and 30 years. These patients had low TG levels, normal LDL-C levels, and the defect was found to be due to a homozygous DNA rearrangement affecting the adjacent APOA1 and APOC3 genes. They had enhanced clearance of very LDL (VLDL) apoB. This disorder is known as familial apolipoprotein A-I/C-III deficiency (17,18).
Matsunaga and colleagues in 1991 described a 56-year-old Japanese woman with premature CVD, planar xanthomas, normal TG and LDL-C levels, marked HDL deficiency, and undetectable plasma apoA-I levels. The defect was a homozygous APOAI codon 84 nonsense mutation, resulting in a lack of normal apoA-I production (17,18). Ng and colleagues in 1994 reported a Portuguese kindred in which a 34-year-old proband had undetectable plasma apoA-I levels, marked HDL deficiency, tendinous xanthomas, premature CVD, cerebellar ataxia, and elevated LDL-C levels. The defect was a codon -2 APOA1 nonsense mutation resulting in lack of apoA-I production. Two other homozygotes in this kindred also had CVD in their 30s (17,18). The elevated LDL-C values were felt to be due to another genetic disorder (17,18). We reported another Portuguese kindred with the same mutation as in the prior kindred (-2 codon nonsense mutation) in two homozygous brothers who presented with undetectable plasma apoA-I (see figure 2), CVD requiring bypass surgery at 38 and 39 years of age, tubo-eruptive and planar xanthomas, corneal arcus and mild corneal opacification (see figure 3), with HDL-C < 5 mg/dL and normal LDL-C and TG levels. They had no detectable apoA-I containing HDL (see figure 3). Multiple heterozygotes in this kindred had HDL-C levels that were about 50% of normal (17,18).
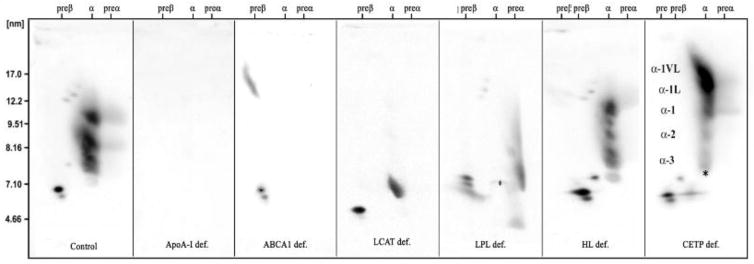
Figure 2.
Two dimensional gel electrophoretic patterns of apoA-I containing HDL particles (from left to right) are shown from a control subject, an apoA-I deficient patient (no particles), a TD patient (only preβ-1 HDL), an FLD patient (preβ-1 and α-4 HDL, with some larger discoidal fusion particles), a LPL deficient patient with lack of large α HDL, an HL deficient patient with decrease in α-2 HDL, and a patient with CETP deficiency with an excess of abnormal very large HDL particles.
Figure 3.
Images of corneal arcus and corneal opacification are shown. In the upper left is a patient with homozygous apolipoprotein A-I deficiency with somewhat atypical arcus juvenilis. In the upper right panel is a patient with homozygous TD showing diffuse corneal opacification only seen by slit lamp examination. In the lower left panel is a patient with homozygous FLD with marked corneal arcus juvenilis and corneal opacification. In the lower right panel is a young patient with compound heterozygous FED with marked arcus juvenilis and some diffuse corneal opacification.
An Iraqi kindred with two probands was reported in 2010 who were noted to have complete apoA-I deficiency and marked HDL deficiency caused by a homozygous nonsense mutation with a stop codon at Arg10. One proband was a 35 year old woman with xanthelasma and xanthomas, but no CVD, while her 37 year old brother also had planar xanthomas, but sustained a myocardial infarction (MI) at age 35 years. Both patients had normal LDL-C and triglyceride levels. In contrast to the sister, the brother was a cigarette smoker and had an Lp(a) of 53 mg/dL (19).
These data indicate that undetectable plasma apoA-I levels and marked HDL deficiency due to homozygous mutations affecting APOA1 are associated with premature CVD. Such patients in our view should have their LDL-C levels optimized with statin therapy, and if necessary with other agents. In the future apoA-I or HDL infusion therapy may be available for such patients since such therapy had been shown to have benefit for CVD in small studies (20).
