Abstract
Background:
Pre-eclampsia as a hypertensive disorder of pregnancy complicates up to 5–10% of pregnancies worldwide. Endothelial dysfunction plays an important role in the pathogenesis of pre-eclampsia. In this study, we aim to evaluate the effect of high-dose folic acid on endothelial dysfunction in pre-eclamptic patients.
Materials and Methods:
In this triple-blinded randomized clinical trial, the enrolled patients were divided randomly into two groups. Folic acid 5.0 mg or placebo was taken daily by oral administration from the initiation of diagnosis until 2 months after delivery by the participants. Every patient's flow-mediated dilation (FMD) was evaluated at the beginning of the study and 2 months after delivery with the same experienced operator at the same period of time (3–5 p.m.) by high-resolution B-mode ultrasonography. Potential confounding variables were included in the independent samples t-test. t-test or Mann–Whitney U-test was used in the comparison of means between the intervention and placebo groups. To compare FMD in each group, before and after the intervention, paired t-test was used.
Results:
Mean value of FMD in intervention (9.64 ± 5.57) and control group (9.30 ± 4.25) has no significant difference before the consumption of drugs (P > 0.05). FMD in intervention group (13.72 ± 7.89) significantly increases after daily consumption of 5 mg folic acid in comparison with control group (10.02 ± 4.81) after daily consumption of placebo (P = 0.002).
Conclusion:
Increased mean of FMD in intervention group shows that this supplement can improve endothelial function and can be significantly affected by maternal blood pressure during pregnancy and some endothelium-dependent disease such as pre-eclampsia and its associated adverse outcomes.
Keywords: Endothelial dysfunction, folic acid, pre-eclampsia
INTRODUCTION
Pre-eclampsia as a hypertensive disorder of pregnancy complicates up to 5–10% of pregnancies worldwide, and constitutes one of the leading causes of maternal and prenatal mortality and morbidity.[1,2,3] In Iran, the prevalence of pre-eclampsia and eclampsia is about 3% and 0.1%, respectively. Pre-eclampsia typically starts with high blood pressure after the 20th week of pregnancy, and the rate has been increased 25% in the last two decades in the United States.[3] Pre-eclampsia is a leading cause of prenatal events including premature delivery, severe hypertension, increasing risk of stroke, and death of mother.[4] Women with a history of pre-eclampsia have a higher risk of future cardiovascular and metabolic disorders.
Endothelial dysfunction plays an important role in the pathogenesis of pre-eclampsia.[5] Several studies have reported that inflammation response and systemic effect during pregnancy on vascular endothelium lead to hypertension and proteinuria as signs and outcomes of pre-eclampsia.[6] Several surveys tried to identify the different aspects of endothelial function and essential role of these cells in the process of endothelial-dependent diseases.[5] Vasodilation measurement due to nitric oxide (NO) effect on endothelium is considered a desirable method to evaluate endothelial function.[7] Flow-mediated dilation (FMD) is used for this purpose by using high-resolution B-mode ultrasonography to measure brachial artery diameter before and after occlusion with a cuff.[8] In fact, it triggers shear stress that stimulates no synthesis.[8] Folic acid as a supplement has an important impact on endothelial function, coagulation cascade, blood pressure, and prevention of atherosclerosis.[9] Some studies evaluate the effect of folic acid on the endothelial function improvement in diabetes mellitus, hypertension, hyperlipidemia, and coronary artery disease.[9] Probably, it affects via tetrahydrobiopterin regeneration, as a major NO cofactor, which causes NO increase and leads to vasodilation and improvement of FMD.[10] Although there are lots of surveys on pre-eclampsia, few studies evaluate the effect of folic acid in pre-eclamptic patients. A cohort study in Denmark showed that folic acid is associated with a reduced risk of pre-eclampsia among normal weight women,[11] but in the results of Li et al.'s study in 2013, there were no findings about the decrease in the risks of gestational hypertension or pre-eclampsia among women who took 400 μg daily folic acid supplementation in the first trimester of pregnancy.[12] In this study, we aimed to evaluate the effect of high-dose folic acid on endothelial dysfunction in pre-eclamptic patients.
