Abstract
Background
α-Amylase plays a pivotal role in a broad range of industrial processes. To meet increasing demands of biocatalytic tasks, considerable efforts have been made to isolate enzymes produced by extremophiles. However, the relevant data of α-amylases from cold-adapted fungi are still insufficient. In addition, bread quality presents a particular interest due to its high consummation. Thus developing amylases to improve textural properties could combine health benefits with good sensory properties. Furthermore, iron oxide nanoparticles provide an economical and convenient method for separation of biomacromolecules. In order to maximize the catalytic efficiency of α-amylase and support further applications, a comprehensive characterization of magnetic immobilization of α-amylase is crucial and needed.
Results
A novel α-amylase (AmyA1) containing an open reading frame of 1482 bp was cloned from Antarctic psychrotolerant fungus G. pannorum and then expressed in the newly constructed Aspergillus oryzae system. The purified recombinant AmyA1 was approximate 52 kDa. AmyA1 was optimally active at pH 5.0 and 40 °C, and retained over 20% of maximal activity at 0–20 °C. The K m and V max values toward soluble starch were 2.51 mg/mL and 8.24 × 10−2 mg/(mL min) respectively, with specific activity of 12.8 × 103 U/mg. AmyA1 presented broad substrate specificity, and the main hydrolysis products were glucose, maltose, and maltotetraose. The influence of AmyA1 on the quality of bread was further investigated. The application study shows a 26% increase in specific volume, 14.5% increase in cohesiveness and 14.1% decrease in gumminess in comparison with the control. AmyA1 was immobilized on magnetic nanoparticles and characterized. The immobilized enzyme showed improved thermostability and enhanced pH tolerance under neutral conditions. Also, magnetically immobilized AmyA1 can be easily recovered and reused for maximum utilization.
Conclusions
A novel α-amylase (AmyA1) from Antarctic psychrotolerant fungus was cloned, heterologous expression in Aspergillus oryzae, and characterized. The detailed report of the enzymatic properties of AmyA1 gives new insights into fungal cold-adapted amylase. Application study showed potential value of AmyA1 in the food and starch fields. In addition, AmyA1 was immobilized on magnetic nanoparticles and characterized. The improved stability and longer service life of AmyA1 could potentially benefit industrial applications.
Electronic supplementary material
The online version of this article (doi:10.1186/s12896-017-0343-8) contains supplementary material, which is available to authorized users.
Keywords: α-Amylase, Antarctic fungus, Biochemical properties, Bread quality, Immobilization
Background
α-Amylase (EC 3.2.1.1) is an endo-acting enzyme which catalyzes random hydrolysis in the interior of the starch molecules [1]. α-Amylase has broad applications in various industries including pharmaceutical, detergent, textile, paper, and of course, the food field [2–5]. In order to meet the increasing demands of biocatalytic tasks, considerable efforts have been made to isolate enzymes produced by organisms living in extreme physicochemical conditions. Although numerous mesophilic and thermophilic amylases have been well studied, cold-adapted enzymes, which possess improved catalytic efficiency at low temperatures that compensates for the inherent reduction in reaction rates, still receive little attention. Only a few bacterial cold-adapted amylases have been successfully expressed in E. coli [6], but there have been scant studies on enzymes from cold-adapted fungi [7].
Fungal amylases are more preferred in the food industry, especially in relation to bread and baking due to their generally recognized as safe status. Among them, cold-adapted fungal amylases are particularly used in industrial processes as energy savers. Geomyces. pannorum, isolated from Victoria Land (continental Antarctica), is a psychrophilic and psychrotolerant saprophytic fungus. It produces abundant cold-adapted α-amylases, indicating its significant role as a desirable candidate in food processing. However, previous studies mainly focused on strain screening and its enzyme-producing ability [8], a rare report intensively researched its biochemical properties and applications.
Several attempts have been made to improve the cost-effectiveness of fungal enzymes. Immobilization is suggested to be advantageous, since it facilitates the recovery and reuse of the expensive enzymes [9]. Recently, iron oxide nanoparticles with uniquely large surface-to-volume ratio and quantum size effects provide an economical and convenient method for immobilization and separation of biomacromolecules [10]. Fe3O4-PVA was prepared and applied in immobilization of transaminase [11]. Fe3O4–κ–carrageenan nanoparticles were produced and adhered to pullulanase [12]. Nanoparticle Fe3O4@SiO2–NH2 carrier was also developed, and alkaline pectinase was successfully coupled on it [13]. A. oryzae α-amylase was also magnetically immobilized, and its parameters for hydrolysis of sweet potato starch were investigated [10]. However, the comparison of free and immobilized α-amylase was not studied in detail. Thus, we tested the nanoparticles immobilization in order to compare and improve the enzymatic properties and expand the potential value of fungal α-amylase in various industrial applications.
In this study, we constructed an A. oryzae expression system based on the pyrG nutritional selection marker. Meanwhile, we reported cloning of a novel fungal, cold-adapted amylase, expressed in a newly constructed A. oryzae system. Here, we also examined the recombinant enzyme (AmyA1) in baking by exploring the role of AmyA1 in bread production. Furthermore, to widen application of AmyA1, we immobilized α-amylase onto magnetic nanoparticles, and investigated the detailed biochemical characters.
Methods
Strains, plasmids, and media
E. coli DH5α (Tiangen, China) was used for construction and routine propagation of plasmids. Aspergillus oryzae auxotrophic host (ΔpyrG, ΔligD and ΔpyrGΔligD) was derivatived from the wild strain A. oryzae RIB40 (ATCC 42149) by gene recombination. Geomyces pannorum AK07KGI1001R1-2(1) (R1-2 for short, CCTCC AF2014016) was kindly donated by University of Malaya [7]. The plasmid pANE and pBC12ANH were preserved in our lab. The pBluescript II SK (+) vector was used as the skeleton for constructing expression vectors.
Potato/Dextrose/Agar (PDA) slant was used for growth of the spores of wild type and transformants. CM medium (Dextrose 1 g, NaNO3 0.6 g, Peptone 0.2 g, Uridine 0.1221 g, Yeast extract 0.1 g, K2HPO4 0.104 g, KH2PO4 0.08 g, MgSO4 0.052 g, KCl 0.05 g, Trace element 100 μL, diluted with water to 100 mL) was applied to cultivate the uracil deficient strains. MM plate (Sucrose 20.52 g, Dextrose 1 g, NaNO3 0.6 g, K2HPO4 0.104 g, KH2PO4 0.08 g, MgSO4 0.052 g, KCl 0.05 g, Trace element 100 μL, agar 1.25 g, diluted with water to 100 mL) was used as a selective medium for transformants. Culture medium added with dextrin (Dextrin 2 g, Yeast extract 0.5 g, Sucrose 0.3 g, Peptone 0.1 g, NaNO3 0.1 g, MgSO4 · 7H2O 0.05 g, FeSO4 · 7H2O 0.001 g, diluted with water to 100 mL) was applied for production of α-amylase. Martin-modified medium (Pepton 0.5 g, Yeast Extract 0.2 g, K2HPO4 0.1 g, MgSO4 0.05 g, supplemented with 2 g starch as the sole carbon when necessary, diluted with water to 100 mL) was used to cultivate G. pannorum.