Apolipoprotein A-I Variants
Funke and colleagues in 1991 reported a 42-yr-old German patient with corneal opacification, marked HDL deficiency and apoA-I deficiency, significantly decreased plasma LCAT activity, increased non-HDL-C and TGs, and lack of CVD (20). Sequencing of his LCAT gene was normal, but the patient was found to be homozygous for an apoA-I frameshift mutation resulting in a truncated 229 amino acid protein instead of full length apoA-I (21). Heterozygotes in the kindred had about 50% of normal HDL-C levels. Takata and colleagues in 1995 reported a 39-year-old Japanese man with corneal opacification, a serum HDL-C of 6 mg/dL, an apoA-I level of < 3.0 mg/dL, increased LDL-C, with normal levels of plasma TG, phospholipid, apoB, apoC-III, and apoE levels. LCAT activity was about 50% of normal. The patient was homozygous for a codon 8 nonsense mutation in exon 3 of the apoA-I gene. Heterozygotes in the family had normal HDL-C values. Coronary angiography showed no significant luminal narrowing (22).
In 2013 we reported a 61-year-old male with significant coronary heart disease since age 42 years, corneal arcus, combined hyperlipidemia, an HDL-C of 1 mg/dL, an apoA-I of 23 mg/dL, and only preβ-1 and α-2 HDL particles present in his HDL particles (23). He was found to have a novel heterozygous inframe insertion mutation with a duplication of nucleotides 1535 through 1552 inserted at position 1553, causing a new amino acid glycine at residue 157 and a duplication of amino acids alanine, arginine, alanine, histidine, and leucine at residues 158–162. His LCAT activity was normal. This novel apoA-I mutation (apoA-INashua) appears to result in the formation of apoA-I that has abnormal lipid binding properties, resulting in probable impaired reverse cholesterol transport, and premature CVD (23). His TG and LDL-C levels have been optimized with rosuvastatin and fenofibrate therapy.
In 2014 we reported a 68-year-old male with premature CVD since age 54 years, corneal arcus, and an HDL-C 14 mg/dL, apoA-I 57 mg/dL, and lack of very large α-1 HDL (24). His TG and LDL-C levels were normal, but have been further optimized with statin therapy. Two other family members also had the same pattern. The affected kindred members were noted to have an apoA-I truncation (apoA-IMytilene) due to a heterozygous nonsense mutation (Gln216termination) resulting in a truncated apoA-I containing only 215 amino acids. Kinetic studies indicated that the proband had an apoA-I production rate that was 40% of normal. The patient had cellular cholesterol efflux capacity that was 65% of normal, and normal LCAT activity (24).
ApoA-I variants are generally heterozygous premature terminations, frameshifts or amino acid substitutions in the 243 amino acid apoA-I sequence. These patients may have HDL-C levels that are low or normal, plasma LCAT activity that is normal or reduced, and they may develop premature CVD or amyloidosis. Heterozygous premature ApoA-I terminations or frameshift mutations with low HDL-C include Trp8termination, Gln32termination, Gln84termination, Glu136termination, and Gln216termination (17,18). Heterozygous apoA-I variants associated with low HDL-C include Arg10Leu, Arg27Thr, Leu144Arg, Arg149Ser, Arg151Cys, Val156Glu, Leu159Arg, Arg160Leu, Pro165Arg, and Arg173Cys (apoA-I Milano). ApoA-I is known to be one of the apolipoproteins that can activate LCAT. Heterozygous APOA1 mutations associated with decreased LCAT activation are located within α helices 5–7, corresponding to amino acids 121 to 187 of apoA-I. Six heterozygous apoA-I missense mutations, namely Leu141ArgPisa, Pro143Arg, Arg151Cys, Val156Arg, Arg160LeuOslo, and Pro165Arg, have been associated with low HDL-C and decreased LCAT activity. None of the affected patients had evidence of premature CVD. A patient with homozygous Val156Glu had corneal opacification consistent with FLD. It is also quite likely that apoA-IMilano (Arg173Cys) falls into this category, and heterozygotes with this disorder appear to be protected from CVD (17,18). Interestingly patients who are compound heterozygotes for Leu141ArgPisa and an apoA-I null allele were reported to have very low HDL-C and apoA-I levels that were 3% of control value. All of these latter subjects had marked corneal opacification and three of the four compound heterozygotes had significant premature CVD (17,18). These data indicate that heterozygous apoA-I variants causing low HDL-C and decreased LCAT activation are not associated with premature CVD. However apoA-I variants associated with low HDL-C and normal LCAT activity have been associated with premature CVD and include Leu141Arg, Arg153Pro, Leu159Pro, and Leu178Pro, as well as those mentioned above (17,18,23,24). An apoA-I variant associated with normal HDL and increased CVD risk is Ala164Ser to be discussed later as well (25).
