Abstract
Background:
Osteoporosis affects quality of life (QoL) and may lead to depression in women. The purpose of this study was to evaluate the effects of zoledronic acid (ZA) treatment on depression and QoL in women with postmenopausal osteoporosis (PO).
Materials and Methods:
A total of 88 newly diagnosed women with PO were included in this study. All patients were treated with once-yearly ZA (5 mg). A QoL questionnaire from the European Foundation for Osteoporosis and Beck Depression Inventory were given to patients at baseline and at 12 months. The results for baseline and post - 12th month were compared, and bone mineral density (BMD) levels were compared.
Results:
The consumption of once-yearly ZA (5 mg) treatment increases BMD at levels of lumbers 1–4 (P = 0.026), total Hip T score's P value is same as femoral neck (P: 0,033). ZA 5 mg treatment also improved QoL (P = 0.001) and reduced depression (P = 0.001).
Conclusion:
ZA treatment increases BMD levels and QoL while reducing depression. Once-yearly ZA (5 mg) may be considered for postmenopausal women as a first-line treatment.
Keywords: Depression, osteoporosis, quality of life
INTRODUCTION
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue. This situation increases the risk of fracture and bone fragility.[1] Bone mineral density (BMD) is responsible for 60%–90% of bone strength. Osteoporotic bones have same size and appearance as the normal bone from the outside but have a thin layer of cortical bone and trabecular bone loss inside and are more fragile than normal bone. Low BMD is the most important predictor of fracture risk.[2]
Estrogen levels begin to fall at menopause. Serum estradiol levels remain fixed at under 30 pg/ml 1 year after menopause. Lower estrogen levels increased osteoclastic activity, reduced osteoblastic activity in the bone, and are responsible for rapid bone loss in women.[3]
The most common symptom is bone pain in patients with postmenopausal osteoporosis (PO). Patients may be asymptomatic until trabecular bone loss reaches 30–40%. The most common physical complaints are pain due to fractures, reduced mobility, spinal deformity, and shortened height. Older age, low-income levels, lack of activity, sedentary lifestyle, duration of menopause, and history of fractures are associated with a decrease in BMD.[2]
Several methods have been developed to assess health status and quality of life (QoL) associated with osteoporosis, including a QoL questionnaire from the European Foundation for Osteoporosis-41 (QUALEFFO-41). It is a simple test which is clearly understandable and repeatable. High scores are associated with low QoL.[4] Validity and reliability studies for QUALEFFO have been conducted in Turkey.[5]
Major depression has been repeatedly reported to be associated with low BMD in the spine and hip. Excessive activity of the hypothalamic-pituitary-adrenocortical axis has been consistently demonstrated in major depressive disorder, whereas depressive symptoms have been reported in patients with osteoporosis, especially in the postmenopausal period.[6,7] Panza et al. reported that depression has frequently been associated with osteoporosis. However, the nature of this link, and whether it is direct or indirect, remains to be explored.[8]
Bisphosphonates are the most commonly used drugs for the prevention and treatment of osteoporosis. Once-yearly zoledronic acid (ZA) 5 mg is a type of bisphosphonate. ZA prevents bone resorption by inhibiting osteoclasts.[9]
The purpose of this study was to investigate the effectiveness of once-yearly ZA 5 mg on depression and QoL in the women with PO.