MATERIALS AND METHODS
Patient setting
This trial has been registered at http://www.irct.ir/with ID “IRCT2016032727135N1” and has been approved by the Isfahan University of Medical Sciences’ Ethics Committee with ID “IR.mui.rec. 1394.3.653.” In this parallel randomized clinical trial, 85 pregnant women with documented pre-eclampsia on the basis of the National High Blood Pressure Education Program working group on high blood pressure in pregnancy, 2000, who visited in St-Zahra Hospital, Isfahan, Iran, by obstetrics-gynecologist, were included in this study. Patients with any clinical evidence of diabetes mellitus, pernicious anemia, megaloblastic anemia, aplastic anemia, known cardiovascular diseases, hypercholesterolemia, hypertriglyceridemia, renal failure, thyroid disease, history of pregnancy-induced hypertension, malignancy, liver dysfunction, infectious disease, migraine, and stroke were excluded from the study. In addition, patients with a history of twin pregnancy, cigarette smoking, alcohol consumption, and drug abuse were also excluded from this study. Women who represented reaction to drug (vertigo, insomnia, rash, severe cough, angioedema, purities, bronchospasm, and drug consumption), poor compliance with prescribed drug and hygiene advice, and who use drugs <2 months during pregnancy were excluded from the study.
Intervention
Enrolled patients were divided randomly (allocation ratio equal to 1:1) into two groups by random allocation service (www.saghaei.net/ra/). Folic acid 5.0 mg or placebo was taken daily by oral administration from the initiation of diagnosis until 2 months after delivery by the trial participant. Intervention was applied by a cardiologist. This trial is triple-blinded (patient, operator, and statistician).
Assessment
Confounding variables included maternal age and prepregnancy body mass index (BMI) which were assessed at the beginning of the study. Baseline amount of triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), fasting plasma glucose (FBS), and hemoglobin was measured. They measured finally at the end of intervention again. Every patient's FMD was evaluated at the beginning of the study and 2 months after delivery with the same experienced operator at the same period of time (3–5 p.m.) by high-resolution B-mode ultrasonography. FMD was estimated by the following formula:
FMD (%) = ([POD − BBD]/BBD) × 100
Basal brachial diameter (BBD) is measured in resting position, and postocclusion diameter (POD) is measured between 30 and 90 s after cuff evacuation (cuff pressure is increased up to 300 mmHg within 5 min before evacuation).
Statistical analysis
Potential confounding variables included in the independent samples t-test were maternal age and prepregnancy BMI. t-test or Mann–Whitney U-test was used in the comparison of means of birth weight and gestational age between the intervention and placebo groups. To compare FMD in each group, before and after the intervention, paired t-test was used by SPSS software, version 21, IL, Chicago, USA.
RESULTS
Eighty-five pre-eclamptic pregnant patients were enrolled in this study between February 2015 and February 2016. Patient enrollment is described in Flowchart 1.
Flowchart 1.
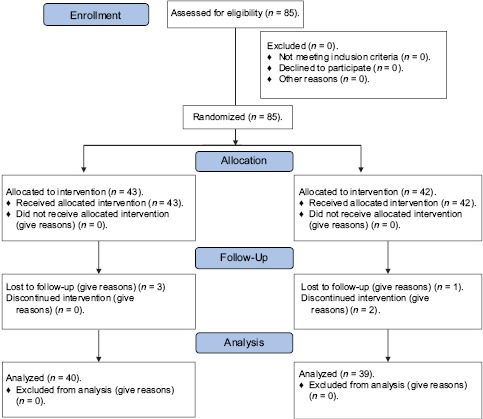
Patient enrollment
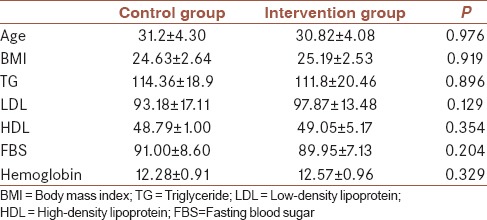
The mean of patients’ age was 30.10 ± 4.16 years (with a range of 21–41 years). The details of patients’ demographic data are summarized in Table 1. As shown in Table 1, the patients have no significant differences in age, BMI, level of serum TG, LDL, HDL, FBS, and hemoglobin.
Table 1.
Demographic and baseline characteristics of patients
Mean value of FMD in intervention (9.64 ± 5.57) and control group (9.30 ± 4.25) has no significant differences before the consumption of drugs (P > 0.05). As demonstrated in Table 2, FMD increased significantly in intervention group after daily consumption of 5 mg of folic acid. In control group, in contrast, FMD showed no significant differences before and after daily consumption of placebo.
Table 2.