ApoA-I mutations can also cause familial visceral amyloidosis, a form of amyloidosis that is an uncommon cause of kidney failure (26). Certain apoA-I mutations lead to the formation of apoA-I–amyloid protein complexes, causing enhanced amyloid proteolysis, and amyloid deposition of 9–11 kd N terminal fragments as fibrils in the kidney, liver, and heart, damaging these organs. In such patients the amyloid deposits exhibit a characteristic green birefringence when viewed by microscopy under polarized light after Congo red staining. These deposits are immunoreactive with antibodies against apoA-I. Das and colleagues have reported that all amyloidogenic apoA-I mutations cluster in residue segments 26–107 and 154–178, and can destabilize lipid free and HDL bound apoA-I (27). The apoA-I variants reported to be associated with amyloidosis include Gly26Arg, Trp50Arg, Leu60Arg. Leu64Pro, Asn74 frameshift, Leu75Pro, Leu90Pro, Ala154 frameshift, Leu170Pro, Arg173Pro, Leu174Ser, Ala175Pro, and Leu178His (17,18,26,27).
Tangier Disease (TD)
TD was first described by Fredrickson and colleagues in 1961 in two young siblings from Tangier Island in the Chesapeake Bay, noted to have very low plasma of HDL-C, moderate hypertriglyceridemia, and decreased LDL-C levels (17,18). They had mild corneal opacification (see figure 3), hepatosplenomegaly and enlarged orange tonsils. Cholesterol-laden macrophages were found in cells in their tonsils, bone marrow, nerves, and smooth muscle cells. The heterozygous parents had approximately half normal levels of HDL-C. Other kindreds were described, and in some cases neuropathy was noted. We noted that homozygotes had markedly increased fractional catabolism of HDL proteins (plasma apoA-I residence times of about 0.5 days), while heterozygotes had enhanced clearance (plasma residence times of about 2.0 days). We also found that Tangier homozygotes had an increased risk of developing premature CVD in their 50s and 60s. They speculated that these patients may not develop CVD earlier in life because their LDL-C levels were about 50% of normal (17,18).
In the 1990s Francis and Oram, as well as Schmitz and Assmann, independently noted that TD fibroblasts were defective in their ability to efflux cellular cholesterol and phospholipids onto HDL or apoA-I (17). In 1998 the chromosomal locus (9q31) for TD was reported by Rust, Assmann, and colleagues. Subsequently it was reported by Schmitz and colleagues that the ABCA1 was involved in the efflux of cellular cholesterol and phospholipid onto HDL and apoA-I. In 1999 and 2000 six different research groups reported that various mutations in ABCA1 were the causes of homozygous TD, with the first three reports by Rust, Assmann and colleagues, Bodzioch, Schmitz and colleagues, and Brooks, Hayden and colleagues appearing simultaneously in 1999 in Nature Genetics (17,18).
We were one of the groups subsequently reporting these mutations, and we also documented that TD homozygotes had only preβ-1 HDL present in their plasma (see figure 2), while heterozygotes had a lack of large α-1 and α-2 HDL particles, normal preβ-1 HDL, and only 50% of normal cellular cholesterol efflux (17,18). We also noted that intestinal cholesterol absorption was normal in a Tangier homozygote, and that the LDL apoB values were about 50% reduced due to enhanced fractional catabolism of small TG-rich, cholesteryl ester-poor LDL. We have speculated that the lack of uptake of cholesterol from cells by HDL in these patients, not only results in the rapid clearance of very small preβ-1 HDL particles by the kidney, but also relative enrichment of the core of LDL by beta-carotene, resulting in enhanced uptake by the reticulo-endothelial cells resulting in the orange coloration of these tissues (17,18).
