Abstract
Recent advances in nanotechnology provide unparalleled flexibility to control the composition, size, shape, surface chemistry, and functionality of materials. Currently available engineering approaches allow precise synthesis of nanocompounds (e.g., nanoparticles, nanostructures, nanocrystals) with both top-down and bottom-up design principles at the submicron level. In this context, these “nanoelements” (NEs) or “nanosized building blocks” can 1) generate new nanocomposites with antibiofilm properties or 2) be used to coat existing surfaces (e.g., teeth) and exogenously introduced surfaces (e.g., restorative or implant materials) for prevention of bacterial adhesion and biofilm formation. Furthermore, functionalized NEs 3) can be conceived as nanoparticles to carry and selectively release antimicrobial agents after attachment or within oral biofilms, resulting in their disruption. The latter mechanism includes “smart release” of agents when triggered by pathogenic microenvironments (e.g., acidic pH or low oxygen levels) for localized and controlled drug delivery to simultaneously kill bacteria and dismantle the biofilm matrix. Here we discuss inorganic, metallic, polymeric, and carbon-based NEs for their outstanding chemical flexibility, stability, and antibiofilm properties manifested when converted into bioactive materials, assembled on-site or delivered at biofilm-surface interfaces. Details are provided on the emerging concept of the rational design of NEs and recent technological breakthroughs for the development of a new generation of nanocoatings or functional nanoparticles for biofilm control in the oral cavity.
Keywords: nanoparticles, dental materials, dental plaque, infection control, dental caries, periodontal diseases
Introduction
The mouth is one of the most complex environments in nature, where a myriad of biological interactions and physicochemical events occur between biomolecules and microorganisms with biotic or abiotic surfaces. It harbors a complex composition of host-derived biomolecules (e.g., salivary proteins), solid and soft tissue surfaces (e.g., teeth and gingival/mucosal tissues), and a diverse microbiota. In particular, a suitable environment exists for bacterial adhesion and biofilm development on nonshedding natural or introduced surfaces and interfaces, such as enamel, restorative materials, dentures, and dental implants (Song et al. 2015).
Essentially, oral microbes are constantly interacting with saliva-coated (or pellicle) surfaces where microorganisms known as early colonizers can bind avidly and then coadhere with other species (Nobbs et al. 2009). Depending on the host diet, immune response, and other systemic factors, this initial colonizing community can transition from a healthy to highly pathogenic state. For instance, frequent consumption of dietary sugars promotes the development of virulent biofilms where highly acidogenic microbiota is enmeshed in a diffusion-limiting biofilm matrix that cause dental caries (Bowen and Koo 2011).
The knowledge about bacteria-surface interactions is evolving, which can provide valuable insights in regard to bacterial adhesion and biofilm formation (Anselme et al. 2010; Belas 2014). Bacteria can respond to oral surface characteristics, such as energy, charge, composition, stiffness, and hydrophobicity, which can modulate their adhesion and further development into biofilms (Anselme et al. 2010; Belas 2014; Kolewe et al. 2015; Song et al. 2015; Teschler et al. 2015). Biological fluids such as saliva also affect these physicochemical properties. This enhanced understanding has provided valuable insights on how to predict and/or control the bacterial growth on solid surfaces, which can also guide rational development of a new generation of antibacterial and antibiofilm materials.
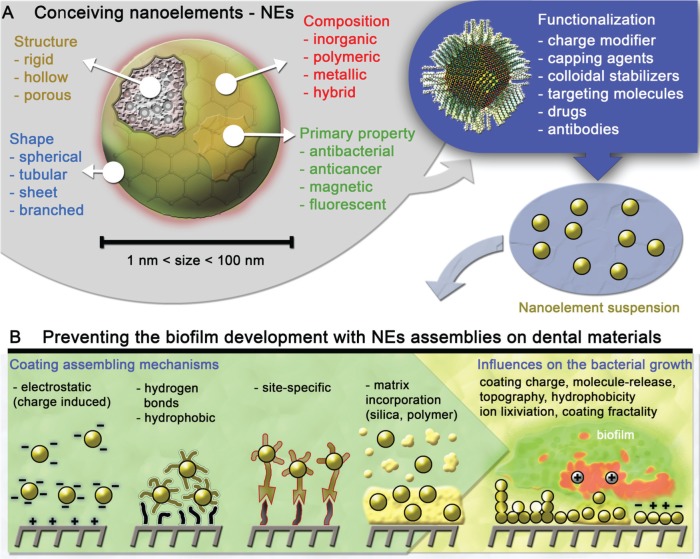
From a technological perspective, rapid advances in nanoscale engineering provide opportunities to develop new nanomaterials against virulent biofilms. By designing the nanoelement (NE; Fig. 1A), it is possible to generate functional structures that emerge as sophisticated superatom/NE building blocks for biomaterials engineering (Thanh et al. 2014; Kovalenko et al. 2015; Boles et al. 2016). In the field of chemistry/material sciences, the physicochemical properties of these NEs are governed by 6 critical parameters: size, shape, surface chemistry, flexibility/rigidity, architecture, and elemental composition (Tomalia and Khanna 2016). By specifically tuning these parameters, NEs can display biological properties, such as antifouling or antibacterial activities (Fig. 1).
Figure 1.
Schematics indicating (A) possible parameters involved in the production of multifunctional nanoelements (NEs) and (B) the mechanisms for generating antibacterial/antibiofilm nanocoatings from NE surface attachment and assembling, which can be directed by several chemical bonds (e.g., hydrogen), through numerous interactions (e.g., electrostatic, hydrophobic), via specific sites (biologically inspired), and through incorporation in matrices. The resulting assembly features that influence the antibacterial properties are represented in panel B.