MATERIALS AND METHODS
A total of 88 newly diagnosed women in 2 years between 2012 and 2014 with PO referred to the endocrinology clinic of Samsun Ondokuz Mayıs University Medicine Faculty and treated with once-yearly ZA 5 mg (ACLASTA® Novartis) were included in this self-controlled prospective cohort study. ZA was chosen for PO treatment because of proven efficacy, ease of use, low cost of treatment, and not requiring follow-up. ZA is the most frequently used drug in the prevention and treatment of osteoporosis. ZA 5 mg was granted the license in 2008 in our country. ZA 5 mg is used as intravenous (IV) infusion once a year. We compared the QoL before and after ZA treatment in the women with PO. Our study does not include control group. Sample size was determined according to year between 2012 and 2014. BMD levels were measured by dual-energy X-ray absorptiometry (DXA) from the lumbar (L1–L4) and femoral neck regions. Diagnosis of osteoporosis was made according to the World Health Organization criteria. Osteoporosis is ≥ 2.5 standard deviations below the mean BMD of healthy adults of the same sex and race.[10] A QoL questionnaire from the QUALEFFO and Beck Depression Inventory (BDI) was given to patients at baseline and 12 months. The QUALEFFO-41 questionnaire has five domains, with 41 items in total: pain (5 - items); physical function (17 - items); social function (7 - items); general health perception (3 - items); and mental function (9 - items). Poor QoL translates to higher scores in QUALEFFO-41. We used Turkish validation of QUALEFFO-41 test for our study.[5] Exclusion criteria were as follows:
Active psychiatric diseases
Active hematological, oncological cancers
Secondary osteoporosis
Active metabolic bone diseases
Previously received treatment for osteoporosis
Fractures (osteoporotic, traumatic)
Stage 4–5 renal failure (creatinine clearance ≤ 30 ml/min)
Hypercalcemia (≥11 mg/dl) or hypocalcemia (≤8 mg/dl)
Bone and joint deformities affecting static standing alone.
Written informed consent was obtained from each participant following a detailed explanation of the objectives and protocol of the study, which was conducted in accordance with the ethical principles stated in the “Declaration of Helsinki” and approved by the Institutional Ethics University Committee Approval Number is 71522473.050.02.05/54.
All patients were supported with daily oral Vitamin D (880 IU) and oral calcium (1000 mg).
Statistical analysis
SPSS version 17 (SPSS Inc., Chicago, IL, U.S.A) software was used to perform statistical analyses. Kolmogorov–Smirnov test was used to determine normality. Mean differences of continuous data were measured through t-test, and median differences of categorical data were measured by Mann–Whitney U and Fisher's exact tests. Differences between parameters before and after treatment were assessed by paired sample t-test. The P value was set at < 0.05 level.
RESULTS
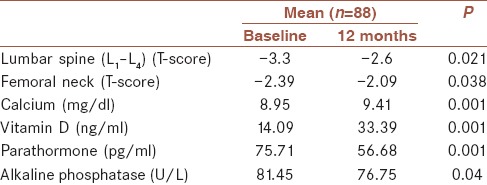
Eighty-eight women with PO agreed to participate in this study. Demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics

Once-yearly ZA 5 mg treatment was found to be effective for treating PO. Baseline mean L1–4 T-scores and femoral neck region were measured at − 3.30 and − 2.39 in DXA. L1–4 T-scores increased − 2.60 and for the femoral neck region increased − 2.09 after treatment at 12 months (P = 0.001) [Table 2].
Table 2.
The effects of once-yearly zoledronic acid 5 (mg)
Pain scores decreased from 17.44 to 13.95 (P = 0.001); physical function scores decreased from 47.29 to 40.51 (P = 0.001); social function scores decreased from 20.4 to 16.67 (P = 0.001); general health perception scores decreased from 20.19 to 16.22 (P = 0.001); mental function scores decreased from 32.1 to 30.9 (P = 0.001); and total scores decreased from 27.48 to 23.65 (P = 0.001) after once-yearly ZA treatment [Table 3].
Table 3.
Changes in domains of quality of life questionnaire from the European Foundation for Osteoporosis-41 and Beck Depression Inventory
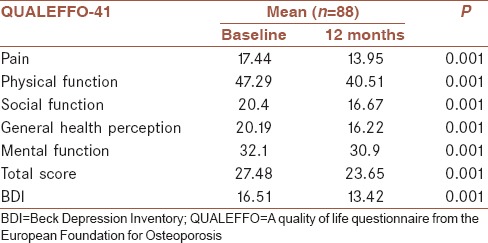
The BDI includes 21 items. A maximum of three points are obtained from one item. The maximum point total is 63 for the inventory. Higher scores indicate depression. BDI points decreased from 16.51 to 13.42 (P = 0.001) after once-yearly ZA treatment [Table 3]. Both BDI point levels are consistent with mild depressive symptoms, but the decrease in BDI points was statistically significant.
DISCUSSION
There are three primary results of this study. Once-yearly ZA treatment is an effective treatment for PO. Once-yearly ZA treatment improved QoL and reduced depression. Our study is the first demonstrating the relationship between once-yearly ZA treatment and both QoL and depression in the literature.