There is significant controversy in the literature about whether patients with homozygous Tangier disease develop premature CVD. We have again recently reviewed this relationship in our own cases and those in the literature (total of 185 cases). In this review 51% of reported cases had peripheral neuropathy and 25% had CVD, which increased to 52% in those between the ages of 40 and 65 years, as compared to 11% in age and gender matched controls. Two main types of patients with homozygous or compound heterozygous Tangier disease and marked HDL deficiency were noted in our recent unpublished review: 1) Group A patients with marked hepatosplenomegaly, anemia, low non-HDL-C levels (<70 mg/dL) and lack of premature coronary heart disease (CHD); and 2) Group B patients without marked hepatosplenomegaly or anemia, normal or near normal non-HDL-C levels (>70 mg/dL), and premature CHD. These data indicate that the presence or absence of marked splenomegaly and the varying non-HDL-C levels appear to account for the variability in CVD risk in homozygous Tangier disease patients. Optimizing the LDL-C levels in Tangier patients with normal LDL-C levels using statin therapy is clearly warranted in our view.
Familial Lecithin:Cholesterol Acyltransferase Deficiency (FLD)
FLD was first described by Norum and Gjone in 1967 in a 33-year-old woman living in Norway, who presented with marked corneal opacification, hyperlipidemia, anemia, proteinuria, and normal kidney function. A kidney biopsy revealed foam cells in the glomeruli (17,18). Plasma cholesterol and TG levels were moderately increased, with most of the cholesterol being unesterified. Two of her sisters were similarly affected, and all three were found to have a marked deficiency of LCAT activity, lacking the ability to transfer a fatty acid from phosphatidylcholine or lecithin to cholesterol to form CE and lysolecithin. Kindreds in other countries were subsequently described, and subsequently were found to have homozygous or compound heterozygous mutations in LCAT.
These patients were found to have not only very low levels of HDL-C, but also were noted to have elevations in free cholesterol-enriched VLDL, which had β instead of pre β mobility on electrophoresis. Moreover their LDL was found to be large and heterogeneous and enriched in free cholesterol, phospholipids, and TG, with a very low CEcontent. These particles have also been found to be low in apoB and enriched in the C apolipoproteins. The corneal opacification observed in FLD presents early in life and consists of numerous, minute, grayish dots in the entire corneal stroma and is much more striking than that observed in TD or the apoA-I deficiency states (see figure 3). The opacification is especially marked near the limbal area, forming a circular band resembling arcus senilis. Surprisingly vision is usually not impaired.
The anemia in these patients is moderate with hemoglobin levels of around 10 g/dl and is associated with enhanced fractional clearance of red cells. The renal disease presents as proteinuria early in life, and increases in the fourth or fifth decades of life as renal function deteriorates. Atherosclerosis has been reported in some patients with familial LCAT deficiency with aortic, carotid, and femoral atherosclerosis but CHD prior to age 60 years has not been reported. Multiple mutations have been reported. Hypercatabolism of HDL proteins, especially apoA-II, has been reported in homozygotes. We have documented that homozygotes have apoA-I in plasma present only in preβ-1 and α-4 discoidal HDL particles (see figure 2) (17,18). Two-dimensional gel electrophoresis of whole plasma followed by immunoblotting with specific antibody for apoA-I can therefore readily be used to distinguish homozygous apoA-I deficiency, ABCA1 deficiency, or LCAT deficiency (see Figure 2). We have reported with Calabresi that heterozygotes for LCAT deficiency have less than 50% of normal large a-1 HDL, but two-fold increases in very small preb-1 HDL (17,18).