The resulting bioactive NEs can be used in multiple approaches in dentistry. New dental materials can be designed by incorporating NEs (e.g., nanocomposites; Table) to impair bacterial adhesion and/or promote their killing. Furthermore, NEs can be used as coating materials (nanocoatings; Table) to protect exogenously introduced or existing dental surfaces (Fig. 1B). Finally, functional NEs can be conceived for improved attachment, penetration, and retention to dismantle established biofilms (Fig. 2A). For example, nanoparticle drug carriers can be designed to release antibiofilm agents when triggered by pathogenic microenvironments for localized and controlled drug delivery. NEs can be also developed to degrade the biofilm matrix to efficiently kill the embedded pathogens. Thus, a rational design of NEs can be performed to achieve specific or combined antiadhesion and antibiofilm properties. Excellent in-depth reviews on nanotechnology applied to dentistry (“nanodentistry”), antimicrobial nanocomposites (e.g., resin composites, adhesives, cements), bacteria-dental surface interactions, and nanotoxicology have been recently published (Allaker and Memarzadeh 2014; Besinis et al. 2015; Padovani et al. 2015; Song et al. 2015). We thus focus on the emerging concept of using functional NEs that could lead to new nanocoatings with antibiofilm properties (Fig. 1B), as well as nanoparticles with improved retention and bioactivity for biofilm prevention or disruption (e.g., “smart release” and biomimetic properties; Fig. 2A). Specifically, we discuss the potential use of promising NEs based on metals (e.g., silver nanoparticles [AgNPs], gold nanoparticles [AuNPs]), polymers (e.g., chitosan, PLGA, DMAEMA), oxides (e.g., zinc, iron, titanium, phosphates), and carbon (e.g., graphene).
Table.
Description of Terms.
| Nanoelement | A specific matter with size in the range of 10−7 to 10−9 m with determined stoichiometries, which can display new properties by tuning 6 critical nanoscale design parameters: size, shape, surface chemistry, flexibility/rigidity, architecture, and elemental composition. |
| Nanocomposite | Solid materials composed of multiple-phase domains, with at least 1 at the nanometric scale. In dentistry, nanocomposites are employed mainly as restorative materials. |
| Nanocoating | A coating material that contains specific chemical and physical characteristics at nanoscale. It is engineered at the nanometric scale (10−9 m) in regard to the composition, morphology, and structure. |
| Surface chemical interactions | Noncovalent interactions manifested between a small entity (e.g., molecule, nanoparticle) and a surface. This phenomenon involves interactions (e.g., electrostatic, van der Waals, hydrophobic, and site specific—such as enzyme-substrate), which are responsible for providing stability for nanocoatings. |
| Surface functionalization | Chemical or physical modification of a surface performed to modify material properties, such as stability, reactivity, biocompatibility, and biological activity. |
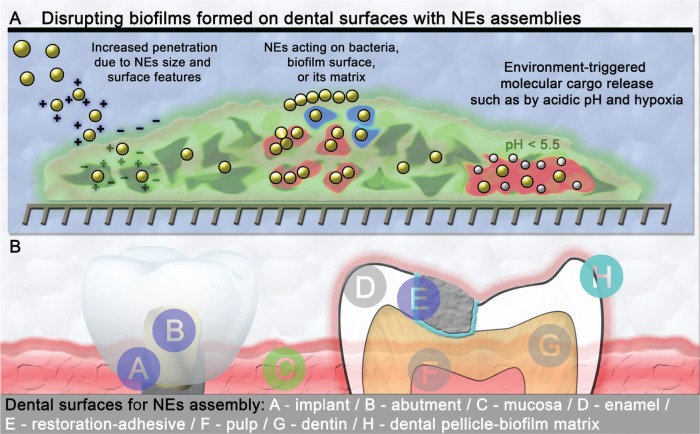
Figure 2.
Nanoelements (NEs) can be conceived with biophysicochemical properties that allow (A) specific assembling at the biofilm-tooth interface, improved biofilm penetration, and sustained/enhanced release or activation of antimicrobial agents in response to microenvironmental changes caused by biofilms (e.g., acidic pH, hypoxia, or pathogen-derived products such as exopolysaccharide). (B) Multiple sites that can be targeted by NEs in the oral cavity for generating antibacterial/antibiofilm action.
Using NEs for Modifications of Surfaces and Interfaces
Modern surface design has been largely conceived through top-down methods such as lithography (e.g., photolithography, electron beam lithography), imprinting, anodization, plasma treatment, thermal vacuum evaporation, melting, etching, molding, and others (Balasundaram et al. 2015; López-Píriz et al. 2015; Thiel et al. 2015; Gilabert-Porres et al. 2016). In contrast, emerging bottom-up procedures using NEs as the building blocks for surface design have been gaining increased attention in biomaterials engineering (Jo et al. 2014; Horev et al. 2015; Zeng et al. 2016). The control of morphology (i.e., shape and size), structure (i.e., composition, porosity, density), and function (e.g., antibacterial, anticancer) is now performed at length scales from a few to hundreds of nanometers (Fig. 1A; Doane and Burda 2012; Thanh et al. 2014; Tonga et al. 2014; Kovalenko et al. 2015). In addition, with the establishment of surface chemistry, NE surface attachment and assembling can be directed by specific chemical bonds (e.g., hydrogen) and interactions (e.g., covalent, electrostatic, hydrophobic, pi stacking), some even biologically driven (Fig. 1B, Table; Borges et al. 2014; Taglietti et al. 2014). Successful strategies for surface attachment and immobilization of molecules and macromolecules, especially proteins (Lee et al. 2014; Zhu and Jun Loh 2015), are now being applied for on-site surface assembling of NEs and for generating surface coatings.
Antibacterial surfaces with silver (Marsich et al. 2013; Borges et al. 2014; Jia et al. 2016) and polymeric (Horev et al. 2015) nanostructures are recent examples of such functional surface design. In particular, a precise manipulation of chitosan layering was recently performed to generate controlled surface patterns containing AgNPs (Shuai et al. 2013). Furthermore, bottom-up surface assemblies through use of NEs can be combined with top-down surface processing. This approach has been recently applied for the surface design of metallic implants, mainly titanium (Fig. 3; Liu et al. 2014; Balasundaram et al. 2015; Jia et al. 2016; Tian et al. 2016). A recent study showed that a surface modification with biocompatible polydimethylsiloxane, combined with pentafluorophenyl methacrylate coating (top-down), allows an efficient deposition of AgNPs (bottom-up; Gilabert-Porres et al. 2016). The resulting nanoengineered surface showed multifunctional properties, such as antifouling, combined with antibacterial properties, thus reducing the adhesion and viability of gram-positive (Staphylococcus aureus) and gram-negative (Pseudomonas aeruginosa) bacteria, without apparent cytotoxicity (i.e., against African green monkey kidney fibroblast-like cells; COS-7).