Flow-mediated dilation in each group before and after consumption of drug/placebo
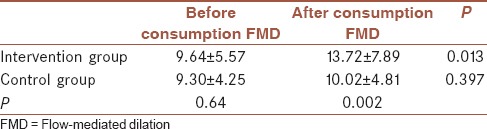
Meanwhile, FMD in intervention group significantly increased after daily consumption of 5 mg folic acid in comparison with control group after daily consumption of placebo.
Mean ± standard error level of serum TG, LDL, HDL, and FBS before and after consumption of drug/placebo in each group is demonstrated in Table 3. Only TG level decreased significantly after folic acid consumption.
Table 3.
Level of serum triglyceride, low-density lipoprotein, high-density lipoprotein, and fasting blood sugar before and after consumption of drug/placebo
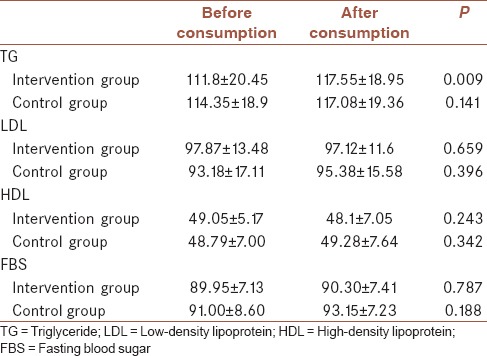
Mean level of serum TG after drug (117.55 ± 18.95) and placebo (117.08 ± 19.36) consumption had no significant differences. Mean level of serum LDL, HDL, and FBS after drug (97.12 ± 11.6, 48.10 ± 7.05, and 90.30 ± 7.41, respectively) and placebo (95.38 ± 15.58, 49.28 ± 7.64, and 93.15 ± 7.29, respectively) consumption had no significant differences (P > 0.05) as well.
DISCUSSION
In this clinical trial on pregnant women suffering from pre-eclampsia who visited St-Zahra Hospital, Isfahan, Iran, 85 enrolled patients were divided randomly into two groups who received folic acid 5.0 mg or placebo daily by oral administration from the initiation of diagnosis until 2 months after delivery. Endothelial function was assessed by FMD. Our results showed that daily oral administration of high-dose folic acid increased FMD significantly which means it can improve endothelial function.
The studies which investigate the effects of using folic acid on cardiovascular system have approved beneficial effect on this system including endothelial function, preventing hypertension, and atherosclerosis.[9,13,14,15,16,17,18,19] Moreover, some studies showed that folic acid can improve endothelial function in different diseases such as hypercholesterolemia, hypertension, diabetes mellitus, coronary artery disease, and hyperhomocysteinemia. They reported that using more folic acid in high-risk populations can cause vasculoprotective effects. On the other hand, epidemic studies have shown that folic acid deficiency can lead to hypertension. Furthermore, there are some reports about a negative association between hypertension and plasma level of folic acid.[20]
In this study, FMD increased significantly in the group who received high-dose folic acid. It may be in consistent with tetrahydrobiopterin regeneration, as a major NO cofactor, which causes NO increase and leads to vasodilation and improvement of FMD.[10] This effect also can be due to decrease in homocysteine by folic acid consumption and therefore causes endothelial function improvement. In addition, folic acid has an antioxidant effect on capillary bed and increases NO bioavailability.[21]
In a meta-analysis study, Mark et al. represented that high-dose folic acid (e.g., 5 mg/day for at least 6 weeks) can decrease blood pressure and improve endothelial function and increase FMD, while supplements containing low-dose folic acid (400–800 mg/day) have no appreciable effect on improving FMD. This study suggests that the primary mechanism proposed for the effect of folic acid on endothelial function is a reduction in plasma homocysteine concentrations by remethylation of homocysteine back to methionine. Homocysteine increases oxidative stress by increasing superoxide anion production that results in the downregulation of NO production.[22,23] While some other studies report that folic acid supplement can significantly improve FMD, some studies did not approve the result.[24] Sandrahirsch et al. investigated that hyperhomocysteinemia in young people is associated with lower serum cobalamin and folate levels but does not impair endothelial function. Supplementation with folic acid during 8 weeks lowers homocysteine levels without effects on vascular function. It seems that using low-dose folic acid has an effect on achieving different results.[25]