Alpha-Core, a company in Ann Arbor, MI developed LCAT enzymatic replacement therapy, and carried out individual treatment studies in CVD patients and prolonged therapy in a 52 year old FLD homozygous patient with moderately impaired renal function that we referred to the National Institutes of Health (28). The weekly or bi-monthly enzyme therapy was well tolerated in the FLD patient, but enzyme supply ran out, and the patient ended up on dialysis. The treatment did result in improvement of his anemia, stabilization of his kidney function, and transient normalization of his HDL particles after treatment (29). The rights to this therapy have been purchased by MediImmune, a subsidiary of AstraZeneca, and hopefully they will develop and commercialize this treatment which may prevent renal failure in homozygous FLD patients.
Fish Eye Disease (FED)
Fish eye disease (FED) was first described by Carlson and Philipson in 1979 in a male Norwegian patient and his three daughters who had all marked corneal opacification (17,18). He and his two living daughters were noted to have normal serum cholesterol, elevated TG, elevated VLDL cholesterol (VLDL-C), and LDL levels, and marked HDL deficiency. This disease was characterized by these authors as being associated with CVD in later life, visual impairment, and dense corneal opacification. The eye findings can be variable. Compared to HDL from control subjects the HDL particles of FED were characterized by their abnormally small size and enrichment of free cholesterol. Findings in these kindreds have led to the formulation of the concept that two different LCAT activities exist in normal plasma. One of these activities, denoted α-LCAT, is specific for HDL, and the other, β-LCAT, is specific for chylomicrons, VLDL, and LDL. FED has been classified as an α-LCAT deficiency, in contrast to the classical LCAT deficiency or FLD, where patients lack both α and β LCAT activities. We described a kindred from Oklahoma with two compound heterozygotes due to mutation in the LCAT gene (30). They had somewhat elevated TGs and LDL-C, and only preβ-1 and α-4 HDL, and responded well to statin therapy to optimize their LDL-C levels and reduce their CVD risk (30).
Genetic Variation in Genes Affecting HDL-C and CVD Risk
In VA-HIT we documented that the prevalence of certain genetic variants in the genes coding for lipoprotein lipase (LPL, D9N, N291S, and S447X), ATP binding cassette transporter A-I (ABCA1, G596A, A2589G and G3456C), and CETP ( TaqI B2B2 genotype) were significantly different in CVD patients with low HDL-C as compared to control subjects (29–33). In VA-HIT in order to identify allelic variants associated with susceptibility to low HDL-C and CVD, we further examined 60 candidate genes with key roles in HDL metabolism, insulin resistance, and inflammation using VA-HIT samples (cases, n = 699) and from normal participants in the Framingham Offspring Study (controls, n = 705). After adjustment for multiple testing within each gene, single-nucleotide polymorphisms (SNP) significantly associated with case status were identified in the genes encoding LIPC (hepatic liase, rs4775065, P < 0.0001); CETP (rs5882, P = 0.0002); ABCA1 (rs2249891, P = 0.0126); CUBN (rs7893395, P = 0.0246); and APOA2 (rs3813627, P = 0.0324), among others (34).
Cohen and colleagues sequenced the APOA1, ABCA1, and LCAT genes in DNA obtained from subjects with HDL-C < 5th percentile and from subjects with HDL-C levels > 95th percentile. Non-synonomous variants were significantly more common (12% versus 2%) in individuals with low HDL-C (n=284) than in those with high HDL-C (n=236). In subjects with low HDL-C 9.9% had ABCA1 mutations, 2.2% had LCAT mutations, and 0.2% had APOA1 mutations (35). Haase and colleagues sequenced the APOA1 gene in 10,330 population-based participants in the Copenhagen City Heart Study (25). Only 0.27% of individuals in the general population were heterozygous for non-synonomous variants which were associated with substantial reductions in apoA-I (up to 39 mg/dL) and/or HDL-C(up to 35 mg/dL). Only 0.41% of the population were heterozygous for variants predisposing to amyloidosis. Non-synonomous variants were associated with a hazard ratio of 1.72 (CI, 1.09–2.70, p<0.01) for MI, largely driven by Ala164Ser, a variant usually not associated with low apoA-I or HDL-C levels (25).
