ABSTRACT
KS-Bcl-2 is a Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded viral Bcl-2 (vBcl-2) homolog which has apoptosis- and autophagy-inhibiting activity when expressed in transfected cells. However, little is known about its function during viral infection. As KS-Bcl-2 is expressed during the lytic replication cycle, we used constitutively lytic and inducibly lytic KSHV mutants to investigate the role of KS-Bcl-2 during the lytic cycle. We show that KSHV cannot complete the lytic replication cycle and produce infectious progeny in the absence of KS-Bcl-2, indicating that the protein is essential for KSHV replication. Replacement of the KS-Bcl-2 coding sequence, ORF16, by sequences encoding a potent cellular apoptosis and autophagy inhibitor, Bcl-XL, or the cytomegalovirus mitochondrial inhibitor of apoptosis, vMIA, did not rescue KSHV replication, suggesting that KS-Bcl-2 has a function that goes beyond apoptosis and autophagy inhibition. Strikingly, the vBcl-2 proteins of the related γ2-herpesviruses murine herpesvirus 68 and herpesvirus saimiri did not rescue the replication of a KS-Bcl-2 deletion mutant, but rhesus rhadinovirus (RRV) vBcl-2 did. Deletion of ORF16 from the RRV genome abrogated viral replication, but its replacement by KSHV ORF16 rescued RRV replication, indicating that the essential vBcl-2 function is conserved between these two primate rhadinoviruses. We further show that the KSHV and RRV Bcl-2 homologs localize to the mitochondria and nuclei of infected cells. Deletion of 17 amino acids from the N terminus of KS-Bcl-2 abrogates nuclear localization and KSHV replication, suggesting that KS-Bcl-2 might execute its essential function in the nuclei of infected cells.
IMPORTANCE Several viruses express proteins homologous to cellular Bcl-2. Viral Bcl-2 proteins have functions similar to those of cellular Bcl-2: they can inhibit apoptosis, a form of programmed cell death, and autophagy, a self-degradative process for the disposal of dysfunctional or unwanted components. This study shows that the vBcl-2 proteins of KSHV and RRV differ from other vBcl-2 proteins in that they are essential for viral replication. The essential function is separate from the apoptosis- and autophagy-inhibiting activity but correlates with an unusual localization within the cell nucleus, suggesting that these proteins exert a novel function in the nucleus.
KEYWORDS: KS-Bcl-2, Kaposi's sarcoma-associated herpesvirus, rhesus rhadinovirus, apoptosis, autophagy, vBcl-2, viral replication
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also referred to as human herpesvirus 8 (HHV-8), is a γ2-herpesvirus and the causative agent of Kaposi's sarcoma (KS), primary effusion lymphoma, and multicentric Castleman's disease (1–4).
The KSHV life cycle is divided into two distinct phases, latency and lytic replication, which allow the virus to establish a long-term persistent infection. During latency, viral genomes are maintained within the host cell nucleus as circular episomes that are replicated by the cellular DNA replication machinery and segregated upon cell division (5). KSHV escapes immune surveillance during latency by limiting gene expression to a few viral genes organized in the so-called major latency cluster (6).
Although latency is the predominant state in which KSHV is found in tumor tissues, lytic replication is detected in a small subpopulation of cells. Lytic replication is a critical step for KSHV tumorigenesis, as the infected cells produce cytokines and virokines that provide a suitable microenvironment for proliferation of latently infected cells and tumor progression (7–9). The lytic cycle is triggered by the expression of the replication and transcription activator (RTA) protein, which activates a tightly regulated cascade of viral gene expression, viral genome replication, production of progeny virus, and host cell death (10–12).
Proteins of the Bcl-2 family are crucial regulators of apoptosis, the most common form of programmed cell death. Antiapoptotic Bcl-2 family proteins execute their function at the outer mitochondrial membrane, where they bind proapoptotic proteins and prevent mitochondrial outer membrane permeabilization, cytochrome c release, and activation of caspases (13–15). Several antiapoptotic Bcl-2 proteins also inhibit autophagy by binding Bcl-2 homology domain 3 (BH3) of the essential autophagy scaffold protein Beclin-1 (16–19).