Development of fungal transformation system
The ΔpyrG strain was initially derived by deleting the orotidine-5-monophosphate decarboxylase gene (pyrG) through targeted gene replacement [14]. Subsequently, the ΔligD strain was generated by homologous recombination with the pyrG marker gene. Then the selected marker pyrG was removed by self-deleting and the ΔpyrGΔligD strain was obtained and used as host for further experiments [15].
A shuttle vector was constructed for expression of recombinant α-amylase in the ΔpyrGΔligD strain. Firstly, pBC12ANH was used as a template to obtain the elements. Fragment I (708 bp) consisting of A. oryzae α-amylase (TAA) promoter, signal peptide and His-tag was amplified using primers FIf/FIr with Kpn I and Xho I restriction sites respectively (Table 1). Secondly, fragment II (875 bp) including TAA terminator and fragment III (1397 bp) containing the pyrG of A. nidulans were obtained by PCR using pBC12ANH and pANE as template with primers FIIf/FIIr and FIIIf/FIIIr, respectively (Table 1). After digestion by Kpn I/Xho I, fragment I was reclaimed and connected with pBluescript II SK (+), generating shuttle vector pSKNH. Then pSKNH was digested with Sma I/Xba I. The linearized vector pSKNH, fragment II and fragment III were integrated by pEASY-Uni SeamLess Cloning And Assembly Kit (Transgen, China) to create recombinant expression vector pSKNHG, which was validated by DNA sequencing.
Table 1.
Primers used in this study
| Designation | Primer sequence (5′-3′) |
|---|---|
| FIf | CGGGGTACCGAATTCATGGTGTTTTGATCa |
| FIr | CCGCTCGAGGCTAGCATGGTGATGGTGAT |
| FIIf | GGGTGGAGAGTATATGATGG |
| FIIr | ATTCTTGAGGACCATTACTG |
| FIIIf | GCAACTTCCTCGAGAACGCG |
| FIIIr | CCCTTTTAGTCAATACCGTTAC |
| AmyA1f | ATGTTTTTCAACTGCCCTGC |
| AmyA1r | TCAAGGGCAATAGCTGCCCT |
| AmyA2f | CTAGCTAGCATGTTTTTCAACTGCCCTGC |
| AmyA2r | TCCCCCGGGTCAAGGGCAATAGCTGCCCT |
aThe sequence underlined was introduced
Cloning of a novel α-amylase gene
DNA manipulations were carried out following the standard procedures. G. pannorum was incubated on the Martin-modified plate supplied with 2% soluble starch as the sole carbon source at 20 °C for 5 days. Total RNA was isolated from mycelia harvested from agar plates using Total RNA Kit I (Omega, USA). Reverse transcription was carried out according to the Primescript™ II 1st strand cDNA Synthesis Kit (TaKaRa, Japan), and the first strand cDNA was used as a template for gene amplification. Afterwards, primers AmyA1f/AmyA1r (Table 1) were synthetized in accordance with the gene sequence of the hypothetical Geomyces (Pseudogymnoascus) pannorum VKM F-4515 (FW-2607) α-amylase (GenBank Accession No. KFY52584.1). Sequence analysis was performed using DNAMAN software (Lynnon Biosoft) and NCBI server (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The isoelectric point (pI) and molecular weight (MW) were predicted on the ExPaSy Server (http://web.expasy.org/compute_pi/). Signal peptide was analyzed by SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/).
Expression and purification of recombinant α-amylase
Primers AmyA2f/AmyA2r (Table 1), with Nhe I and Sma I restriction sites respectively, were synthesized to amplify the complete ORF of AmyA1 gene. The purified PCR products were digested, reclaimed and cloned into plasmid pSKNHG, generating recombinant expression vector pSKNHG-AmyA1. Afterwards, PEG-CaCl2 mediated transformation was completed as previously described [16]. Transformants were selected according to their growth capability on MM plates, and then confirmed by PCR. Transformants were cultured for 6 days with an inoculum (about 5 × 106 spores/50 mL) in fermentation medium at 20 °C, 200 rpm.
The supernatant was separated by vacuum filtration and subjected to a HisTrap HP column (GE Healthcare, USA) pre-equilibrated with buffer A (50 mM sodium phosphate buffer, pH 7.4, containing 0.5 M NaCl and 20 mM imidazole). Then the column was washed with 10 ml of buffer A, and 10 ml of buffer B (50 mM sodium phosphate buffer, pH 7.4, containing 0.5 M NaCl and 100 mM imidazole) in order to wash out completely the unbounded protein. Subsequently, the target protein AmyA1 was eluted with buffer C (50 mM sodium phosphate buffer, pH 7.4, containing 0.5 M NaCl and 200 mM imidazole). Eluted protein showing α-amylase activity was pooled, desalted using desalting column (GE, Healthcare, USA), and determined by enzyme assays and SDS-PAGE. Protein concentration was calculated by the Lowry method using bovine serum albumin (BSA) as the standard.
Enzyme assays and biochemical characterization
The amylolytic activity was assayed using the DNS method [17]. The optimum pH and temperature of recombinant α-amylase (AmyA1) was determined by incubating enzyme with 1% (w/v) starch in a range of buffers (pH 2.0–9.0, 0.1 M) at 40 °C, or at different temperatures (20–70 °C) in 0.1 M citrate buffer (pH 5.0). In order to evaluate the thermostability of AmyA1, purified α-amylase was incubated at different temperatures (40–60 °C, pH 5.0). Samples were withdrawn at different time intervals and residual enzymatic activity against control was determined. The pH stability was studied by preincubating AmyA1 in various buffers ranging from pH 2.0 to 9.0 at 30 °C for 30 min. Residual activity after incubation was assayed using standard procedures.
To assess the effects of metal ions and chemical reagents on enzymatic activity, AmyA1 was preincubated with the individual reagents in 50 mM sodium phosphate buffer (pH 6.0) at 30 °C for 60 min. The residual activity was then determined in a standard assay medium. The enzyme with no metal ions was tested as the control. Additionally, the purified AmyA1 has been dialyzed at 4 °C for 12 h against 5 mM EGTA or 5 mM EDTA, respectively. The residual enzyme activity was checked with or without 5 mM calcium ions. The substrate specificity of AmyA1 was studied using soluble starch, potato starch, maize starch, glycogen, amylopectin, amylose, pullulan and maltooligosaccharides as substrates through the DNS method. The hydrolysis products arising from the action of AmyA1 on soluble starch were analyzed by HPLC. The conversion was evaluated on series 1200 HPLC system (Agilent Technologies, USA) using an NH2 column (Ultimate XB-NH2-3.5 μm USA), eluting with acetonitrile/water (85:15) at a flow rate of 0.8 mL/min. The average of triplicate measurements was used as each value.
The kinetic properties K m and V max values of the purified AmyA1 toward soluble starch were determined by Lineweaver-Burk double reciprocal plot at pH 5.0, 40 °C.