Fricke-Schmidt and colleagues genotyped 9259 individuals in a Danish general population followed for 25 years. Two ABCA1 variants (V771M and V825I) were previously associated with increases in HDL-C, and one (R1587K) with decreased HDL-C, whereas three variants (R219K, I883M and E1172D) did not affect HDL-C levels. Despite this, 5 out of 6 SNPs (V771M, V825I, I883M, E1172D, R1587K) predicted increased risk of IHD. Similar results were obtained in a verification sample with 932 CVD cases versus 7999 controls. A stepwise regression approach identified V771M, I883M, and E1172D as the most important predictors of CHD and additive effects on CHD risk were present for V771M/I883M and I883M/E1172D pairs (36).
In another large population analysis these same investigators looked at CVD risk in heterozygotes versus noncarriers for four ABCA1 variants (P1065S, G1216V, N1800H, R2144X). These variants reduced HDL-C levels by 17-mg/dL, and this reduction was associated with a multifactorially adjusted hazard ratio for CVD of 1.70 (p<0.001). However after multiple adjustments, the lower plasma levels of HDL-C due to heterozygosity for loss-of-function mutations in ABCA1 were not associated with an increased risk of CVD (37). These same investigators reported that the ABCA1 variant N1800H tested in 92,726 individuals for Alzheimer’s disease risk and in 64,181 individuals to examine risk of cerebrovascular disease. They documented that N1800H AC (0.2%) versus AA (99.8%) was associated with a 13% lower plasma level of apoE (p<0.00001), and a multifactorially adjusted hazard ratios of 4.13 for cerebrovascular disease, and 8.28 for the hemorrhagic stroke subtype (both p<0.00001). Moreover they documented that this ABCA1 loss of function variant, present in 1:500 individuals, was associated with low plasma levels of apoE and with high risk of Alzheimer’s disease in the general population (38).
We assessed the ABCA1 Arg219Lys variant in 5414 participants in PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) (mean age 75.3 years), who had been randomized to pravastatin 40 mg/day or placebo and were followed for a mean of 3.2 years. Of these subjects 47.6% carried the variant, with 40.0% carrying one allele, and 7.6% carrying both alleles. A modest but significant effect was seen on HDL-C levels (p = 0.024). On trial those with the variant had an overall adjusted hazard ratio for new CVD that was 1.22 versus those without the variant (p = 0.006), and in the pravastatin group it was 1.41 (p = 0.001) versus those without the variant. These data indicate that subjects with the variant may get significantly less CVD risk reduction from pravastatin treatment than those without the variant (39).
Based on a mendelian randomization analysis it was concluded that HDL is not a causative factor for atherosclerosis and CVD (40). However in our view these studies were highly flawed because they excluded the most important genes affecting HDL metabolism, namely APOAI, ABCA1, LCAT, LPL, CETP, and endothelial lipase gene (LIPC), focusing instead mainly on the effects of a single genetic variant (Asn396Ser) in the LIPG gene, whose role in human HDL metabolism is uncertain. Their analysis indicated that this variant while affecting HDL-C levels, had no significant effect on CVD risk. They did note a significant relationship of genetic variation at many of the other gene loci with CVD, however they were excluded from the analysis because of their role in modulating the levels of other lipoproteins such as VLDL. In our view one has to consider the effects of genetic variation at each gene locus because the metabolism of individual lipoproteins are highly linked (40).
Conclusions
Low HDL-C levels < 40 mg/dL are clearly an important CVD risk factor, and are often associated with hypertriglyceridemia, obesity, insulin resistance and DM. Marked HDL deficiency with HDL-C levels < 20 mg/dL is rare and can be associated with a wide variety of clinical disorders. Patients who lack apoA-I due to APOA1 mutations have marked HDL deficiency, normal LDL-C, normal LCAT activity, and premature CVD. Patients with marked apoA-I deficiency due to apoA-I variants with normal LDL-C, and normal LCAT activity, also usually have premature CVD. However such patients with decreased plasma LCAT activity, usually do not have premature CVD. TD patients with defects in ABCA1 have only preβ-1 HDL in their plasma. TD patients with marked splenomegaly usually have significant anemia and thrombocytopenia, and very low LDL-C levels (< 30 mg/dL). These patients usually do not develop premature CVD. In contrast TD patients without significant splenomegaly usually do not have anemia, but have normal LDL-C levels and usually develop premature CVD. Patients with homozygous FLD due to LCAT defects do not form normal HDL or LDL, and have only preβ-1 and α-4 HDL in their plasma. They do not develop premature CVD, but do develop kidney failure in the 4th and 5th decades of life. In contrast patients with homozygous FED do not form normal HDL, but do form normal LDL, and can develop premature CVD. All patients in the above categories at increased CVD risk with normal LDL-C levels in our view should have these levels reduced to < 50 mg/dL with statin therapy to decrease their risk.