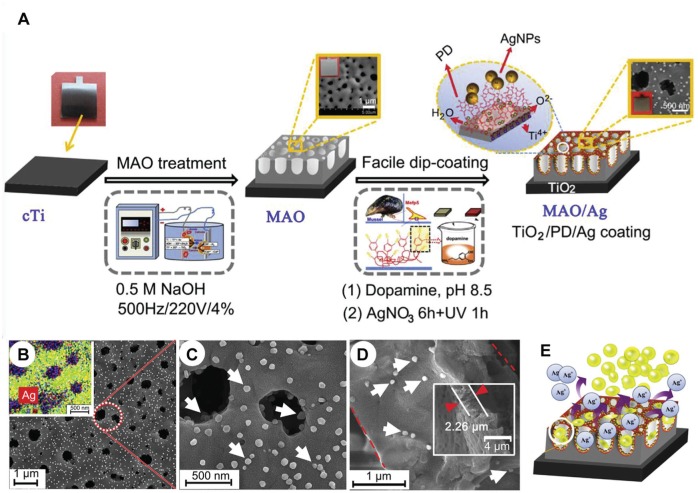
Figure 3.
Conjugation of bottom-up and top-down approaches for engineering antimicrobial surfaces on metallic implants. (A) Process for assembling silver nanoparticles (AgNPs) on micronanoporous TiO2 formed on titanium (Ti) substrates. The process associates the top-down micro-arc oxidation (MOA) technique for generating the micronanoporous TiO2 (by oxidation) and a substrate immersion into an AgNO3 solution (dip coating). The formation of the AgNP coating (through the Ag+ reduction) was favored by the presence of adsorbed polydopamine (PD) films on TiO2. Scanning electron microscopy of the resulting coatings (B, C) at different magnification and (D) from a cross section (white arrows indicate AgNPs; red arrowheads indicate the TiO2 nanopore film thickness). The inset in panel b contains the elemental map from energy-dispersive x-ray spectroscopy: Ag (red), O (green), C (green), N (yellow), and Ti (blue). (E) A scheme displaying the trap-killing contribution to the biofilm control, induced by the presence of nanopores (topography effect) associated with the presence of AgNPs (Ag+ releasing effect). Adapted from Jia et al. (2016) with the permission of Elsevier. This figure is available in color online.
NE attachment is usually induced by noncovalent bonds, especially with electrostatic (i.e., +/– charge combination) and other interactions (e.g., hydrogen bonds, hydrophobic, host-guest chemistry; Marsich et al. 2013; Alghamdi et al. 2014; Borges et al. 2014; Horev et al. 2015; Jia et al. 2016). In some cases, an NE-surface attachment through covalent bonds can provide more resilient assemblies for preventing deterioration and undesired detachment of NEs (Mokkaphan et al. 2014). However, the surface attachment can also be driven through site-specific interactions through use of biomolecules such as proteins and enzymes, providing a highly specific interaction (e.g., enzyme-substrate) and thus allowing a precise control of their surface-assembling process (Fig. 1B; Hu et al. 2015).
Besides tuning the intrinsic NE bioactivity (e.g., antiadhesion, antibiofilm), alterations in the surface charge, surface energy, stiffness, and topography resulting from their attachment may have a key role on the adhesion of cells and microbes (Mahmoudi et al. 2011). In general, bacteria adhere to a lesser extent on negatively charged (Song et al. 2015), soft (Kolewe et al. 2015), superhydrophobic (Zhang et al. 2013), and smooth (Anselme et al. 2010) surfaces. For example, less biofilm can be formed on hydrophobic surfaces, while the surface topography and physical feature can affect the dimensional aspects of the biofilm developed, including its size, shape, and 3-dimensional architecture (Rzhepishevska et al. 2013; Song et al. 2015).
NE Coatings on Dental Surfaces to Prevent Biofilm Formation
In the oral cavity, the antiadhesion properties of the biomaterial surface can be useful for preventing the attachment of several harmful microorganisms, including cariogenic and periodontopathogenic bacteria as well as opportunistic fungi (e.g., Candida albicans; Xiao et al. 2012; Hwang et al. 2015). In this sense, zinc oxide nanoparticles have been successfully assembled on the surface as coatings with antiadhesion properties. Zinc oxide–modified surfaces reduced viable bacteria (e.g., S. aureus and streptococci) without cytotoxic effect on osteoblasts and human mesenchymal cells (Abdulkareem et al. 2015). When the zinc oxide nanoparticle coatings were applied to titanium anodized surfaces, another interesting effect was observed: the coating disrupted the expression of Streptococcus mutans adhesion genes (Liu et al. 2014). Nevertheless, it is noteworthy that preventing bacterial adhesion altogether in the complex oral environment is extremely challenging—particularly due to presence of saliva and other host-derived components as well as a complex microbiota—and yet to be achieved clinically (Song et al. 2015).
Although AgNPs have been described extensively as an antibacterial nanoparticle/material, their use as building blocks for surface modification have recently emerged as a promising approach. For instance, AgNPs exhibited both high antiadhesion and antibacterial actions against S. mutans when they were coated on the surface of BisGMA/TEGDMA methacrylic resins (Ionescu et al. 2015). After being coated on the surface, the attached AgNPs can induce death of bacteria either through cell membrane–nanoparticle interactions or through secondary effects induced by long-term released AgNPs or Ag+ ions, by the production of reactive oxygen species, by alteration of the membrane integrity, and by protein-site specific interactions that could prevent DNA replication (de Lima et al. 2012; Padovani et al. 2015).
