Abstract
Background:
This study attempted to determine the effects of long-term use of Vitamin C on vascular endothelial function.
Materials and Methods:
During a pilot clinical trial study conducted at Imam Hussein Hospital (Isfahan) in 2014–2015, a total of forty diabetic patients were selected and then assigned randomly into two twenty-subject groups receiving Vitamin C and placebo tablets. The patients were treated with Vitamin C or placebo for 6 months. All patients were examined through echocardiography in terms of cardiac function before and after treatment. To evaluate the endothelial function (flow-mediated dilatation [FMD], intima-media thickness), they underwent arterial Doppler. Moreover, the chemical indices of vascular function were tested through intercellular adhesion molecule and vascular cell adhesion molecule (VCAM). Finally, the results were compared between the two groups.
Results:
Based on the results, the mean left ventricular mass significantly reduced after the intervention in the group treated with Vitamin C (from 76.35 ± 25.6–68.62 ± 22.66; P = 0.015) while there was no significant difference observed in the control group (from 67.58 ± 25.38–71.63 ± 26.84; P = 0.19) but no statistically difference between the two groups-based repeated measures ANOVA test (P = 0.6). In addition, the mean of VCAM changes was significantly difference between the two groups (P < 0.001).
Conclusion:
Long-term use of Vitamin C in diabetic patients can improve certain echocardiographic parameters such as ejection fraction, fractional shortening, and FMD, which in turn enhances vascular endothelial function.
Keywords: Adolescents, children, diabetes, endothelial function, Vitamin C
INTRODUCTION
Over the last two decades, it has been proven that endothelium plays an essential role in homeostasis.[1] In addition to atherosclerosis, endothelial dysfunction has been observed in various pathological conditions such as hypercholesterolemia, diabetes, hypertension, heart failure, smoking, and aging.[1] Under basic conditions, endothelial functions are supposed to sustain a relatively dilated vascular system. Although endothelial capacity responds to physical stimuli such as shear stress, blood vessels are dilated in response to stress.
Endothelial dysfunction induced by dyslipidemia is the initial step in atherosclerosis.[1,2] Among patients with normal coronary arteries or minimal coronary artery disease, positive family history of coronary artery disease can be a significant predictor of the risk of a coronary artery low blood flow, reflecting any potential coronary endothelial dysfunction in the microcirculation.[3]
Brachial artery ultrasound imaging during reactive hyperemia provides a tool for vascular tone measurement associated with endothelium and may verify vascular endothelial dysfunction.[4] Measurement of vascular tone impairment (endothelial dysfunction) plays an important role as a screening tool. Vasodilatation through blood flow is technically called flow-mediated dilatation (FMD). FMD measurement is a clinical method to assess endothelial function. This endothelium-dependent response is essentially regulated by the release of nitric oxide from the endothelium.[1]
In patients with hypercholesterolemia, Vitamin C, folate, 5-methyl tetra-dihydrofolate (active form of folic acid) can improve endothelial function without changes in plasma lipid levels.[4] Vitamin C can also reverse the microcirculatory coronary response, thus delaying coronary flow damage in smokers with normal coronary arteries.[5] Vitamin C and folate may reduce oxidative stress and prevent the destruction of nitric oxide.
Vitamin C is a water-soluble vitamin and is a nutritional supplement essential for collagen formation and tissue repair. Moreover, it engages in a number of other metabolic reactions such as reductase reactions.[6]
Vitamin C is a potent endogenous antioxidant, regulating the intracellular reductase through glutathione, capable of destroying oxygen-derived free radicals.[7] Vitamin C can reverse damages caused by smoking, including oxidative stress and increased monocyte adhesion to endothelium.[8] Vitamin C may affect coronary artery disease through curtailing lesion activity.[9] Observational studies of Vitamin C have yielded mixed results in the prevention of coronary heart disease (CHD). A few have demonstrated the beneficial effects of Vitamin C.[10] Others identified small benefits.[11,12,13] However, there has been no consensus about the effects of Vitamin C on improving endothelial function, since the previous studies have been limited. Considering the high prevalence of cardiovascular diseases in diabetes patients and the role of endothelial dysfunction in this process, this study intended to determine the long-term administration of Vitamin C on vascular endothelial function in children and adolescents with diabetes.