Abbreviations
- ABC
ATP binding cassette
- APO
apolipoprotein
- CE
Cholesterol ester
- CETP
Cholesterol ester transport protein
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- FED
Fish Eye Disease
- FH
Familial Hypercholesterolemia
- FLD
Familial Lecithin: Cholesteryl Ester Acyltransferase Deficiency
- HDL
High –density lipoprotein
- HDL-C
High –density lipoproteincholesterol
- LCAT
lecithin:cholesterol acyl transferase
- LDL
Low-density lipoprotein
- LDL-C
Low-density lipoprotein cholesterol
- LIPG
endothelial lipase gene
- Lp(a)
Lipoprotein a
- MI
Myocardial infarction
- SR
Scavenger receptor
- TD
Tangier Disease
- TG
Triglyceride
- VLDL
Very low-density lipoprotein
- VLDL-C
Very low-density lipoprotein cholesterol
Footnotes
Disclosures/Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;29:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Morris PM, Ballantyne CM, et al. 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. JACC. 2016:S0735–1097. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. ACCORD Study Group. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1580–4. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito Y, Yokoyama M, Origasa H. JELIS Investigators Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS) Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JL, Hazzard WR, Schrott HG, et al. Hyperlipidemia in coronary heart disease. II. Genetic analyses of lipid levels in 176 families and delineation of a new inheroited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genest JJ, Martin-Munley S, McNamara JR, et al. Prevalence of familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 9.Genest J, Jr, Bard JM, Fruchart JC, et al. Familial hypoalphalipoproteinemia in premature coronary artery disease. Arterioscler Thromb. 1993;13:1728–37. doi: 10.1161/01.atv.13.12.1728. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer EJ, Levy RI, Anderson DW, et al. Plasma-triglycerides in regulation of H.D.L.-cholesterol levels. Lancet. 1978;2(8086):391–3. doi: 10.1016/s0140-6736(78)91863-9. [DOI] [PubMed] [Google Scholar]
- 11.Brinton EA, Eisenberg S, Breslow JL. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991;87:536–44. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer EJ, Lamon-Fava S, Ordovas JM, et al. Factors associated with low and elevated plasma high density lipoprotein cholesterol and apolipoprotein A-I levels in the Framingham Offspring Study. J Lipid Res. 1994;35:871–82. [PubMed] [Google Scholar]
- 13.Robins SJ, Rubins HB, Faas FH, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–7. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 14.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol. 2011;22:176–85. doi: 10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos RD, Asztalos BF, Martinez LR, et al. Clinical presentation, laboratory values, and coronary heart disease risk in marked high-density lipoprotein-deficiency states. J Clin Lipidol. 2008;2:237–47. doi: 10.1016/j.jacl.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Current Opinion in Lipidology. 2010;21:289–297. doi: 10.1097/MOL.0b013e32833c1ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Sarraf A, Al-Ghofaili K, Sullivan DR, et al. Complete Apo AI deficiency in an Iraqi Mandaean family: case studies and review of the literature. J Clin Lipidol. 2010;4:420–6. doi: 10.1016/j.jacl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 21.Funke H, von Eckardstein A, Pritchard PH, et al. A frameshift mutation in the human apolipoprotein A-I gene causes high density lipoprotein deficiency, partial lecithin: cholesterol-acyltransferase deficiency, and corneal opacities. J Clin Invest. 1991;87:371–6. doi: 10.1172/JCI114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takata K, Saku K, Ohta T, et al. A new case of apoA-I deficiency showing codon 8 nonsense mutation of the apoA-I gene without evidence of coronary heart disease. Arterioscler Thromb Vasc Biol. 1995;15:1866–74. doi: 10.1161/01.atv.15.11.1866. [DOI] [PubMed] [Google Scholar]