In terms of the Ag+ release as a major mechanism for antimicrobial action of AgNPs, the local chemical microenvironment in which they are exposed can influence their biological action. AgNPs act against microbes through nonspecific binding of Ag+ to moieties in several biomolecules (e.g., DNA, cofactors), thus altering a variety of biochemical pathways (Le Ouay and Stellacci 2015). Besides the intrinsic AgNP features (e.g., size, morphology), recent studies indicate that the pH and composition of the medium alter the AgNP dissolution (Le Ouay and Stellacci 2015). Since the concentration of dissolved O2 has a direct implication on the AgNP dissolution via the Ag0 oxidation, a low (or absence of) antimicrobial activity has been reported in anaerobic conditions (Loza et al. 2014).
When deposited on nanoporous silica surfaces formed on titanium substrates (Ti6Al4V alloy), AgNPs induced antiadhesion action against Aggregatibacter actinomycetemcomitans (Massa et al. 2014). A 2-step bottom-up approach was used for generating the coatings. AgNPs were first dispersed in a solution of silicon monomers (tetraethyl orthosilicate) and pore structure–directing agents (Pluronic P123 and polyethylene glycol), and then Ti substrates were immersed into it. The resulting surface-engineered Ti surface containing AgNPs and silica nanotubes reduced by more than half the amount of biofilm formed on the surface. The formation of AgNP coatings along with silica layers is a promising approach for biomaterial functionalization, as it can provide a physical and chemically stable coating.
Similar efficiency of AgNP surface coating was achieved on a hydroxyapatite-modified surface with different patterns, including net-plate (SHAC), oriented nanorod arrays (RHAC), and oriented nanoplate arrays (PHAC; Tian et al. 2016). The surfaces exhibited antibacterial activity against S. aureus and Escherichia coli (significantly superior than titanium surfaces) and induced no cytotoxic effect toward human bone marrow stromal cells.
Inorganic NEs based on hexametaphosphate associated with chlorhexidine also displayed antibacterial effects when coating pure grade II titanium. Coatings were obtained by immersing the substrates for 30 s into hexametaphosphate suspensions. The antimicrobial efficacy of the coated surfaces appears to be associated with long-term release of chlorhexidine (>99 d; measured in vitro), which was capable of inhibiting the growth of Streptococcus gordonii, an early colonizer (Wood et al. 2015).
NE coatings for dental applications can also benefit from advances on graphene oxide (GO), which has been shown to have antiadhesion and antibacterial properties (Zeng et al. 2016). The mechanism in which GO induces antimicrobial effects are not yet fully understood, although the size of the GO sheet appears to play an important role (Perreault et al. 2015). Recently, graphene-wrapped silver nanowires were introduced as promising coatings for dentures (Zhao et al. 2016). Silver nanowire coatings on flexible and transparent plastic films made of ethylene vinyl acetate and polyethylene terephthalate resulted in high antimicrobial activity against both gram-negative (E. coli) and gram-positive bacteria (S. aureus), as well as against fungi (C. albicans).
In antimicrobial coatings, the killing effect may occur essentially by physicochemical interactions at the microbe-coating interface (Perreault et al. 2015; Zeng et al. 2016) or by the release of antimicrobial ions/agents (Massa et al. 2014; Jo et al. 2014; Jia et al. 2016). Therefore, relevant parameters that influence the nanoparticle decomposition/dissolution must be considered (Le Ouay and Stellacci 2015), since these processes alter the bioactive agent release. Once the parameters are established, the antimicrobial efficiency on the coatings can be quantitatively compared. Furthermore, because NE coatings are assembled in very small-length scales (nanometers), there is little influence on the mechanical properties of the bulk upon which they are assembled (e.g., hardness, strength, flexibility) and thereby exert limited action against mechanically induced failures (Spencer et al. 2014). However, antimicrobial coatings may help prevent interface (e.g., adhesive) failure from the deleterious effects of biofilms. Importantly, multiple or specific sites can be targeted by NEs in the oral cavity for generating coatings in situ. Enamel, dentin, and dental materials can be coated depending on the NE composition, capping, and ligands present on these oral surfaces for antifouling or antibiofilm activity (Fig. 2B).
NEs in the Oral Cavity for Biofilm Inhibition
Even if bacteria adhere, there are promising antibacterial approaches to prevent further growth and biofilm initiation through NE-based assemblies that target bacteria or biofilm components. The bioactivity can be “programmed” through physicochemical mechanisms (i.e., cell membrane rupture, charge interaction, blockage of membrane channels) and biochemical mechanisms (i.e., enzyme inhibition, prevention of DNA replication; Padovani et al. 2015) or toward the biofilm matrix by disrupting exopolysaccharide (EPS) synthesis (Koo et al. 2013). NEs with long-term cargo-releasing properties of bioactives (antimicrobials or enzyme inhibitors) can be used in combination with remineralizing agents such as fluoride to reduce biofilm accumulation while helping to control caries development.
Surface attachment in situ can be attained with the use of mouthwashes and dentifrices containing NEs. The efficiency of this approach was explored in a recent clinical study by using formulations with AgNPs (Santos et al. 2014). In this particular case, a formulation containing chitosan-stabilized AgNPs suspended in a fluoride solution was able to arrest carious lesions in children when topically applied as drops on the decayed teeth (4 µg of AgNPs on each tooth). The effect was observed even after 12 mo of the application, thus suggesting a long-term therapeutic effect. However, the role of the proteinaceous pellicle layer on the NE binding properties and its retention or stability at tooth-biofilm interface needs further investigation (Besinis et al. 2015; Song et al. 2015).
In addition, AuNPs can bind avidly to pathogens C. albicans and P. aeruginosa, forming AuNP assemblies on the microbial surface, which in turn prevent the biofilm formation (Yu et al. 2016). Interestingly, AuNPs did not have significant killing effects against the microbes, suggesting distinctive antibiofilm mechanisms. Furthermore, these surface-associated nanoparticles activated immune response–related genes in dental pulp stem cells. The possibility of using multifunctional nanoparticles on bacterial surfaces or within biofilms opens promising avenues for controlling microbial accumulation and expression of virulence while activating stem cells critical for tissue regeneration.