There are strong evidences in several animal and human studies supporting the hypothesis of preventive effect of folic acid on pre-eclampsia and its outcomes.[26,27] In these studies, pre-eclampsia was considered as a two-stage disorder. At Stage I, we have a decrease in placental perfusion, and at Stage II, the maternal syndrome of pre-eclampsia, secondary to systemic endothelial dysfunction, develops. Supplementation of high-dose folic acid in early gestation may affect both the stages. Folic acid, or folate, as a Vitamin B coenzyme, is required in the production of nucleic acid, and cell growth and folate supply play an important role in the implantation and development of placenta. While another cohort study in Denmark showed that periconceptional multivitamin consists of folic acid which is associated with a reduced risk of pre-eclampsia among normal weight women,[11] there are other studies in 2009 and 2013 conducted in the Netherlands[28] and China,[12] respectively, that did not confirm this association. Li et al. in 2013 declared the results of their cohort study on 193,554 pregnant women. They did not find a decrease in the risks of gestational hypertension or pre-eclampsia among women who took 400 μg daily folic acid supplementation in the first trimester of pregnancy. This study suggests that folic acid is not able to improve endothelial function or prevent vascular changing due to hyperhomocysteinemia in pregnancy.[12] The discrepancy between the results of this study and previous studies, and our study may be the result of various factors, including the timing of the intervention, the dose of folic acid, the other components in the supplements, and the population. Finally, Hamad et al. reported persistent decrease in FMD in pre-eclamptic patients after 1 year. Meanwhile, in our study, FMD was increased only 2 months after delivery in intervention group who took oral daily high dose of folic acid.[29] Hua et al. assessed the effect of folic acid supplementation during pregnancy on gestational hypertension/pre-eclampsia in a meta-analysis. Whether folic acid supplementation in pregnancy can prevent the occurrence of gestational hypertension/pre-eclampsia remains uncertain.[30]
CONCLUSION
This study demonstrated an association between high-dose folic acid supplementation and increased flow-mediated vasodilation in the brachial artery in pre-eclamptic patients. Based on the appropriate setting of the trial, the study population, and the types of interventions used, we can conclude that increase in the mean of FMD in groups who took regular daily oral 5 mg folic acid starting right at the initiation of diagnosis until 2 months after delivery shows that this supplement through biological pathway can improve endothelial function and can significantly affect maternal blood pressure during pregnancy and some endothelium-dependent disease such as pre-eclampsia and its associated adverse outcomes. The first and most important limitation of our study is small sample size due to difficulties to diagnosis pure pre-eclamptic patients who met inclusion criteria. In addition, cultural difficulties limited our study. The last but not the least was unequal period of drug administration by participants due to emergency indications for cesarean section which some of the participants did meet.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
KH contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MH contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. EZ contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FB contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. ZM contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Schroeder BM American College of Obstetricians and Gynecologists. ACOG practice bulletin on diagnosing and managing preeclampsia and eclampsia. American College of Obstetricians and Gynecologists. Am Fam Physician. 2002;66:330–1. [PubMed] [Google Scholar]
- 2.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–72. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 3.Wen SW, Champagne J, Rennicks White R, Coyle D, Fraser W, Smith G, et al. Effect of folic acid supplementation in pregnancy on preeclampsia: The folic acid clinical trial study. J Pregnancy 2013. 2013 doi: 10.1155/2013/294312. 294312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 5.Guimarães MF, Brandão AH, Rezende CA, Cabral AC, Brum AP, Leite HV, et al. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet. 2014;290:441–7. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 6.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 7.Hirata K, Amudha K, Elina R, Hozumi T, Yoshikawa J, Homma S, et al. Measurement of coronary vasomotor function: Getting to the heart of the matter in cardiovascular research. Clin Sci (Lond) 2004;107:449–60. doi: 10.1042/CS20040226. [DOI] [PubMed] [Google Scholar]
- 8.Sankatsing RR, de Groot E, Jukema JW, de Feyter PJ, Pennell DJ, Schoenhagen P, et al. Surrogate markers for atherosclerotic disease. Curr Opin Lipidol. 2005;16:434–41. doi: 10.1097/01.mol.0000174400.68938.f6. [DOI] [PubMed] [Google Scholar]
- 9.Chambers JC, Ueland PM, Obeid OA, Wrigley J, Refsum H, Kooner JS. Improved vascular endothelial function after oral B Vitamins: An effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000;102:2479–83. doi: 10.1161/01.cir.102.20.2479. [DOI] [PubMed] [Google Scholar]