Effects of AmyA1 on bread quality
The dough mixture was added with the enzyme preparation (AmyA1 or TAA) in a ratio of 10 U/g of dough. The flour and enzyme were manually mixed for 20 min until homogeneous dough was achieved [18]. Control dough was prepared without the addition of enzyme. Afterwards, doughs were kept for panning and proofing for 75 min at 38 °C and 85% relative humidity. Baking was performed in electric oven for 20 min at 180 °C (surface temperature) and 200 °C (bottom temperature). Subsequently, bread loaves were cooled at room temperature and packaged in polyethylene bags. The crumb texture of the loaves (hardness, springiness, cohesiveness, and gumminess) was measured by texture profile analysis (TPA) on a texture analyzer (TMS-PRO, FTC, USA) using a 35 mm flat-end plate with an aluminum compression disk (probe P/35). An acrylic cylindrical probe was used to compress the samples (2 cm thickness slices) by 40% of their original height (40 mm) at a speed of 1 mm/s, with a 30 s delay between the 1st and 2nd compression. All values are means from three independent experiments.
Magnetic immobilization of AmyA1
Magnetic nanoparticles (IONPs) were synthesized by the solvothermal method [10]. Briefly, 0.2 M FeCl3 and 0.1 M FeSO4 were dissolved in 100 mL ultrapure water and blended together. The solution was vigorously stirred with drop wise addition of 50 mL of 10% NaOH until black precipitates were obtained. The resultant precipitates were washed a couple of times using ultrapure water and separated by magnetic decantation. Precipitates were dispersed in 50 mL Tris-HCl buffer (10 mM, pH 8.5) and ultrasonicated for 15 min, after which large precipitates were removed. α-Amylase was mixed with dried IONPs in the ratio of 1:1 in phosphate buffer (pH 4.0) and shook for 30 min. Gluteraldehyde (1%, v/v) was then added and shaken continually for 2 h. Finally, the AmyA1-IONPs were collected through magnetic decantation.
Characterization of immobilized AmyA1
The magnetic effect of IONPs and AmyA1-IONPs was checked using a strong magnet. The aggregate morphology was analyzed with scanning electron microscopy (SEM) on a Hitachi S4800 scanning electron microscope (Hitachi, Japan). Energy dispersive spectroscopy (EDS) was carried out in an X-ray spectrometer from Falion, Japan. X-ray diffraction (XRD) identification of phases structure was studied using a Rigaku D/MAX 2550 X-ray diffractometer (Rigaku, Japan) at a wavelength of 0.154 nm.
The characteristics of magnetically immobilized AmyA1 were determined subsequently. The effect of pH on the immobilized enzyme was evaluated through pre-incubating AmyA1-IONPs at room temperature in various buffers (pH 2.0–9.0, 0.1 M) for 2 h. The residual enzymatic activity was determined as described in Enzyme assays and biochemical characterization section. The effect of temperature on the immobilized enzyme was determined by detecting the residual enzymatic activity after pre-incubating in phosphate buffer (0.1 M, pH 7.0) at temperatures ranging from 20 to 70 °C for 2 h. The reusability of the magnetically immobilized AmyA1 was investigated under constant conditions. The AmyA1-IONPs were recovered by magnetic separation after each batch reaction, and washed with PBS for latter batches. The residual activity of the immobilized enzyme after each cycle was normalized to the initial value, which was taken as 100%.
The mRNA sequence of AmyA1 has been deposited into GenBank database under accession number KU925863.
Results
Cloning and sequence analysis of the α-amylase gene from G. pannorum
Based upon the genome sequence of Geomyces (Pseudogymnoascus) pannorum VKM F-4515 (FW-2607), the AmyA1 gene, encoding a putative α-amylase, was obtained from G. pannorum 1-2. The cDNA of AmyA1 consisted of 1482 nucleotides, corresponding to a protein of 493 amino acid residues (Additional file 1: Figure S1). Comparing the genomic DNA (analyzed from the genome sequence of G. pannorum in GenBank) and cDNA of AmyA1, two introns with lengths of 52 and 56 bp were identified, which are less than that of mesophilic α-amylases such as α-amylase (TAA) from A. oryzae containing 8 introns. However, the GU-AG classical rule of exon/intron splice junctions in filamentous fungi was strictly followed.
According to the homology search of the deduced amino acid sequences by BLAST, AmyA1 attaches to the GH 13 family of glycoside hydrolase. It shares the highest amino acid identity of 66% with that from Geomyces pannorum (GenBank Accession No. KFZ10929.1), and only 54 and 44% identity with the α-amylases from Lipomyces kononenkoae (GenBank Accession No. AAO12212.1) and Aspergillus kawachii (GenBank Accession No. XP_007584707.1), respectively, suggesting that AmyA1 is a novel α-amylase (Fig. 1).
Fig. 1.

Multiple-sequence alignment of amino acids of AmyA1. The sequences of the mature proteins from G. pannorum (Gp, AHN65136.1), Lipomyces kononenkoae (LK, AAO12212.1), Aspergillus kawachii IFO 4308 (AK, GAA91738.1), Neofusicoccum parvum UCRNP2 (NU, XP_007584707.1), Penicillium sp. 3-5 (PS, AIF73124.1) have been aligned by introducing gaps to maximize the similarity. Regions (I, II, III, IV) are the four conserved sequence regions within α-amylase family. The structures are denoted as follows: three proposed catalytic residues (*), the potential active sites for substrate binding (△)
Visual inspection of the alignment indicated seven common conserved regions (I-VII) associated with catalytic activity and characteristics the of α-amylase family (Fig. 1) [19]. The first 25 residues of AmyA1 encoding a potential canonical signal peptide coincides with the prediction online. The putative catalytic active site Asp228, Glu252, and Asp317 are correlated well with those in the α-amylase family. The calculated pI/Mw of the AmyA1 was estimated to be 4.48/53,607 and 4.45/50,992 for the native protein (containing signal peptide) and mature peptide, respectively.
Expression and purification of AmyA1 in A. oryzae
The A. oryzae auxotrophic host (ΔpyrGΔligD) was obtained by homologous recombination and used as the host. The general vector designed for heterologous protein expression was then constructed (Additional file 2: Figure S2). The produced vector, pSKNHG, contained a complete expression cassette together with a signal peptide, His-tag and pyrG gene as the selectable marker. The expression vector pSKNHG harboring the AmyA1 gene was introduced into the ΔpyrGΔligD strain. The positive transformants were fermented at 20 °C and a superior strain (A1-3) was selected by enzymatic activity assay. The recombinant α-amylase was purified by affinity chromatography and the purified enzyme showed a clear band of 52 kDa according to SDS-PAGE analysis (Additional file 3: Figure S3), which is similar to that of TAA (54 kDa). The specific activity of purified AmyA1 was 12.8 × 103 U/mL. The kinetic parameters were evaluated, and the K m and V max values of purified AmyA1 were 2.51 mg/mL and 8.24 × 10−2 mg/(mL min) towards soluble starch, respectively.
Biochemical characterization of AmyA1
The temperature activity and stability profiles of the purified AmyA1 are illustrated in Fig. 2a and b. The enzyme was optimally active at 40 °C and exhibited over 20% of its maximal activity at 0–20 °C. The activity declined sharply when temperature exceeded 50 °C. AmyA1 maintained over 70% of initial activity after incubation at 40 °C for more than 30 min, while losing most activity when incubated above 50 °C. The optimum pH of AmyA1 was determined over a pH range of 2.0–9.0. According to the pH-activity profile shown in Fig. 2c, maximum activity was observed at pH 5.0 and more than 70% activity was retained at pH 4.0–6.0. AmyA1 was relatively stable during treatment in neutral and basic buffers. The residual activities were nearly 80% of the control value after incubated in the pH range of 5.0–9.0 for 30 min (Fig. 2d).