ZA is an IV bisphosphonate containing nitrogen. It has a prolonged dosing interval and fewer side effects than other bisphosphonates.[11] Once-yearly ZA can be used in treatment and prevention of PO and glucocorticoid-induced osteoporosis as well as treatment of male osteoporosis.[12] A meta-analysis of nine clinical trial showed that ZA is an effective treatment for osteoporosis. Significantly, higher BMD and lower fractures were found.[9] In our study, ZA treatment was found to be effective in terms of BMD levels as in 9's clinical trial.
There are many studies examining the effects of osteoporosis on QoL in the literature.[12,13,14,15,16,17,18] Some of these studies were conducted with patients with osteoporosis,[13,15,16,17,18] and some were conducted with patients with osteoporotic fractures.[14,19] In all of these studies, osteoporosis with or without fracture was found to be related with low QoL. In the literature, we found only one study demonstrating the relationship between once-yearly ZA treatment and QoL.[20] ZA treatments were found to be effective in PO and increase QoL, but the QUALEFFO-41 questionnaire was not used in that study. Ours is the first study showing the relationship between once-yearly ZA treatment and QoL using the QUALEFFO-41 questionnaire in terms of depression. Our results are similar to those of previous studies.
Depression is associated with several chronic conditions such as diabetes mellitus,[21] cardiovascular diseases,[22] menopause,[23,24] and osteoporosis.[25,26,27] The most common symptom is bone pain due to osteoporosis. Osteoporosis may cause limitations in movement as well as fractures. These limitations in movement, as well as pain, lead to breaks in daily activity, and patients may become isolated. Development of depression often begins in osteoporotic patients. In our study, our patients scored 16.51 points for BDI at the beginning of the once-yearly ZA treatment. These results are consistent with mild depressive symptoms. Our patients’ BDI points decreased to 13.42 in the 12th month. These results are consistent with some mild depressive symptoms as well, but the decrease in BDI points was statistically significant.
Our study also suffers from certain limitations. The most important one is the small number of participants and the short study duration. The other is that we did not compare our participants with participants who not using ZA treatment for osteoporosis. It would be unethical to deny active treatment to patients with osteoporosis.
CONCLUSION
Once-yearly ZA treatment is effective in PO while also increasing QoL and decreasing depression. Once-yearly ZA treatment is easy to use and has fewer side effects. This treatment may be used as the first choice for patients with PO. More extensive studies are needed to further examine this subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
FG; found the subject and the patients
CV wrote the article
AA; made the statistical analysis
MHA; made the tables
RC; made the last corrections.
Acknowledgments
The authors would like to thank the clinical staffs for their contribution to this work.
REFERENCES
- 1.Rizzoli R, Bruyere O, Cannata-Andia JB, Devogelaer J, Lyritis G, Ringe JD, et al. Management of osteoporosis in the elderly. Curr Med Res Opin. 2009;25:2373–87. doi: 10.1185/03007990903169262. [DOI] [PubMed] [Google Scholar]
- 2.Kamienski M, Tate D, Vega M. The silent thief: Diagnosis and management of osteoporosis. Orthop Nurs. 2011;30:162–71. doi: 10.1097/NOR.0b013e318219ab9d. [DOI] [PubMed] [Google Scholar]
- 3.Hall JE. Endocrinology of the menopause. Endocrinol Metab Clin North Am. 2015;44:485–96. doi: 10.1016/j.ecl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Schoor NM, Knol DL, Glas CA, Ostelo RW, Leplège A, Cooper C, et al. Development of the Qualeffo-31, an osteoporosis-specific quality-of-life questionnaire. Osteoporos Int. 2006;17:543–51. doi: 10.1007/s00198-005-0024-7. [DOI] [PubMed] [Google Scholar]