NEs with Microenvironment-Triggered and Biomimetic Properties for Biofilm Disruption
Improvements in retention and bioactivity of NEs can be achieved by tuning their specific physicochemical interactions with surface and interfaces. Furthermore, advanced designing of NEs can include multifunctionality for 1) enhanced accumulation at the biofilm-tooth interface, 2) improved biofilm penetration, and 3) sustained/enhanced release or activation of antimicrobial agents (Fig. 2A). For example, the presence of EPS-rich matrix and acidic conditions found in caries-causing biofilms provide unique opportunities to exploit the microchemical environment for customizing NE targeting and drug delivery. With these properties, NEs can deliver precise amounts that are effective against bacteria but harmless against oral cells and mucosal tissues without causing enamel alterations/staining. Recently, functional polymeric nanostructures (PNs) were developed capable of binding to tooth pellicle and EPS matrix with enhanced drug release when triggered by acidic pH (Horev et al. 2015). By tuning the PNs outer corona surface, they displayed outstanding affinities to the negatively charged pellicle and EPS surfaces (Fig. 4). Owing to hydrophobic cores, nanoparticles can load nonpolar antibacterial drugs such as farnesol (~22 wt%) in aqueous solution. With this specific polymer conjugation, the nanoparticles undergo core destabilization at acidic pH, thus releasing drugs in a pH-responsive manner, resulting in a “smart drug-releasing” mechanism, triggered by the acidification of the biofilm microenvironment, a hallmark of pathogenic (cariogenic) condition. These polymeric nanocarriers were able to enhance the antibiofilm activity of farnesol (at 0.3 mg·mL−1) and reduce caries development in vivo (by up to 20-fold vs. free farnesol) following 1-min topical treatment twice daily.
Figure 4.
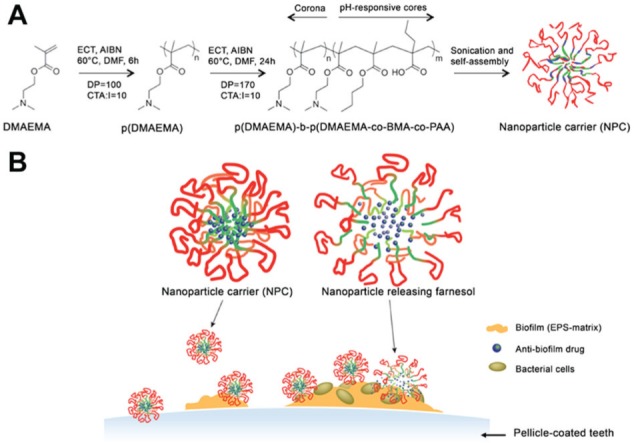
Scheme depicting (A) the self-assembly of p(DMAEMA)-b-p(DMAEMA-co-BMA-co-PAA) diblock copolymers that results in cationic polymeric nanoelements of around 21 nm (each block with ~20 kDa). The polymer conjugation and its subsequent physicochemical features results in nanoelements with antagonist microchemical features: positively charged hydrophilic surface and hydrophobic core, in which hydrophobic antibacterial agent (e.g., farnesol) was incorporated. (B) Assemblies could be formed from the nanoelement affinity to the dental pellicle and exopolysaccharide (EPS) matrix (multisite affinity). Microchemical environment changes toward an acidic pH (found in cariogenic biofilms) trigger rapid farnesol release and bacterial death within biofilms. Adapted from Horev et al. (2015) with the permission of the American Chemical Society. The polymeric nanostructure and biofilm diagrams were designed by Michael Osadciw, University of Rochester.
Similarly, nanocarriers were designed with mixed-shell polymeric micelles, where the shell was composed of PEG conjugated with a pH-responsive element (i.e., poly[β-amino ester]; Liu et al. 2016). In the acidic microenvironment found in staphylococcal biofilms (pH 5), the protonation of the NE surface due to the presence of poly(β-amino ester) increases its penetration and further accumulation at the bacterial cell–medium interface. Only with the enzymatic degradation of the nanocarrier is the antibacterial agent released (i.e., triclosan), thus resulting in a selective drug delivery mechanism (Fig. 5). Bactericidal action for this system against S. aureus biofilms was observed at 133 μg·mL−1 of NEs (triclosan at 40 μg·mL−1). Furthermore, PNs have been produced to enhance binding of antibiotics to negatively charged bacterial cell (e.g., S. aureus and E. coli), from the pH-triggered protonation of NEs (pH < 6.5; Radovic-Moreno et al. 2012).
Figure 5.
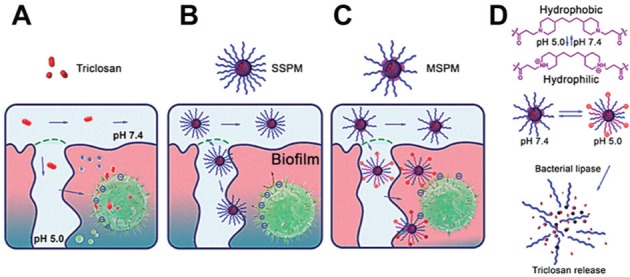
Schematics showing the mechanisms of action of the mixed-shell polymeric micelles (MSPMs) composed of poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) conjugated with a pH-responsive element (i.e., poly[β-amino ester]) localized at the nanoelement shell. The nonencapsulated antibacterial agent triclosan (A) and the single-shell polymeric micelles (SSPMs; without the poly[β-amino ester]) incorporated with the antibacterial agent (B) can penetrate the biofilm, but the latter achieve deeper layers of the biofilm. MSPMs, however, (C) can specifically target the bacteria cell wall and liberate the antibacterial agent at its vicinity due to a pH-triggered mechanism (D), thus increasing the efficiency. Adapted from Liu et al. (2016) with the permission of the American Chemical Society.