Fig. 2.
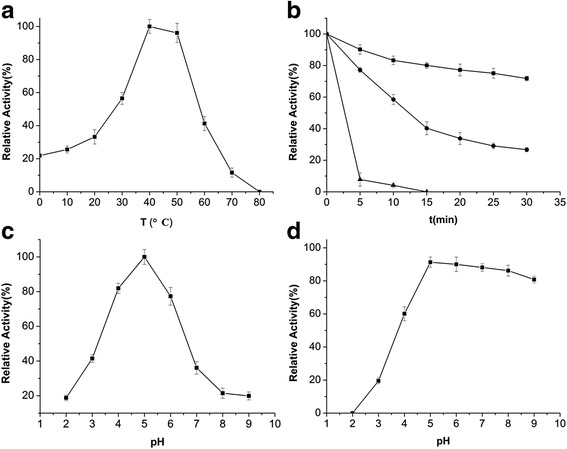
Effect of temperature and pH on purified recombinant α-amylase. a Temperature dependence of AmyA1; b Thermostability of AmyA1. Incubation at 40 °C (■), 50 °C (●), 60 °C (▲). c Relative activity profile of AmyA1 at different pH conditions; d Relative stability of AmyA1 at different pH conditions. The values shown are the means of three independent experiments and the error bars represent the standard deviations
The effects of different metal ions and reagents at a final concentration of 5 mM and 0.5% (v/v) on AmyA1 activity were analyzed (Table 2). The activity was slightly enhanced by Ca2+, Mg2+, and mildly decreased by EDTA, which was not consistent with previous reports that most α-amylases require calcium or magnesium for activity or stability [20]. Additionally, the catalytic efficiency of AmyA1 was negligibly effected in the presence of Zn2+, Cu2+, and slightly inhibited by Co2+, Mn2+, Fe2+, and isopropanol, DMSO (retaining above 80% of activity), but strongly reduced by Ni2+, Tween-80, Tween-20, Triton X-100, and methanol. Moreover, the activity of dialyzed AmyA1 was investigated with or without Ca2+. In accordance with our previous result, EDTA and EGTA did not affect the activity of AmyA1 significantly, and Ca2+ had mildly effect on the enzymatic activity (Table 3).
Table 2.
Effect of different metal ions and chemical reagents on recombinant AmyA1
| Metal ions/chemical reagents | Concentration (mM) | Relative activity (%)a |
|---|---|---|
| Blank | 5 | 100 ± 3.7 |
| Mg2+ | 5 | 117.1 ± 4.2 |
| Co2+ | 5 | 91.1 ± 3.2 |
| Mn2+ | 5 | 93.1 ± 3.1 |
| Fe2+ | 5 | 95.9 ± 2.9 |
| Cu2+ | 5 | 99.4 ± 5.3 |
| Ni2+ | 5 | 61.3 ± 1.8 |
| Ca2+ | 5 | 113.0 ± 4.8 |
| Zn2+ | 5 | 100.7 ± 3.5 |
| EDTA | 5 | 81.7 ± 2.1 |
| Teween-80 | 0.5% (v/v) | 63.2 ± 1.5 |
| Teween-20 | 0.5% (v/v) | 45.3 ± 1.7 |
| Triton X-100 | 0.5% (v/v) | 58.8 ± 2.2 |
| Methanol | 0.5% (v/v) | 53.2 ± 1.5 |
| Isopropanol | 0.5% (v/v) | 85.0 ± 3.0 |
| DMSO | 0.5% (v/v) | 81.3 ± 2.7 |
aThe data are the means of three independent experiments
Table 3.
Effect of calcium ions on recombinant AmyA1
| Metal ions/chemical reagents | Concentration (mM) | Relative activity (%)a |
|---|---|---|
| Blankb | 5 | 100 ± 1.0 |
| EDTAc | 5 | 73.0 ± 1.8 |
| EGTAd | 5 | 82.2 ± 3.5 |
| Ca2++ EDTAe | 5 | 81.9 ± 2.7 |
| Ca2++ EGTAf | 5 | 89.1 ± 3.8 |
aThe data are the means of three independent experiments
bThe activity of AmyA1 was determined in standard assay condition in the absent of metal ions
cThe enzyme was dialyzed against 5 mM EDTA, then the activity was determined in standard assay condition
dThe enzyme was dialyzed against 5 mM EGTA, then the activity was determined in standard assay condition
eThe enzyme was dialyzed against 5 mM EDTA, then the activity was determined in the presence of 5 mM Ca2+
fThe enzyme was dialyzed against 5 mM EGTA, then the activity was determined in the presence of 5 mM Ca2+
The substrate specificity of AmyA1 was ascertained using various carbohydrates at final concentration of 1% (w/v). As illustrated in Table 4, AmyA1 displayed broad substrate specificity that could efficiently hydrolyze all the tested substrates, except pullulan. The highest activity of AmyA1 was achieved on substrates maize starch and potato starch. Notably, glycogen could also be hydrolyzed effectively by AmyA1.
Table 4.
Substrate specificity of recombinant AmyA1
| Substrate (5 g/L) | Main linkage/monomer | Relative activity (%)a |
|---|---|---|
| Soluble starch | α- (1 → 4)-α- (1 → 6) glucose | 100 ± 3.1 |
| Maize starch | α- (1 → 4)-α- (1 → 6) glucose | 74.1 ± 4.2 |
| Potato starch | α- (1 → 4)-α- (1 → 6) glucose | 81.3 ± 2.8 |
| Amylose | α- (1 → 4) glucose | 54.1 ± 1.9 |
| Amylopectin | α- (1 → 4)-α- (1 → 6) glucose | 69.2 ± 3.5 |
| Glycogen | α- (1 → 4)-α- (1 → 6) glucose | 46.1 ± 2.6 |
| Pullulan | α- (1 → 6) glucose | 8.2 ± 1.5 |
| maltohexaose | α- (1 → 4) glucose | 38.9 ± 3.8 |
| maltopentaose | α- (1 → 4) glucose | 30.5 ± 3.5 |
| maltotetraose | α- (1 → 4) glucose | 7.7 ± 1.7 |
| maltotriose | α- (1 → 4) glucose | 6.1 ± 1.3 |
aThe data are the means of three independent experiments
The hydrolyzed products were detected by HPLC with standard sugar solutions. As presented in Fig. 3a, the soluble starch was converted into glucose, maltose, maltotriose, maltotetraose and maltopentaose. During the early phase of the reaction (8 h), 19.4% (w/w) glucose, 13.5% maltose, 6.6% maltotriose, 4.5% maltotetraose and 11.7% maltopentaose were obtained, demonstrating that AmyA1 preferentially cleaves at the internal a-1,4 linkage between adjoining glucose units. As the reaction time prolonged, the amount of glucose, maltose and maltotriose increased, but the amount of maltopentaose decreased. Interestingly, the amount of maltotriose almost stayed the same (Fig. 3b). The highest hydrolysis yield of 86.4% was achieved at 48 h, and the soluble starch was catalyzed into glucose, maltose and maltotetraose accounting for 36.4, 31.3 and 16.7% respectively. Subsequently, different substrates were tested to confirm the type of amylases of AmyA1. The catalytic rate enhanced according to the increase in substrate length, indicating a higher affinity for longer substrates. Besides, HPLC analysis illustrated that AmyA1 could hydrolyze different polysaccharides and maltooligosaccharides into glucose, maltose, maltotriose, and small amounts of maltotetraose and maltopentaose (Table 5). These data confirmed that AmyA1 was an α-amylase.