- 5.Koçyigit H, Gülseren S, Erol A, Hizli N, Memis A. The reliability and validity of the Turkish version of quality of life questionnaire of the European Foundation for osteoporosis (QUALEFFO) Clin Rheumatol. 2003;22:18–23. doi: 10.1007/s10067-002-0653-6. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam JD, Hooper MB. Bone density measurement in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:267–77. doi: 10.1016/s0278-5846(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 7.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15:1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 8.Panza EK, Güven Z, Akyüz G, Ofluolu D. The relationship between depression and lifestyle in osteoporotic and osteopenic patients. World of Osteoporosis. 2004;10:29–31. [Google Scholar]
- 9.Zhang J, Wang R, Zhao YL, Sun XH, Zhao HX, Tan L, et al. Efficacy of intravenous zoledronic acid in the prevention and treatment of osteoporosis: a meta-analysis. Asian Pac J Trop Med. 2012;5:743–8. doi: 10.1016/S1995-7645(12)60118-7. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Guidelines for Preclinical Evaluation and Clinical Trials in Osteoporosis. Geneva: WHO; 1998. [Google Scholar]
- 11.Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–33. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 12.Package insert. Reclast injection. East Hanover: Novartis Pharmaceuticals Corp; 2010. [Google Scholar]
- 13.Silverman SL. The osteoporosis assessment questionnaire (OPAQ): A reliable and valid disease-targeted measure of health-related quality of life (HRQOL) in osteoporosis. Qual Life Res. 2000;9:767–74. [Google Scholar]
- 14.Helmes E, Hodsman A, Lazowski D, Bhardwaj A, Crilly R, Nichol P, et al. A questionnaire to evaluate disability in osteoporotic patients with vertebral compression fractures. J Gerontol A Biol Sci Med Sci. 1995;50:M91–8. doi: 10.1093/gerona/50a.2.m91. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Cook DJ. Osteoporosis quality of life study group: Measuring quality of life in women with osteoporosis. Osteoporos Int. 1997;7:478–87. [PubMed] [Google Scholar]
- 16.Cook DJ, Guyatt GH, Adachi JD, Epstein RS, Juniper EF, Austin PA, et al. Development and validation of the mini-osteoporosis quality of life questionnaire (OQLQ) in osteoporotic women with back pain due to vertebral fractures. Osteoporosis quality of life study group. Osteoporos Int. 1999;10:207–13. doi: 10.1007/s001980050217. [DOI] [PubMed] [Google Scholar]
- 17.Marquis P, Cialdella P, De la Loge C. Development and validation of a specific quality of life module in post-menopausal women with osteoporosis: the QUALIOST. Qual Life Res. 2001;10:555–66. doi: 10.1023/a:1013041206433. [DOI] [PubMed] [Google Scholar]
- 18.Kumamoto K, Nakamura T, Suzuki T, Gorai I, Fujinawa O, Ohta H, et al. Validation of the Japanese osteoporosis quality of life questionnaire. J Bone Miner Metab. 2010;28:1–7. doi: 10.1007/s00774-009-0125-z. [DOI] [PubMed] [Google Scholar]
- 19.Lips P, Leplege A. Development and validation of a quality of life questionnaire for patients with vertebral fractures: Qualeffo-41. Qual Life Res. 2000;9:763–6. [Google Scholar]
- 20.Huang S, Lin H, Zhu X, Chen X, Fan L, Liu C. Zoledronic acid increases bone mineral density and improves health-related quality of life over two years of treatment in Chinese women with postmenopausal osteoporosis. Endokrynol Pol. 2014;65:96–104. doi: 10.5603/EP.2014.0014. [DOI] [PubMed] [Google Scholar]
- 21.Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torréns JI, Kravitz HM, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–62. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 22.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: A meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 23.Timur S, Sahin NH. The prevalence of depression symptoms and influencing factors among perimenopausal and postmenopausal women. Menopause. 2010;17:545–51. doi: 10.1097/gme.0b013e3181cf8997. [DOI] [PubMed] [Google Scholar]
- 24.Dørmænen A, Heimdal MR, Wang CE, Grimsgaard AS. Depression in postmenopause: A study on a subsample of the acupuncture on hot flushes among menopausal women (ACUFLASH) study. Menopause. 2011;18:525–30. doi: 10.1097/gme.0b013e3181f9f89f. [DOI] [PubMed] [Google Scholar]
- 25.Cizza G, Ravn P, Chrousos GP, Gold PW. Depression: A major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20:367–73. doi: 10.1016/j.tem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams LJ, Berk M, Henry MJ, Stuart AL, Brennan SL, Jacka FN, et al. Depression following fracture in women: a study of age-matched cohorts. BMJ Open. 2014;4:e004226. doi: 10.1136/bmjopen-2013-004226. [DOI] [PMC free article] [PubMed] [Google Scholar]