MATERIALS AND METHODS
The current study was a pilot randomized, double-blind study carried out at Imam Hussein Hospital in Isfahan during 2014–2015.
Inclusion criteria were age range 5–18 years, diagnosed with diabetes, at least 5 years of diabetes, absence of any other systemic diseases, no intake of Vitamin C in the 3 months before the start of the study, no smoking, no pregnancy, normal diet regimen (no special diet such vegetarian habit), and no suffering to dietary diseases.[6]
The exclusion criteria were nonintake of Vitamin C or placebo during the study for any reason, failure to attend upcoming sessions, and development of any new disease during the study.
The smallest sample size was estimated to be twenty patients using the sample size formula taking into account the 95% confidence level, 80% test power, and 10% standard deviation of FMD estimated to be 0.9. The minimum significant difference between the two treatment procedures was 0.8. Moreover, the samples were selected through a convenient procedure.
After approval of the proposal and obtaining permission from the University Ethics Committee, forty patients fulfilling the inclusion criteria were visited in Imam Hussein Hospital where they were diagnosed with diabetes. Having spoken with patients and their parents and having obtained their written consent to participate in the study, the patients were distributed into two twenty-member groups through randomized block design. In fact, the first patient was randomly by lot placed in one of the groups supplemented with Vitamin C or placebo while the patients were in order of inclusion distributed consecutively into the two groups until the sample size was realized.
The patients were blinded since they were unaware of the type of drug or placebo administered. Moreover, the drug or placebo was similarly supplied by an identical pharmaceutical company and then coded by a third person unaware of the study details while the project researcher was unaware of the drug specifications.
At baseline, demographic data and patient records were gathered together with heights and weights recorded in the data collection form. Before intervention, all patients were examined through echocardiography in terms of cardiac function. The endothelial function was evaluated through arterial Doppler. FMD, intima-media thickness (IMT), and chemical indices of vascular function were tested through intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecules (VCAM). Before the intervention, the level of hemoglobin A1C was measured to determine glycemic control, where 7 and lower values were considered desirable blood glucose control and 7 and above values were considered undesirable blood glucose control.
Patients in intervention group were treated with 250 mg of single daily dose of oral Vitamin C. Patients in the control group received a placebo with the same shape and size of Vitamin C tablets and with the same duration.[11] At the end of the 6th-month period, the tests were repeated for all patients.
Given that, many factors affect the FMD and IMT (such as medicines, food, sympathetic stimulation, etc.) patients were fast for at least 6 h before doing FMD. Moreover, the test took place in a quiet environment at a constant temperature of 25°C.[3] In addition, all cardiovascular-effective drugs such as nitrates were cutoff for at least four half-life. Moreover, 24 h before the FMD test, prohibition was imposed on any exercise and consumption of foods containing caffeine and similar substances and high-fat foods and Vitamin K (either natural or supplementary) and folic acid. For women in the first 7 days of menstrual cycle, FMD test was not performed to reduce the impact of hormones on the test.[3]
Patients underwent full echocardiogram to evaluate cardiac chambers, systolic and diastolic functions, valvular dysfunction, and hypertrophy.
The thickness of the carotid artery intima and media was measured through two-dimensional high-resolution ultrasound images by a 7–12 MHz transducer of EKO 7 machine by Samsung Medison Company. The patients rested in the supine position for 10 min at room temperature 24–26°C. The right carotid artery was scanned while the patient looked to the left. An artery length where intima can be clearly seen was used to measure the thickness of intima. That was repeated three times until the average thickness value was achieved for thickness of carotid intima and media.