Fig. 3.
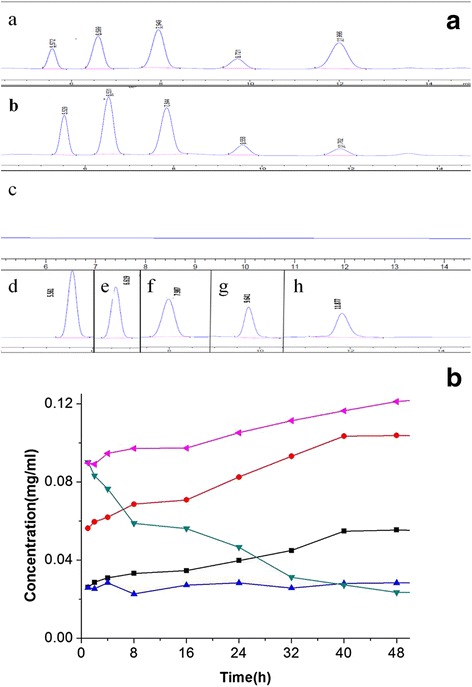
HPLC analysis of end products catalyzed by AmyA1 on soluble starch. (a) Reaction time: 1 h (a) and 48 h (b); Control: Reaction for 48 h without AmyA1 (c); Standards: glucose (d), maltose (e), maltotriose (f), maltotetraose (g), maltopentaose (h). The retention time of major peaks represented glucose (5.6 min), maltose (6.6 min), maltotriose (7.9 min), maltotetraose (9.6 min), maltopentaose (11.9 min) respectively; (b) Effect of reaction time on hydrolysis products, Abbreviations: G1 (◄), glucose; G2 (●), maltose; G3 (■), maltotriose; G4 (▲), maltotetraose, G5 (▼), maltopentaose
Table 5.
Product specificity profile of AmyA1a
| Substrate (1%, w/w) | End hydrolysis products (48 h) |
|---|---|
| Amylose | G1 G2 G3 G4 G5 |
| Amylopectin | G1 G2 G3 G4 G5 |
| maltohexaose | G1 G2 G3 G4 |
| maltopentaose | G1 G2 G3 |
| maltotetraose | G1 G2 G3 |
| maltotriose | G1 G2 |
a Abbreviations: G1 glucose, G2 maltose, G3 maltotriose, G4 maltotetraose, G5 maltopentaose
Effects of AmyA1 on bread
Acidic amylases, which could produce fermentable sugars and dextrin for further use by yeast, are applied in the baking industry to improve bread quality. Supplementation of AmyA1 and TAA had a significant effect on bread, which is clearly visible in the bread slices (Fig. 4). In comparison with the control, addition of AmyA1 and TAA improved bread specific volume of 26 and 18.6%, respectively. Besides, crumb structure was more uniform with homogeneous small cells when adding AmyA1. The quality of bread was illustrated directly by TPA evaluation (Table 6). These mechanical characteristics are correlated well with the sensory perception of bread and can define bread quality [18, 21, 22]. Table 6 shows that significant changes were observed in all the tested parameters of bread: the hardness and springiness increased notably, the cohesiveness increased by 14.5%, and the gumminess decreased by 14.1% as compared with the control. In particular, the effect was more pronounced with AmyA1 than that of TAA, indicating the potential value of AmyA1 in the food and starch fields.
Fig. 4.

Crumb structure of the loaf supplemented with: control (without enzyme) (a); AmyA1 (b); α-amylase TAA from A. oryzae (c)
Table 6.
Difference in TPA supplemented with TAA and AmyA1
| Substrate | Hardness (gf) | Springiness | Gumminess (gf) | Cohesiveness |
|---|---|---|---|---|
| Control | 1314.09 ± 15.62a | 0.812 ± 0.016 | 792.23 ± 11.52 | 0.228 ± 0.011 |
| TAA | 1439.31 ± 17.49 | 0.839 ± 0.013 | 793.00 ± 17.38 | 0.252 ± 0.013 |
| AmyA1 | 1588.44 ± 17.55 | 0.837 ± 0.016 | 681.03 ± 15.86 | 0.111 ± 0.006 |
aValues are mean ± standard deviation of triplicates data
Magnetic immobilization and characterization of AmyA1
Magnetic separation of the AmyA1-IONPs aggregates was achieved by placing a magnet adjacent to a vial, in which the immobilized enzyme was suspended (Fig. 5). The AmyA1-IONPs were completely accumulated near to the magnet within 5 s, and the solution became clear and transparent, demonstrating the simple and rapid method to effectively collect aggregated immobilized enzyme and reuse. In addition, removal of the magnet followed by agitation resulted in resuspension of the aggregates.
Fig. 5.
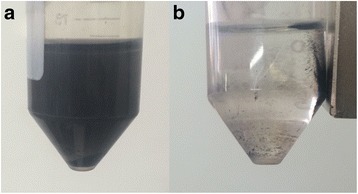
a AmyA1 immobilized on pretreated IONPs; b AmyA1-IONPs under magnetic field
The surface characteristics of magnetically immobilized AmyA1 were investigated by SEM (Fig. 6a). Most of the magnetite particles obtained have a nearly spherical shape and uniform size. The diameter of the spheres was generally under 100 nm. The surface of AmyA1-IONPs was found to be porous in nature, which might reinforce the catalytic activity of α-amylase. Results of EDS analysis illustrated the elemental composition of AmyA1-IONPs as shown in Fig. 6B. The presence of carbon, oxygen and iron was confirmed, proving the magnetic effect of the nanocomposite of AmyA1. The identification of material phases was studied by XRD analysis (Fig. 6c). Six high peaks in the diffraction angles of 30.1°, 37.1°, 43.1°, 53.4°, 57.0°, and 62.6°, mirrored typical Fe3O4 crystal plane (220, 311, 400, 422, 511, and 440. JCPDS file, No. 85-1436), demonstrating the pure inversed spinel Fe3O4 and crystalline AmyA1-IONPs in structure. The average diameter of the crystal plane was about 40 nm, which was calculated by Debye-Scherrer formular [23].
Fig. 6.