- 10.Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. 1997;237:340–4. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- 11.Catov JM, Nohr EA, Bodnar LM, Knudson VK, Olsen SF, Olsen J. Association of periconceptional multivitamin use with reduced risk of preeclampsia among normal-weight women in the Danish National Birth Cohort. Am J Epidemiol. 2009;169:1304–11. doi: 10.1093/aje/kwp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61:873–9. doi: 10.1161/HYPERTENSIONAHA.111.00230. [DOI] [PubMed] [Google Scholar]
- 13.Mangoni AA, Arya R, Ford E, Asonganyi B, Sherwood RA, Ouldred E, et al. Effects of folic acid supplementation on inflammatory and thrombogenic markers in chronic smokers. A randomised controlled trial. Thromb Res. 2003;110:13–7. doi: 10.1016/s0049-3848(03)00295-0. [DOI] [PubMed] [Google Scholar]
- 14.Deol PS, Barnes TA, Dampier K, John Pasi K, Oppenheimer C, Pavord SR. The effects of folic acid supplements on coagulation status in pregnancy. Br J Haematol. 2004;127:204–8. doi: 10.1111/j.1365-2141.2004.05172.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangoni AA, Ouldred E, Swif CG, Jackson SH, Draper RP, Sherwood RA, et al. Vascular and blood pressure effects of folic acid in older patients with cardiovascular disease. J Am Geriatr Soc. 2001;49:1003–4. doi: 10.1046/j.1532-5415.2001.49196.x. [DOI] [PubMed] [Google Scholar]
- 16.Mangoni AA, Sherwood RA, Swift CG, Jackson SH. Folic acid enhances endothelial function and reduces blood pressure in smokers: A randomized controlled trial. J Intern Med. 2002;252:497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 17.Mangoni AA, Sherwood RA, Asonganyi B, Swift CG, Thomas S, Jackson SH. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005;18(2 Pt 1):220–6. doi: 10.1016/j.amjhyper.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Schutte AE, Huisman HW, Oosthuizen W, van Rooyen JM, Jerling JC. Cardiovascular effects of oral supplementation of Vitamin C, E and folic acid in young healthy males. Int J Vitam Nutr Res. 2004;74:285–93. doi: 10.1024/0300-9831.74.4.285. [DOI] [PubMed] [Google Scholar]
- 19.Undas A, Domagala TB, Jankowski M, Szczeklik A. Treatment of hyperhomocysteinemia with folic acid and Vitamins B12 and B6 attenuates thrombin generation. Thromb Res. 1999;95:281–8. doi: 10.1016/s0049-3848(99)00043-2. [DOI] [PubMed] [Google Scholar]
- 20.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293:320–9. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 21.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 22.American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: Summary statement. Muscle Nerve. 2002;25:918–22. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 23.McRae MP. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: A meta-analysis of randomized controlled clinical trials. J Chiropr Med. 2009;8:15–24. doi: 10.1016/j.jcm.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch S, Pia De la Maza M, Yañez P, Glasinovic A, Petermann M, Barrera G, et al. Hyperhomocysteinemia and endothelial function in young subjects: Effects of vitamin supplementation. Clin Cardiol. 2002;25:495–501. doi: 10.1002/clc.4960251105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch S, de la Maza P, Mendoza L, Petermann M, Glasinovic A, Paulinelli P, et al. Endothelial function in healthy younger and older hyperhomocysteinemic subjects. J Am Geriatr Soc. 2002;50:1019–23. doi: 10.1046/j.1532-5415.2002.50255.x. [DOI] [PubMed] [Google Scholar]
- 26.Wen SW, Chen XK, Rodger M, White RR, Yang Q, Smith GN, et al. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198:45.e1–7. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 27.Wen SW, Zhou J, Yang Q, Fraser W, Olatunbosun O, Walker M. Maternal exposure to folic acid antagonists and placenta-mediated adverse pregnancy outcomes. CMAJ. 2008;179:1263–8. doi: 10.1503/cmaj.080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmermans S, Jaddoe VW, Silva LM, Hofman A, Raat H, Steegers-Theunissen RP, et al. Folic acid is positively associated with uteroplacental vascular resistance: The Generation R study. Nutr Metab Cardiovasc Dis. 2011;21:54–61. doi: 10.1016/j.numecd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25:2301–7. doi: 10.1097/HJH.0b013e3282ef5fc0. [DOI] [PubMed] [Google Scholar]
- 30.Hua X, Zhang J, Guo Y, Shen M, Gaudet L, Janoudi G, et al. Effect of folic acid supplementation during pregnancy on gestational hypertension/preeclampsia: A systematic review and meta-analysis. Hypertens Pregnancy. 2016;1:1–14. doi: 10.1080/10641955.2016.1183673. [DOI] [PubMed] [Google Scholar]