The right-hand brachial artery located in the extension position was scanned in longitudinal cross-section of approximately 2–5 cm above right elbow hole. Transducer was placed at an angle of 70–80° to the body. Settings were made for optimum lumen artery wall. Electrocardiogram (ECG) monitor was attached to the patient along with ultrasound machine. The size of the lumen was recorded at the end of diastole coincident with R wave in ECG. All images were stored in the hard drive of the echo machine. A pneumatic tourniquet was inflated on the forearm distal to the scanning area, 50 mmHg over the systolic blood pressure of the patient for 5 min. An increase in blood flow was observed by sudden emptying of cuff while the measurements from the anterior to posterior in the midline at the end of diastole were calculated again. The lumen diameter was scanned 60 s after cuff was deflated. The average vascular diameter and dilatation percentage were obtained for each patient. FMD was calculated during hyperemia as percentage increase in diameter compared with the resting period.[3] Moreover, after 6 months of intervention, FMD, IMT, and echocardiographic indices of heart as well as biochemical markers of endothelial function (ICAM, VCAM) were measured for all subjects.
After completion of the study, the drug codes were recognized, and the data were analyzed with SPSS 23 (IBM corporation, USA). Statistical tests used for data analysis included Chi-square (comparison of the two groups in terms of qualitative and nominal data), t-test (for comparison of continuous data between the two groups), T-paired (to compare continuous data in each group), and repeated measures ANOVA (for examining how variables changed in the two groups).
RESULTS
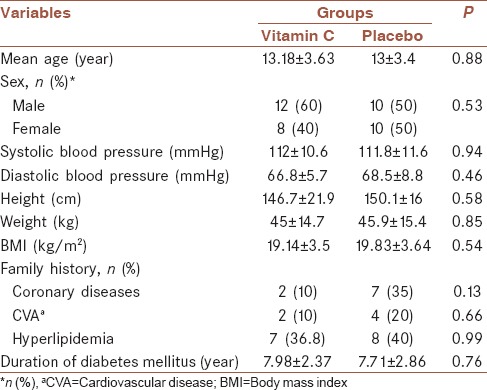
In this study, forty patients with diabetes were examined in two groups of twenty patients receiving Vitamin C and placebo. During the study period, no patient was excluded from the study, and forty patients participated to the end. Table 1 displays the distribution and demographic characteristics of the two groups. Based on Chi-square and t-test, there were no differences between the two groups in terms of age, gender, blood pressure, height, weight, body mass index, family history of the disease, and duration of diabetes.
Table 1.
Distribution of the demographic and general variables in the two groups
Hypoglycemia occurred in the case and control groups in two and four cases, respectively (10% vs. 20%). However, it was not statistically significant. One patient had coma in the control group.
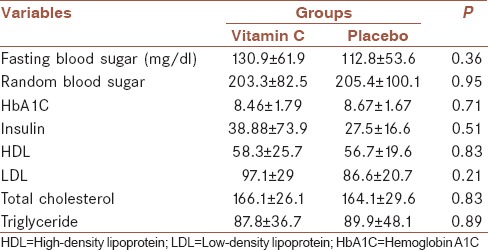
Table 2 displays the biochemical findings in patients of both groups. Based on t-test, the mean 2-h fasting blood glucose level, HbA1C, insulin, high-density lipoprotein and low-density lipoprotein cholesterol, and triglyceride levels had no significant statistical differences between the groups.
Table 2.
The mean and standard deviation of glucose and lipid profiles in the two groups before intervention
The mean hemoglobin A1C at baseline and after intervention in the group supplemented with Vitamin C were 8.5 ± 1.9 and 8.4 ± 2.15 (P = 0.82). Moreover, hemoglobin A1C levels at baseline and after intervention in the placebo group were 9.61 ± 1.75 and 8.21 ± 1.4 (P = 0.18). In addition, according to repeated measures ANOVA test, changes of echocardiography parameters and biochemical findings aren’t significant between the two groups except VCAM value that was ststisticaly decreased in the intervention groups.
Table 3 shows the mean and standard deviation of echocardiographic parameters in both groups before and after the intervention. Based on the results of paired t-test, the mean left ventricular (LV) mass significantly reduced after the intervention in the group treated with Vitamin C while there was no significant difference observed in the control group. The mean ejection fraction (EF), systolic function (SF), FMD, and Myocardial Performance Index (MPI) significantly altered in the intervention group while there was no significant difference in the control group.