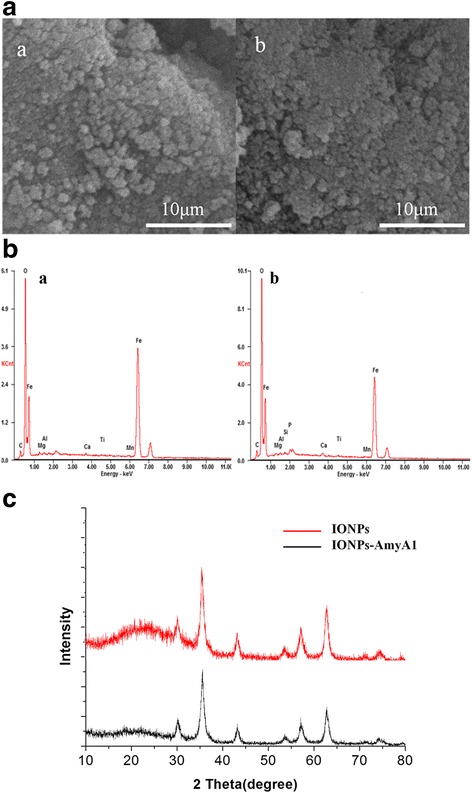
(a) SEM analysis of IONPs and AmyA1-IONPs (a: IONPs, b: AmyA1-IONPs); (b) EDS analysis of IONPs and AmyA1-IONPs (a: IONPs, b: AmyA1-IONPs); (c) XRD analysis of IONPs and AmyA1-IONPs
The effect of temperature on the activity and stability of free and immobilized AmyA1 was investigated. Both free and AmyA1-IONPs had an optimal temperature of 40 °C. However, the immobilized enzyme showed comparatively higher activity when the temperature increased or decreased. The AmyA1-IONPs kept more than 40% of the optimal activity at 20 and 70 °C, whereas the free AmyA1 maintained only 23.2 and 11.6%, respectively (Fig. 7a). The stability of free and immobilized AmyA1 at various temperatures suggested that AmyA1-IONPs were obviously more stable than free AmyA1 after being incubated at temperatures ranging 20–60 °C for 2 h (Fig. 7b). The magnetic immobilized α-amylase retained nearly 20% of the optimal activity at 60 °C, but the free AmyA1 lost its activity within 15 min. The effect of pH was also investigated. The immobilized AmyA1 exhibited higher activity in neutral and basic conditions, with an optimal pH of 6.0–7.0, which is higher than that of free AmyA1 (pH 5.0) (Fig. 7c). Meanwhile, AmyA1-IONPs displayed better pH tolerance and maintained its vitality slightly better (Fig. 7d).
Fig. 7.
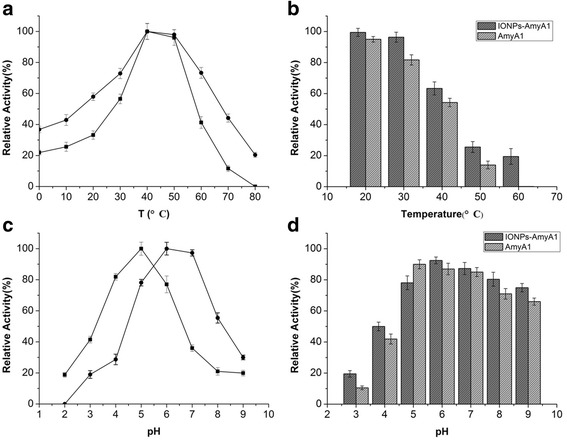
a Temperature dependence of AmyA1 (■) and AmyA1-IONPs (●); b Thermostability of AmyA1 and AmyA1-IONPs; c Relative activity of AmyA1 (■) and AmyA1-IONPs (●) at different pH conditions; d pH stability of AmyA1 and AmyA1-IONPs
The activity of AmyA1-IONPs was 11.9 × 103 U/mL while the activity of free enzyme was found to be 12.8 × 103 U/mL. The immobilized AmyA1 retained 93.1% of its original activity as compared to free enzyme. The α-amylase loading on IONPs was found to be 190.9 mg/g. To investigate the reusability, AmyA1-IONPs were recovered using magnetic separation after each batch reaction and reused for hydrolysis of soluble starch. The magnetic immobilized α-amylase maintained more than 90% activity in the first 3 cycles, and 60% of its initial hydrolytic activity even after 8 cycles (Fig. 8).
Fig. 8.
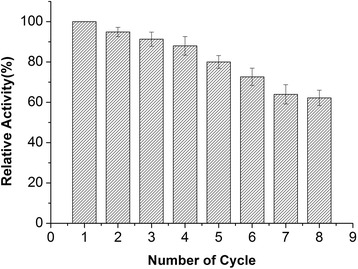
Reusability of immobilized AmyA1
Discussion
Alpha-amylase, one of the most versatile enzymes, holds a large market share with applications in a broad range of industrial processes. Diverse α-amylases from various genera have already been cloned and well characterized. To date, cold-adapted amylases from bacteria have drawn attention, such as thermolabile α-amylase from Pseudoalteromonas arctica and cold-adapted α-amylase from Alteromonas haloplanctis [24, 25]. However, the amylases from psychrophilic fungi have been poorly investigated at the molecular level. G. pannorum, a psychrotolerant fungus thriving in Antarctica, secretes many kinds of hydrolytic enzymes, like amylase, protease, cellulase, and esterase. In this work, a cold-adapted amylase gene AmyA1 was successfully cloned from G. pannorum R1-2. Sequence analysis showed that AmyA1 shares relatively low homology with α-amylase from other genera, revealing its research significance as a novel enzyme.
Note that no enzymatic activity was detected when AmyA1 was expressed in E. coli and Pichia pastoris. However, AmyA1 was functionally heterologous expressed in A. oryzae and efficiently secreted into the medium, demonstrating that the A. oryzae system we constructed has distinct advantages for fungal genes expression. Subsequently, the AmyA1 was purified for enzymatic properties exploration. Similar to fungal α-amylases from A. oryzae, A. niger, and Rhizopus oryzae, AmyA1 exhibited the highest activity at neutral environment (pH 5.0–7.0) with a sharp peak in pH curve. Generally, lower optimum temperature and greater thermosensitivity than most reported fungal amylases indicate broad potential in industrial applications, such as additives in processed food, waste-water treatment, cold-washing detergents, cold-climate bioremediation, molecular-biological applications. The optimum temperature of AmyA1 is slightly lower than that of other α-amylases, and its thermolabile feature (unstable above 40 °C) are consistent with the cold-adapted amylases [24, 25], thus providing an opportunity of AmyA1 for further use. It is worth mentioning that the wild strain G. pannorum R1-2 could not present α-amylase activity above 30 °C, but the AmyA1 showed its optimum temperature at 40–50 °C, indicating the abundant enzyme reserves of microorganism, and its powerful resistance to harsh environment.
Generally, the main products of starch catalyzed by fungal α-amylase were maltose with a small amount of glucose [26, 27]. However, our study revealed a high-degradation rate and high-glucose product catalyzed by AmyA1, which could simplify the syrup industry by eliminating the saccharification process. Meanwhile, AmyA1 also produced abundant maltotriose as well as certain amounts of maltotetraose and maltopentaose, suggesting that AmyA1 could serve as a potential candidate for functional oligosaccharide production. In addition, substrate specificity experiments demonstrated that AmyA1 had broad substrate scope and could act on diverse kinds of starch. Note that AmyA1 could catalyze glycogen effectively, which is distinct from other α-amylases [28].