Recently, biomimetic iron oxide nanoparticles with enzyme-like (catalytic) activity have been shown to prevent dental caries in vivo (Gao et al. 2016). Nanoparticles containing Fe3O4 (with peroxidase-like activity) were developed to catalyze hydrogen peroxide (H2O2), a commonly used antiseptic agent, in a pH-dependent manner (high catalytic activity at low pH vs. no activity at neutral pH). At acidic conditions, the nanoparticles can generate free radicals from H2O2 in situ that simultaneously degrade the biofilm matrix and kill the embedded bacteria with exceptional efficacy (>5-log reduction of cell viability). Daily topical treatments of iron oxide NP/H2O2 combination (500 μg·mL−1 of Fe3O4 followed by 1% H2O2) effectively reduces the initiation and severity of dental caries (preventing cavitation) while sparing normal tissues in vivo (Gao et al. 2016). Because iron oxide activation of H2O2 is triggered only in acidic (pathologic) conditions, it prevents catalytic reaction at physiologic (neutral) pH and unmitigated free radical production, thereby providing a biocompatible treatment.
Another promising biomimetic approach against microbial infections comprises the use of NEs for transporting and releasing nitric oxide (NO) and its molecular donors. NO acts toward the formation of oxidative/nitrosative reactive species that interact with microbial proteins, DNA, and metabolic enzymes, thus resulting in antimicrobial effects. However, there remain challenges related to the NO storage and release to attain effective concentrations for bioactivity. This limitation was recently addressed by the exogenous administration of NO through molecular vehicles based on NEs. In this context, sub–200 nm silica nanoparticles co-condensed with aminosilanes that had their secondary amine moieties converted into N-diazeniumdiolate NO donors (NONOates) exhibited antibacterial effects on periodontopathogens Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis at pH 7.4 (with silica nanoparticles at 8 mg·mL−1). NO release occurs via decomposition of NONOate moiety in the presence of protons (H3O+). Thus, at lower pH (6.4), an antibacterial effect was observed against S. mutans (Backlund et al. 2015). A similar approach was taken against S. mutans by using PNs based on generation 1 poly(amidoamine) dendrimers with the surface modified with alkyl epoxides of varied chain lengths, from propyl to dodecyl (Backlund et al. 2016). Antimicrobial activity against S. mutans biofilms was observed at pH 6.4 due to NO release and dendrimer-bacterium membrane interactions.
The presence of a complex oral microbiota, where commensals coexist with potential pathogens, provides a significant challenge to develop antimicrobial NEs against a particular species or a specific group of microorganisms. Specificity can be achieved by, for example, adding species-specific antibodies or peptides in the outer corona of the nanoparticle (Monopoli et al. 2011; Forier et al. 2014), although higher costs and additional chemistry could be limiting factors. Furthermore, aspects related to the penetrability of NEs in existing oral biofilms and their affinity to the oral surfaces should be accounted, since these factors affect both antibacterial efficacy and cytotoxicity. In this context, the key factor may not be to target specific bacteria but rather to trigger antimicrobial activity in response to pathogenic microenvironments (e.g., acidic pH, hypoxia, or pathogen-derived products such as EPS). Thus, the biological effects of NE can be tuned to specifically target the biofilm microenvironment to kill the resident bacteria, degrade the matrix, and thereby eradicate the pathogenic niche.
Conclusion and Perspectives
Recent technological breakthroughs and emerging concepts in physics, chemistry, and material science/engineering are providing unlimited resources to design a new generation of functional NEs for precise biofilm control in the oral cavity. Enhanced stability of nanocoatings on dental surfaces may arise from a rational selection of chemical and physical interactions (e.g., formation of covalent bonds, electrostatic and hydrophobic interactions), to overcome the coating deterioration and dissolution. A myriad of available NEs should be selected per the coating properties as well as the enhanced mechanochemical stability on the surface.
Furthermore, the physicochemical characteristics of the NE (e.g., size, shape, surface charge) can be finely tuned to allow enhanced penetration in oral biofilms formed on dental surfaces, for both acting directly on bacteria and disrupting the matrix. For example, different biocompatible polymers can be used, with variable chain lengths and through different methods of conjugation and condensation for fabrication of PNs that can reach deeper layers of the biofilm structure while facilitating formulation development. With the evolution of organic and inorganic chemistry, the molecular mechanisms for cargo release from NEs as well as for drug activation can be programmed (Horev et al. 2015). These “sensing” or “triggering” mechanisms of functional NEs for rapid drug release or activation in situ could be designed to respond not only to environmental pH or oxygen level changes but also to bacterial- or host-derived factors associated with disease.
Despite remarkable advancements and development of new nanotechnologies, there remain major challenges in regard to the efficiency and stability of nanoengineered coatings or particles, mostly due to the complex nature of the oral cavity. In the mouth, all the available surfaces are coated by a salivary pellicle composed of host and bacterially derived biomolecules while being subjected to mechanical-hydrodynamic forces. Other variables, such as diet and complex oral microbiota, directly influence bacterial adhesion and biofilm development. Once the biofilm is established, the resident microorganisms display remarkable strategies to overcome antibacterial agents and develop drug resistance. Furthermore, the bacteria are embedded in a protective matrix with diffusion-limiting properties that confer viscoelasticity, making them highly adherent and difficult to remove from surfaces. Therefore, these biological factors must be taken into consideration when designing NE properties. Importantly, further development of NE-based formulations for clinical use will require in-depth efficacy, toxicity, and cost/benefit studies using appropriate in vivo models, which remain sparse in the current literature.
A practical issue of how NE coatings can be replenished as they eventually wear off from dental surfaces remains to be determined. Furthermore, the exact molecular/chemical interactions at the tooth/pellicle-biofilm interface are unclear. Nevertheless, the highly customizable NE chemistry provides endless possibilities to retain and release bioactive agents where and when they are most needed for an exciting path toward precision medicine to promote oral health. A multidisciplinary effort would ensure that NEs are low cost and use simple yet high-yield synthetic chemistry that is biocompatible and efficacious. The concerted actions with chemists, engineers, and oral biologists, combined with toxicology and safety studies, will help clinicians to evaluate the efficacy of these new nanotechnologies in clinical trials and pave the way for effective antifouling/antibiofilm approaches in the field of dental medicine.