Table 3.
The mean and standard deviation of echocardiographic parameters and vascular endothelial function indicators before and after intervention in both groups

The results indicated that 13 patients (32.5%) had adequate control of blood glucose levels, and 27 patients (67.5%) had inadequate control of blood glucose levels during the 6-months period based on the level of hemoglobin A1C. On the other hand, 13 patients in the intervention group and 14 patients in the control group had improper control blood glucose levels (65% vs. 70%) (P = 0.73).
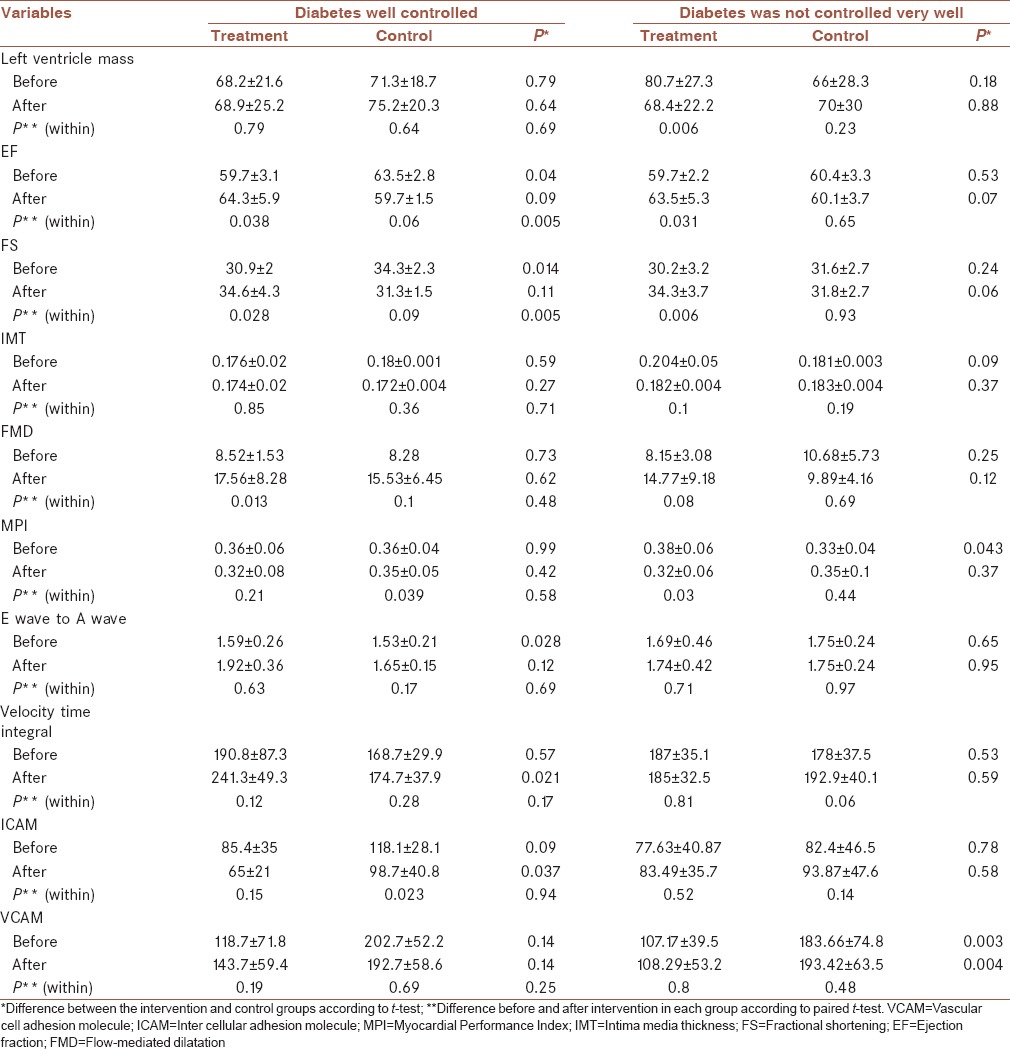
Table 4 displays the mean and standard deviation of echocardiographic findings and indicators related to vascular endothelial function in two groups of intervention and control as well as blood glucose control over the 6 months of intervention. Based on the group of patients with well-controlled diabetes, there was a significant difference between the two groups receiving Vitamin C and placebo in terms of EF and SF, whereas the group of patients whose diabetes was not controlled, showed a significant difference in EF and SF as well as LV mass changes.