Nowadays, bread quality received a particular attention on account of its high consumption. Thus considerable efforts have given rise to improve breads that combine health benefits with good sensory properties. For instant, addition of glycogen branching enzyme led to bread volume increase, while adding esterase resulted in a decreased bread volume [22, 29]. The water and sucrose SRC values of flour reduced with the increasing level of endoxylanases [30]. Amylase action and properties also significantly affect starch structure in bread. The supplementation of Bacillus subtilis α-amylase caused an increase in hardness, gumminess, and a decrease in springiness, cohesiveness [18]. In comparison, addition of AmyA1 led to decrease in gumminess and increase in springiness and cohesiveness, which favor better bread quality. Furthermore, lower optimum temperature, greater thermosensitivity, and its neutral optimal pH indicate that AmyA1 would be more favored in the baking industry or related applications, because its characteristics coincide well with the condition of dough fermentation [22].
Easy separation and repeated reuse perform significant roles in utilization of enzymes. Recently, a convenient and economical method using iron oxide nanoparticles is thriving for separation of biomacromolecules [10, 31]. Several successful applications on proteins as well as cells were reported [32–34]. In order to expand the potential value of this enzyme in other areas, the purified fungal α-amylase AmyA1 was efficiently immobilized on magnetic nanoparticles and the characteristics of immobilized AmyA1 were investigated. The AmyA1-IONPs displayed more stability than free AmyA1, mainly due to the fact that IONPs made the conformation of α-amylase more rigid and lead to less denaturation of protein. Moreover, fixing the enzyme on a matrix could also decrease the protein autolysis [23]. Compared with the free enzyme, the immobilized enzyme exhibited better pH tolerance and stability under neutral conditions. This notable shift of optimum pH may be owing to the difference between H+ and OH− concentrations in the microenvironment, which is formed by the electrostatic interactions with the carrier [35]. Meanwhile, the binding interaction between IONPs and AmyA1 could reduce drastic conformational changes during pH shifting [36]. The magnetically immobilized AmyA1 can be effectively separated, recovered and reused for maximum utilization. The immobilized α-amylase showed good stability over cycles and 60% of its initial hydrolytic activity was retained after the eighth cycle. The favorable stability and longer service life of the magnetically immobilized AmyA1 provide an opportunity for large-scale application and cost reduction.
Conclusions
The presented study described the gene cloning, expression, purification, and characterization of a novel α-amylase from Antarctic mycelial fungus G. pannorum. The detailed report of the enzymatic properties of AmyA1 gives new insights into fungal cold-adapted amylase. Moreover, different application prospects of α-amylase are investigated. Application study showed the potential value of AmyA1 in traditional baking and food industry. By the means of magnetic immobilization, the improved thermostability and favorable reusability of AmyA1 could potentially support industrial production.
Acknowledgements
We are grateful to Institute of Ocean and Earth Sciences, University of Malaya for offering strain Geomyces pannorum.
Funding
This work was funded by Natural Science Foundation of Shanghai (No. 16ZR1449500), the National High Technology Research and Development Program of China (No. 2013AA102109), the National Natural Foundation of China (No. 31571786), and the National Natural Foundation of China (No. 21406068/B060804).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files. The dataset of sequence generated during the current study is available in the GenBank repository (http://www.ncbi.nlm.nih.gov/genbank/). Accession number of the mRNA sequence of AmyA1: KU925863.
Authors’ contributions
LH carried out cloning of a novel α-amylase, heterologous expression, characterization and drafted the manuscript. YM carried out construction of Aspergillus oryzae system. LZ carried out application of the recombinant α-amylase in baking. HW participated in the immobilization of enzyme. SAA performed the statistical analysis. BG and DW participated in the design of the study and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional files
Nucleotide and deduced amino-acid sequence of the cDNA of AmyA1. The bases of lowercase interrupting the coding region are introns (Intron I, Intron II). (TIF 7270 kb)
Scheme for construction of the vector pSKNHG for α-amylase expression in Aspergillus sp. (TIF 12,481 kb)
SDS-PAGE analysis of AmyA1. Lane 1, protein molecular mass markers; Lane 2, cultured supernatant of transformant A1-3; Lane 3-6, NPI 20, 50, 200, 500 elution of cultured supernatant of transformant A1-3; Lane 7-8, the purified recombinant AmyA1. (TIF 1590 kb)
Contributor Information
Lei He, Email: 303402935@qq.com.
Youzhi Mao, Email: maoyz08@yeah.net.
Lujia Zhang, Email: ljzhang@ecust.edu.cn.
Hualei Wang, Email: hlwang@ecust.edu.cn.
Siti Aisyah Alias, Email: saa@um.edu.my.
Bei Gao, Email: gaobei@ecust.edu.cn.
Dongzhi Wei, Email: 25363720@qq.com.
References
- 1.Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38(11):1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- 2.Geertrui B, Bert L, Ellen F, Jan D. Impact of amylases on biopolymer dynamics during storage of straight-dough wheat bread. J Agric Food Chem. 2013;61:6525–6532. doi: 10.1021/jf402021g. [DOI] [PubMed] [Google Scholar]
- 3.Rana N, Walia A, Gaur A. α-Amylases from microbial sources and its potential applications in various industries. Natl Acad Sci Lett. 2013;36:9–17. doi: 10.1007/s40009-012-0104-0. [DOI] [Google Scholar]
- 4.Hans G, Pedro L, Annabel B, Jan D. Antifirming effects of starch degrading enzymes in bread crumb. J Agric Food Chem. 2009;57:2346–2355. doi: 10.1021/jf803058v. [DOI] [PubMed] [Google Scholar]
- 5.Leon AE, Duran E, Benedito De Barber C. Utilization of enzyme mixtures to retard bread crumb firming. J Agric Food Chem. 2002;50(6):1416–1419. doi: 10.1021/jf0106446. [DOI] [PubMed] [Google Scholar]
- 6.Feller G, Le Bussy O, Gerday C. Expression of psychrophilic genes in mesophilic hosts: assessment of the folding state of a recombinant alpha-amylase. Appl Environ Microbiol. 1998;64(3):1163–1165. doi: 10.1128/aem.64.3.1163-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan A, Alias SA, Wong CMVL, Pang KL, Convey P. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar Biol. 2011;34(10):1535–1542. doi: 10.1007/s00300-011-1012-3. [DOI] [Google Scholar]
- 8.Fenice M, Selbmann L, Zucconi L, Onofri S. Production of extracellular enzymes by Antarctic fungal strains. Polar Biol. 1996;17(3):275–280. doi: 10.1007/s003000050132. [DOI] [Google Scholar]
- 9.Cao L. Immobilised enzymes: science or art? Curr Opin Chem Biol. 2005;9(2):217–226. doi: 10.1016/j.cbpa.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Baskar G, Afrin Banu N, Helan Leuca G, Gayathri V, Jeyashree N. Magnetic immobilization and characterization of α-amylase as nanobiocatalyst for hydrolysis of sweet potato starch. Biochem Eng J. 2015;102:18–23. doi: 10.1016/j.bej.2015.02.020. [DOI] [Google Scholar]