Author Contributions
A.J. Paula and H. Koo, contributed to conception, design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The research related to Paula’s laboratory is supported by the grants from the Brazilian Council for Scientific and Technological Development (CNPq), Projeto Universal 446800/2014-7, and from FUNCAP, Nucleus of Excellence on Physico-Chemistry at Extreme Conditions (Edital 02/2015). Part of the work in the Koo’s laboratory is supported by the U.S. National Science Foundation (EFRI-1137186), the National Institute for Dental and Craniofacial Research (grants DE18023, DE25220, and DE25848), and the University of Pennsylvania Research Foundation.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abdulkareem EH, Memarzadeh K, Allaker RP, Huang J, Pratten J, Spratt D. 2015. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J Dent. 43(12):1462–1469. [DOI] [PubMed] [Google Scholar]
- Alghamdi HS, Bosco R, Both SK, Iafisco M, Leeuwenburgh SC, Jansen JA, van den Beucken JJ. 2014. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials. 35(21):5482–5490. [DOI] [PubMed] [Google Scholar]
- Allaker RP, Memarzadeh K. 2014. Nanoparticles and the control of oral infections. Int J Antimicrob Agents. 43(2):95–104. [DOI] [PubMed] [Google Scholar]
- Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. 2010. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 6(10):3824–3846. [DOI] [PubMed] [Google Scholar]
- Backlund CJ, Worley BV, Schoenfisch MH. 2016. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 29:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund CJ, Worley BV, Sergesketter AR, Schoenfisch MH. 2015. Kinetic-dependent killing of oral pathogens with nitric oxide. J Dent Res. 94(8):1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram G, Storey DM, Webster TJ. 2015. Molecular plasma deposition: biologically inspired nanohydroxyapatite coatings on anodized nanotubular titanium for improving osteoblast density. Int J Nanomedicine. 10:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R. 2014. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 22(9):517–527. [DOI] [PubMed] [Google Scholar]
- Besinis A, De Peralta T, Tredwin CJ, Handy RD. 2015. Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano. 9(3):2255–2289. [DOI] [PubMed] [Google Scholar]
- Boles MA, Ling D, Hyeon T, Talapin DV. 2016. The surface science of nanocrystals. Nat Mater. 15(2):141–153. [DOI] [PubMed] [Google Scholar]
- Borges J, Rodrigues LC, Reis RL, Mano JF. 2014. Layer-by-layer assembly of light-responsive polymeric multilayer systems. Adv Funct Mater. 24(36):5624–5648. [Google Scholar]
- Bowen WH, Koo H. 2011. Biology of Streptococcus mutans –derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45(1):69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima R, Seabra AB, Durán N., Seabra AB, Duran N. 2012. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 32(11):867–879. [DOI] [PubMed] [Google Scholar]
- Doane TL, Burda C. 2012. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev. 41(7):2885–2911. [DOI] [PubMed] [Google Scholar]
- Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. 2014. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 190:607–623. [DOI] [PubMed] [Google Scholar]
- Gao L, Liu Y, Kim D, Li Y, Hwang G, Naha PC, Cormode DP, Koo H. 2016. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 101:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Porres J, Marti S, Calatayud L, Ramos V, Rosell A, Borros S. 2016. Design of a nanostructured active surface against Gram-positive and Gram-negative bacteria through plasma activation and in situ silver reduction. ACS Appl Mater Interfaces. 8(1):64–73. [DOI] [PubMed] [Google Scholar]
- Horev B, Klein MI, Hwang G, Li Y, Kim D, Koo H, Benoit DS. 2015. PH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 9(3):2390–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, et al. 2015. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 526(7571):118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang G, Marsh G, Gao L, Waugh R, Koo H. 2015. Binding force dynamics of Streptococcus mutans–glucosyltransferase B to Candida albicans. J Dent Res. 94(9):1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu AC, Brambilla E, Travan A, Marsich E, Donati I, Gobbi P, Turco G, Di Lenarda R, Cadenaro M, Paoletti S, et al. 2015. Silver-polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces Streptococcus mutans biofilm formation in vitro. J Dent. 43(12):1483–1490. [DOI] [PubMed] [Google Scholar]
- Jia Z, Xiu P, Li M, Xu X, Shi Y, Cheng Y, Wei S, Zheng Y, Xi T, Cai H, et al. 2016. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials. 75:203–222. [DOI] [PubMed] [Google Scholar]
- Jo YK, Seo JH, Choi B-H, Kim BJ, Shin HH, Hwang BH, Cha HJ. 2014. Surface-independent antibacterial coating using silver nanoparticle-generating engineered mussel glue. ACS Appl Mater Interfaces. 6(22):20242–20253. [DOI] [PubMed] [Google Scholar]
- Kolewe KW, Peyton SR, Schiffman JD. 2015. Fewer bacteria adhere to softer hydrogels. ACS Appl Mater Interfaces. 7(35):19562–19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. 2013. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 92(12):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko MV, Manna L, Cabot A, Hens Z, Talapin DV, Kagan CR, Klimov VI, Rogach AL, Reiss P, Milliron DJ, et al. 2015. Prospects of nanoscience with nanocrystals. ACS Nano. 9(2):1012–1057. [DOI] [PubMed] [Google Scholar]
- Le Ouay B, Stellacci F. 2015. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 10(3):339–354. [Google Scholar]
- Lee WH, Loo CY, Rohanizadeh R. 2014. A review of chemical surface modification of bioceramics: effects on protein adsorption and cellular response. Colloids Surf B Biointerfaces. 122:823–834. [DOI] [PubMed] [Google Scholar]
- Liu W, Su P, Chen S, Wang N, Ma Y, Liu Y, Wang J, Zhang Z, Li H, Webster TJ. 2014. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale. 6(15):9050–9062. [DOI] [PubMed] [Google Scholar]
- Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, van der Mei HC, Ren Y, Shi L. 2016. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 10(4):4779–4789. [DOI] [PubMed] [Google Scholar]
- López-Píriz R, Solá-Linares E, Rodriguez-Portugal M, Malpica B, Díaz-Güemes I, Enciso S, Esteban-Tejeda L, Cabal B, Granizo JJ, Moya JS, et al. 2015. Evaluation in a dog model of three antimicrobial glassy coatings: prevention of bone loss around implants and microbial assessments. PLoS One. 10(10):e0140374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza K, Diendorf J, Sengstock C, Ruiz-Gonzalez L, Gonzalez-Calbet JM, Vallet-Regi M, Köller M, Epple M. 2014. The dissolution and biological effects of silver nanoparticles in biological media. J Mater Chem B. 2(12):1634–1643. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Laurent S, Shokrgozar M, Hosseinkhani M. 2011. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano. 5(9):7263–7276. [DOI] [PubMed] [Google Scholar]
- Marsich E, Travan A, Donati I, Turco G, Kulkova J, Moritz N, Aro HT, Crosera M, Paoletti S. 2013. Biological responses of silver-coated thermosets: an in vitro and in vivo study. Acta Biomater. 9(2):5088–5099. [DOI] [PubMed] [Google Scholar]
- Massa MA, Covarrubias C, Bittner M, Fuentevilla IA, Capetillo P, Von Marttens A, Carvajal JC. 2014. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater Sci Eng C. 45:146–153. [DOI] [PubMed] [Google Scholar]
- Mokkaphan J, Banlunara W, Palaga T, Sombuntham P, Wanichwecharungruang S. 2014. Silicone surface with drug nanodepots for medical devices. ACS Appl Mater Interfaces. 6(22):20188–20196. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. 2011. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 133(8):2525–2534. [DOI] [PubMed] [Google Scholar]
- Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus adherence and colonization. Microbiol Mol Biol Rev. 73(3):407–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani GC, Feitosa VP, Sauro S, Tay FR, Durán G, Paula AJ, Duran N. 2015. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 33(11):621–636. [DOI] [PubMed] [Google Scholar]
- Perreault F, Faria AF, Nejati S, Elimelech M. 2015. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano. 9(7):7226–7236. [DOI] [PubMed] [Google Scholar]
- Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. 2012. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 6(5):4279–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhepishevska O, Hakobyan S, Ruhal R, Gautrot J, Barbero D, Ramstedt M. 2013. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater Sci. 1:589–602. [DOI] [PubMed] [Google Scholar]
- Santos VE, Jr, Vasconcelos Filho A, Targino AG, Flores MA, Galembeck A, Caldas AF, Jr, Rosenblatt A. 2014. A new “silver-bullet” to treat caries in children—nano silver fluoride: a randomised clinical trial. J Dent. 42(8):945–951. [DOI] [PubMed] [Google Scholar]
- Shuai HH, Yang CY, Harn HI-C, York RL, Liao TC, Chen WS, Yeh JA, Cheng CM. 2013. Using surfaces to modulate the morphology and structure of attached cells—a case of cancer cells on chitosan membranes. Chem Sci. 4:3058–3067. [Google Scholar]
- Song F, Koo H, Ren D. 2015. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 94(8):1027–1034. [DOI] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS. 2014. Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res. 93(12):1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglietti A, Arciola CR, D’Agostino A, Dacarro G, Montanaro L, Campoccia D, Cucca L, Vercellino M, Poggi A, Pallavicini P, et al. 2014. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials. 35(6):1779–1788. [DOI] [PubMed] [Google Scholar]
- Teschler JK, Zamorano-Sánchez D, Utada AS, Warner CJ, Wong GC, Linington RG, Yildiz FH. 2015. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol. 13(5):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh NT, Maclean N, Mahiddine S. 2014. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 114(15):7610–7630. [DOI] [PubMed] [Google Scholar]
- Thiel K, Grunwald I, Wittig L, Westphal N, Salz D, Vogel K, Ciacchi LC. 2015. Dental implants coated with a durable and antibacterial film. Surf Innov. 3(1):27–38. [Google Scholar]
- Tian B, Chen W, Dong Y, Marymont JV, Lei Y, Ke Q, Guo Y, Zhu Z. 2016. Silver nanoparticle-loaded hydroxyapatite coating: structure, antibacterial properties, and capacity for osteogenic induction in vitro. RSC Adv. 6(11):8549–8562. [Google Scholar]
- Tomalia DA, Khanna SN. 2016. A systematic framework and nanoperiodic concept for unifying nanoscience: hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive mendeleev-like nanoperiodic tables. Chem Rev. 116(4):2705–2774. [DOI] [PubMed] [Google Scholar]
- Tonga GY, Saha K, Rotello VM. 2014. Interfacing nanoparticles and biology: new strategies for biomedicine. Adv Mater. 26(3):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NJ, Jenkinson HF, Davis SA, Mann S, O’Sullivan DJ, Barbour ME. 2015. Chlorhexidine hexametaphosphate nanoparticles as a novel antimicrobial coating for dental implants. J Mater Sci Mater Med. 26(6):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8(4):e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M. 2016. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep. 6:26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Yu D, He Z, Liu J, Xiao FX, Zhang Y, Wang R, Bhattacharyya D, Tan TT. 2016. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci Rep. 6:20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang L, Levänen E. 2013. Superhydrophobic surfaces for reduction of bacterial adhesion. RCS Adv. 3:12003–12020. [Google Scholar]
- Zhao C, Deng B, Chen G, Lei B, Hua H, Peng H, Yan Z. 2016. Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 9(4):963–973. [Google Scholar]
- Zhu X, Jun Loh X. 2015. Layer-by-layer assemblies for antibacterial applications. Biomater Sci. 3(12):1505–1518. [DOI] [PubMed] [Google Scholar]