Table 4.
The mean and standard deviation of echocardiographic parameters and endothelial function indicators in the two groups receiving Vitamin C and placebo separately in terms of controlled and uncontrolled diabetes
DISCUSSION
There have been numerous studies demonstrating that the incidence of atherosclerosis and cardiovascular diseases in diabetic patients is by far more than that in the general population[1] and such a phenomenon mostly arises from vascular endothelial dysfunction. On the other hand, the studies have shown that antioxidants can improve the vascular endothelial function. In this regard, Vitamin C is a powerful antioxidant applied in some studies. Hence, this study aimed to determine the effect of long-term Vitamin C intake on vascular endothelial function in pediatric and adolescent patients with diabetes.
Our results showed that taking Vitamin C for 6 months can affect and improve a number of echocardiographic and sonographic parameters such as EF, SF, LV mass, MPI, and FMD and the significantly higher in case rather than the control group. However, chemical parameters of vascular function including ICAM and VCAM showed no significant difference between the two groups, although changes in the level of ICAM and VCAM was higher in the intervention group than the control group.
In another study by Sabri et al. at Isfahan University of Medical Sciences on 18 patients with type I diabetes and 19 healthy children, IMT was reduced in both groups of patients and healthy subjects after taking Vitamin C for 1 month.[14] Furthermore, FMD increased in both groups, but it was significant only in the control group. The results of this study and the previous one could indicate the fact that the use of Vitamin C may have a protective effect on endothelial dysfunction. At the same time, in another study conducted by Erbs et al., administration of Vitamin C on patients with coronary artery disease and congestive heart failure was associated with improved vascular endothelial function.[15] In a review study by Ashor et al., evaluation of 14 randomized clinical trials demonstrated a positive effect of Vitamin C on EF, which was evident in three groups of patients with atherosclerotic disease, diabetes, and heart failure.[16] Moreover, the results of this study had a significant correlation with an increase in EF with the prescribed dose of Vitamin C. In another review in 2015, Ashor et al. examined the effect of Vitamins C and E to improve cardiac EF, concluding that the effect of Vitamin C; this parameter was significant and more dependent on the patient's age.[17] Raitakari et al. (1999) administered supplementation of Vitamin K improved vascular endothelial function in smoking patients.[18] Ceriello et al. showed that administration of Vitamin C in diabetic patients improved the endothelial dysfunction and oxidative stress in diabetic patients.[19] In a study conducted by Ling et al., the postprandial serum triglyceride concentration increased significantly at 2–5 h after the high-fat meal in all groups. The fasting FMD (P < 0.02) and nitroglycerin-induced dilatation (NID) (P < 0.05) of patients with CHD were impaired compared with those of non-CHD subjects. Postprandial FMD was significantly aggravated in the non-CHD/control group (P < 0.01) and the CHD/control group (P < 0.001), but the postprandial FMD in patients and subjects taking Vitamin C showed no significant change; however, the CHD/Vitamin C group had a mild tendency toward improvement (P = 0.064) and non-CHD/Vitamin C group had a mild tendency toward aggravation (P = 0.852). The change of NID after a high-fat meal did not reach statistical significance in the four groups. The decrement of postprandial FMD correlated positively with the increment of 2-h serum triglyceride concentration in the patients without Vitamin C (n = 62, r = 0.545, P < 0.001).[20]
CONCLUSIONS
The results of the current study showed that long-term use of Vitamin C in diabetic patients can improve certain echocardiographic and sonographic parameters such as EF, fractional shortening, MPI, and FMD, which in turn may show improvement in vascular endothelial function. However, because this study was done in a pilot and due to the lack of significant difference in the biochemical markers, such as ICAM and VCAM as well as the limitations of this study, including a small sample size and low dose of Vitamin C, it is recommended that further studies could be carried out with larger sample size and higher Vitamin C dosage in diabetic patients.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences (Research project number: 393852).