- 11.Jia H, Huang F, Gao Z, Zhong C, Zhou H, Jiang M, Wei P. Immobilization of ω-transaminase by magnetic PVA-Fe3O4 nanoparticles. Biotechnol Rep. 2016;10:49–55. doi: 10.1016/j.btre.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long J, Xu E, Li X, Wu Z, Wang F, Xu X, Jin Z, Jiao A, Zhan X. Effect of chitosan molecular weight on the formation of chitosan-pullulanase soluble complexes and their application in the immobilization of pullulanase onto Fe3O4-kappa-carrageenan nanoparticles. Food Chem. 2016;202:49–58. doi: 10.1016/j.foodchem.2016.01.119. [DOI] [PubMed] [Google Scholar]
- 13.Fang G, Chen H, Zhang Y, Chen A. Immobilization of pectinase onto Fe3O4@SiO2-NH2 and its activity and stability. Int J Biol Macromol. 2016;88:189–195. doi: 10.1016/j.ijbiomac.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Ling SO, Storms R, Zheng Y, Rodzi MR, Mahadi NM, Illias RM, Abdul Murad AM, Abu Bakar FD. Development of a pyrG mutant of Aspergillus oryzae strain S1 as a host for the production of heterologous proteins. Sci World J. 2013;2013:634317. doi: 10.1155/2013/634317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama J, Kitamoto K. Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (DeltaligD) in Aspergillus oryzae. Biotechnol Lett. 2008;30:1811–1818. doi: 10.1007/s10529-008-9763-9. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez EQ, Moyer DL, Maiyuran S, Labaro A, Brody H. Vector-initiated transitive RNA interference in the filamentous fungus Aspergillus oryzae. Fungal Genet Biol. 2012;49:294–301. doi: 10.1016/j.fgb.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Gail M. Use of dinitrosalicylic acid reagent for determintation of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 18.Roy JK, Borah A, Mahanta CL, Mukherjee AK. Cloning and overexpression of raw starch digesting α-amylase gene from Bacillus subtilis strain AS01a in Escherichia coli and application of the purified recombinant α-amylase (AmyBS-I) in raw starch digestion and baking industry. J Mol Catal B Enzym. 2013;97:118–129. doi: 10.1016/j.molcatb.2013.07.019. [DOI] [Google Scholar]
- 19.Janecek S. How many conserved sequence regions are there in the α-amylase family? Biol Bratislava. 2002;57(Suppl.11):29–41. [Google Scholar]
- 20.Salimi A, Ghollasi M, Tavoli H, Khajch K. Investigations on possible roles of C-terminal propeptide of a Ca-independent α-amylase from Bacillus. J Microbiol Biotechnol. 2012;22(8):1077–1083. doi: 10.4014/jmb.1112.11085. [DOI] [PubMed] [Google Scholar]
- 21.Ben Halima N, Borchani M, Fendri I, Khemakhem B, Gosset D, Baril P, Pichon C, Ayadi MA, Abdelkafi S. Optimised amylases extraction from oat seeds and its impact on bread properties. Int J Biol Macromol. 2015;72:1213–1221. doi: 10.1016/j.ijbiomac.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Liu Y, Yan Q, Jiang Z. Gene cloning, functional expression and characterisation of a novel glycogen branching enzyme from Rhizomucor miehei and its application in wheat breadmaking. Food Chem. 2014;159:85–94. doi: 10.1016/j.foodchem.2014.02.161. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Ni K, Wei D, Ren Y. One-step purification and immobilization of his-tagged protein via Ni2+-functionalized Fe3O4@polydopamine magnetic nanoparticles. Biotechnol Bioprocess Eng. 2015;20(5):901–907. doi: 10.1007/s12257-015-0136-7. [DOI] [Google Scholar]
- 24.Lu M, Wang S, Fang Y, Li H, Liu S, Liu H. Cloning, expression, purification, and characterization of cold-adapted alpha-amylase from Pseudoalteromonas arctica GS230. Protein J. 2010;29(8):591–597. doi: 10.1007/s10930-010-9290-0. [DOI] [PubMed] [Google Scholar]
- 25.Feller G, Lonhienne T, Deroanne C, Libioulle C, Van Beeumen J, Gerday C. Purification, characterization, and nucleotide sequence of the thermolabile alpha-amylase from the antarctic psychrotroph Alteromonas haloplanctis A23. J Biol Chem. 1992;267(8):5217–5221. [PubMed] [Google Scholar]
- 26.Li S, Zuo Z, Niu D, Singh S. Gene cloning, heterologous expression, and characterization of a high maltose-producing α-amylase of Rhizopus oryzae. Appl Biochem Biotechnol. 2011;164:581–592. doi: 10.1007/s12010-011-9159-5. [DOI] [PubMed] [Google Scholar]
- 27.Khoo S, Amirul AA, Kamaruzaman M, Nazalan N, Azizan M. Purification and characterization of α-amylase from Aspergillus flavus. Folia Microbiol. 1994;39:392–398. doi: 10.1007/BF02814445. [DOI] [PubMed] [Google Scholar]
- 28.Sahnoun M, Bejar S, Sayari A, Triki MA, Kriaa M, Kammoun R. Production, purification and characterization of two α-amylase isoforms from a newly isolated Aspergillus oryzae strain S2. Process Biochem. 2012;47:18–25. doi: 10.1016/j.procbio.2011.09.016. [DOI] [Google Scholar]
- 29.Nieter A, Kelle S, Takenberg M, Linke D, Bunzel M, Popper L, Berger RG. Heterologous production and characterization of a chlorogenic acid esterase from Ustilago maydis with a potential use in baking. Food Chem. 2016;209:1–9. doi: 10.1016/j.foodchem.2016.03.106. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Hou GG, Chen Z, Gehring K. Effects of endoxylanases, vital wheat gluten, and gum Arabic on the rheological properties, water mobility, and baking quality of whole-wheat saltine cracker dough. J Cereal Sci. 2013;58(3):437–445. doi: 10.1016/j.jcs.2013.09.006. [DOI] [Google Scholar]
- 31.Ni K, Lu H, Wang C, Black KC, Wei D, Ren Y, Messersmith PB. A novel technique for in situ aggregation of Gluconobacter oxydans using bio-adhesive magnetic nanoparticles. Biotechnol Bioeng. 2012;109(12):2970–2977. doi: 10.1002/bit.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman MA, Culsum U, Kumar A, Gao H, Hu N. Immobilization of a novel cold active esterase onto Fe3O4 approximately cellulose nano-composite enhances catalytic properties. Int J Biol Macromol. 2016;87:488–497. doi: 10.1016/j.ijbiomac.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Saravanakumar T, Palvannan T, Kim DH, Park SM. Optimized immobilization of peracetic acid producing recombinant acetyl xylan esterase on chitosan coated-Fe3O4 magnetic nanoparticles. Process Biochem. 2014;49:1920–1928. doi: 10.1016/j.procbio.2014.08.008. [DOI] [Google Scholar]
- 34.Xia TT, Liu CZ, Hu JH, Guo C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem Eng J. 2016;295(11):201–206. doi: 10.1016/j.cej.2016.03.044. [DOI] [Google Scholar]
- 35.Lee KM, Kalyani D, Tiwari MK, Kim TS, Dhiman SS, Lee JK, Kim IW. Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresour Technol. 2012;123:636–645. doi: 10.1016/j.biortech.2012.07.066. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Kaskel S, Shi JL, Wage T, van Pee KH. Immobilization of trametes versicolor laccase on magnetically separable mesoporous silica spheres. Chem Mater. 2007;19:6408–6413. doi: 10.1021/cm071265g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. The dataset of sequence generated during the current study is available in the GenBank repository (http://www.ncbi.nlm.nih.gov/genbank/). Accession number of the mRNA sequence of AmyA1: KU925863.