- 23.Lee EY, Klementowicz PT, Hegele RA, et al. HDL deficiency due to a new insertion mutation (ApoA-INashua) and review of the literature. J Clin Lipidol. 2013;7:169–73. doi: 10.1016/j.jacl.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthanont P, Polisecki B, Asztalos BF, et al. A novel ApoA-I truncation (ApoA-IMytilene) associated with decreased ApoA-I production. Atherosclerosis. 2014;235:470–476. doi: 10.1016/j.atherosclerosis.2014.05.935. [DOI] [PubMed] [Google Scholar]
- 25.Haase CL, Frikke-Schmidt R, Nordestgaard BG, et al. Population-based resequencing of APOA1 in 10,330 individuals: spectrum of genetic variation, phenotype, and comparison with extreme phenotype approach. PLoS Genetics. 2012;8:e1003063. doi: 10.1371/journal.pgen.1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soutar AK, Hawkins PN, Vigushin DM, et al. Apolipoprotein AI mutation Arg-60 causes autosomal dominant amyloidosis. Proc Natl Acad Sci USA. 1992;89:7389–93. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das M, Wilson CJ, Mei X, et al. Structural Stability and Local Dynamics in Disease-Causing Mutants of HumanApolipoprotein A-I: What Makes the Protein Amyloidogenic? J Mol Biol. 2016;428:449–62. doi: 10.1016/j.jmb.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol. 2011;5:493–9. doi: 10.1016/j.jacl.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shamburek RD, Bakker-Arkema R, Auerbach BJ, et al. Familial lecithin:cholesterol acyltransferase deficiency: First-in-human treatment with enzyme replacement. J Clin Lipidol. 2016;10:356–67. doi: 10.1016/j.jacl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimick SM, Sallee B, Asztalos BF, et al. A kindred with fish eye disease, corneal opacities, marked high-density lipoprotein deficiency, and statin therapy. J Clin Lipidol. 2014;8:223–30. doi: 10.1016/j.jacl.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Brousseau ME, Goldkamp AL, Collins D, et al. Polymorphisms in the gene encoding lipoprotein lipase in men with low HDL-C and coronary heart disease: the Veterans Affairs HDL Intervention Trial. J Lipid Res. 2004;45:1885–91. doi: 10.1194/jlr.M400152-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Brousseau ME, Bodzioch M, Schaefer EJ, et al. Common variants in the gene encoding ATP-binding cassette transporter 1 in men with low HDL cholesterol levels and coronary heart disease. Atherosclerosis. 2001;154:607–11. doi: 10.1016/s0021-9150(00)00722-x. [DOI] [PubMed] [Google Scholar]
- 33.Brousseau ME, O’Connor JJ, Jr, Ordovas JM, et al. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler Thromb Vasc Biol. 2002;22:1148–54. doi: 10.1161/01.atv.0000024566.57589.2e. [DOI] [PubMed] [Google Scholar]
- 34.Peloso GM, Demissie S, Collins D, et al. Common genetic variation in multiple metabolic pathways influences susceptibility to low HDL-cholesterol and coronary heart disease. J Lipid Res. 2010;51:3524–32. doi: 10.1194/jlr.P008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen JC, Kiss RS, Pertsemlidis A, et al. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004;305:869–72. doi: 10.1126/science.1099870. [DOI] [PubMed] [Google Scholar]
- 36.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, et al. Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol. 2008;28:180–6. doi: 10.1161/ATVBAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 37.Frikke-Schmidt R, Nordestgaard BG, Stene MC, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–32. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 38.Nordestgaard LT, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimers Dement. 2015;11:1430–8. doi: 10.1016/j.jalz.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Akao H, Polisecki E, Schaefer EJ, et al. ABCA1 gene variation and heart disease risk reduction in the elderly during pravastatin treatment. Atherosclerosis. 2014;235:176–81. doi: 10.1016/j.atherosclerosis.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]