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
All of the authors contributed in all stages of this work.
Acknowledgments
The authors want to highly appreciate the cooperation's of Applied Physiology Research Center staffs, especially Dr. Shaghayegh Haghjooy Javanmard in completing in this research project.
REFERENCES
- 1.Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adults. 7th ed. philidelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 2.Schächinger V, Britten MB, Elsner M, Walter DH, Scharrer I, Zeiher AM. A positive family history of premature coronary artery disease is associated with impaired endothelium-dependent coronary blood flow regulation. Circulation. 1999;100:1502–8. doi: 10.1161/01.cir.100.14.1502. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients<40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–4. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 4.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–2. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schäfers KP, Lüscher TF, Camici PG. Coronary heart disease in smokers: Vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–8. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board Staff, Panel on Dietary Antioxidants, and Institute of Medicine Staff. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. National Academies Press. 2000 [Google Scholar]
- 7.Piper P, Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971;180:363–85. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–74. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Arnet U, Bauer E, von Segesser L, Siebenmann R, Turina M, et al. Thrombin-induced endothelium-dependent inhibition and direct activation of platelet-vessel wall interaction. Role of prostacyclin, nitric oxide, and thromboxane A2. Circulation. 1994;89:2266–72. doi: 10.1161/01.cir.89.5.2266. [DOI] [PubMed] [Google Scholar]
- 11.Shahraz S, Ghaziani T. A Comprehensive Text Book of Drug Information. 2nd ed. Tehran: Teymourzadeh Corp; 2008. p. 801. [Google Scholar]
- 12.Hatton MW, Moar SL, Richardson M. Deendothelialization in vivo initiates a thrombogenic reaction at the rabbit aorta surface. Correlation of uptake of fibrinogen and antithrombin III with thrombin generation by the exposed subendothelium. Am J Pathol. 1989;135:499–508. [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein G, Bughdady Y, Kandil ME, Bazaraa HM, Taher H. Doppler assessment of brachial artery flow as a measure of endothelial dysfunction in pediatric chronic renal failure. Pediatr Nephrol. 2008;23:2025–30. doi: 10.1007/s00467-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 14.Sabri M, Tavana EN, Ahmadi A, Hashemipoor M. Effect of Vitamin C on endothelial function of children. IJPM. 2015;23:75–9. [Google Scholar]
- 15.Erbs S, Gielen S, Linke A, Möbius-Winkler S, Adams V, Baither Y, et al. Improvement of peripheral endothelial dysfunction by acute Vitamin C application: Different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J. 2003;146:280–5. doi: 10.1016/S0002-8703(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 16.Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235:9–20. doi: 10.1016/j.atherosclerosis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of Vitamin C and Vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2015;113:1182–94. doi: 10.1017/S0007114515000227. [DOI] [PubMed] [Google Scholar]
- 18.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Stocker R, Celermajer DS. Oral Vitamin C and endothelial function in smokers: Short-term improvement, but no sustained beneficial effect. J Am Coll Cardiol. 2000;35:1616–21. doi: 10.1016/s0735-1097(00)00576-3. [DOI] [PubMed] [Google Scholar]
- 19.Ceriello A, Piconi L, Esposito K, Giugliano D. Telmisartan shows an equivalent effect of Vitamin C in further improving endothelial dysfunction after glycemia normalization in type 1 diabetes. Diabetes Care. 2007;30:1694–8. doi: 10.2337/dc07-0318. [DOI] [PubMed] [Google Scholar]
- 20.Ling L, Zhao SP, Gao M, Zhou QC, Li YL, Xia B. Vitamin C preserves endothelial function in patients with coronary heart disease after a high-fat meal. Clin Cardiol. 2002;25:219–24. doi: 10.1002/clc.4950250505. [DOI] [PMC free article] [PubMed] [Google Scholar]