All sequenced gammaherpesviruses encode one or more homologs of the cellular Bcl-2 protein. These include the BHRF1 and BALF1 proteins of Epstein-Barr virus (EBV) (20, 21) and the viral Bcl-2 (vBcl-2) proteins of KSHV (22, 23), rhesus rhadinovirus (RRV) (24), herpesvirus saimiri (HVS) (25), and murine gammaherpesvirus 68 (MHV-68) (26, 27). The vBcl-2 protein of KSHV, usually referred to as KS-Bcl-2, is encoded by ORF16 and expressed early during lytic replication (8). When it was expressed in human cells, KS-Bcl-2 displayed functions similar to those of cellular Bcl-2 family proteins: it inhibited apoptosis induced by several stimuli (22, 23) and suppressed autophagy by interacting with Beclin-1 (16, 28). Similarly to cellular Bcl-2, KS-Bcl-2 can interact with Aven, a cellular protein that inhibits Apaf-1/caspase-9-mediated apoptosis and regulates ATM activation (29, 30). It has also been shown that KS-Bcl-2, unlike its cellular homolog, is not phosphorylated and inactivated by the KSHV-cyclin/CDK6 complex (31), nor can it be cleaved by caspases and converted into a proapoptotic protein (32). A more recent study showed that KS-Bcl-2 can interact with the nucleolar protein GLTSCR2/PICT1, resulting in KS-Bcl-2 accumulation in nucleoli, and that the N terminus of KS-Bcl-2 is necessary for this interaction (33).
Functional studies of KSHV lytic gene products, such as KS-Bcl-2, have been hampered by the fact that KSHV remains predominantly latent in cultured cells. Induction of the lytic replication cycle is possible, for instance, by treatment with phorbol esters or sodium butyrate, but it is inefficient. We previously showed that KSHV can be modified to become a lytically replicating virus by insertion of a constitutively active promoter in front of ORF50, the gene encoding RTA (34). In the present study, we investigated the importance of KS-Bcl-2 for viral replication by using constitutively and inducibly lytic KSHV mutants. We show that KS-Bcl-2 differs from other vBcl-2 proteins in that it is essential for viral replication. Replacement of ORF16 by the coding sequences of potent antiapoptotic and antiautophagic proteins did not rescue KSHV replication, suggesting that KS-Bcl-2 has a function that goes beyond inhibition of apoptosis and autophagy. While this work was in progress, the essential nature of KS-Bcl-2 was reported by others (35, 36). These two papers reported that KS-Bcl-2 was required for viral progeny production upon KSHV reactivation from iSLK cells harboring the latent viral genome. In addition, the study by Liang et al. used a comprehensive mutagenesis approach to show that domains mediating the antiapoptotic and antiautophagic functions of KS-Bcl-2 were not needed to rescue replication of a KSHV ORF16 deletion mutant but that a glutamic acid residue at position 14 was essential (36). Our results, which were obtained by different methods, are largely consistent with these published findings. Moreover, we show that KSHV replication can be rescued by replacement of ORF16 with the RRV vBcl-2 gene, but not the HVS or MHV-68 vBcl-2 gene, indicating that the essential vBcl-2 function is conserved between KSHV and RRV but not in the more distantly related rhadinoviruses HVS and MHV-68. We further show that RRV-Bcl-2 is essential for RRV replication and that RRV replication is rescued upon replacement of the RRV-Bcl-2 gene by the KS-Bcl-2 gene. Unlike other vBcl-2 proteins, KS-Bcl-2 and RRV-Bcl-2 localize to the nuclei (in addition to mitochondria) of infected cells, suggesting that these proteins might execute their essential function in the nucleus.
RESULTS
Construction of BAC16-based KSHVLYT and KSHVIND.
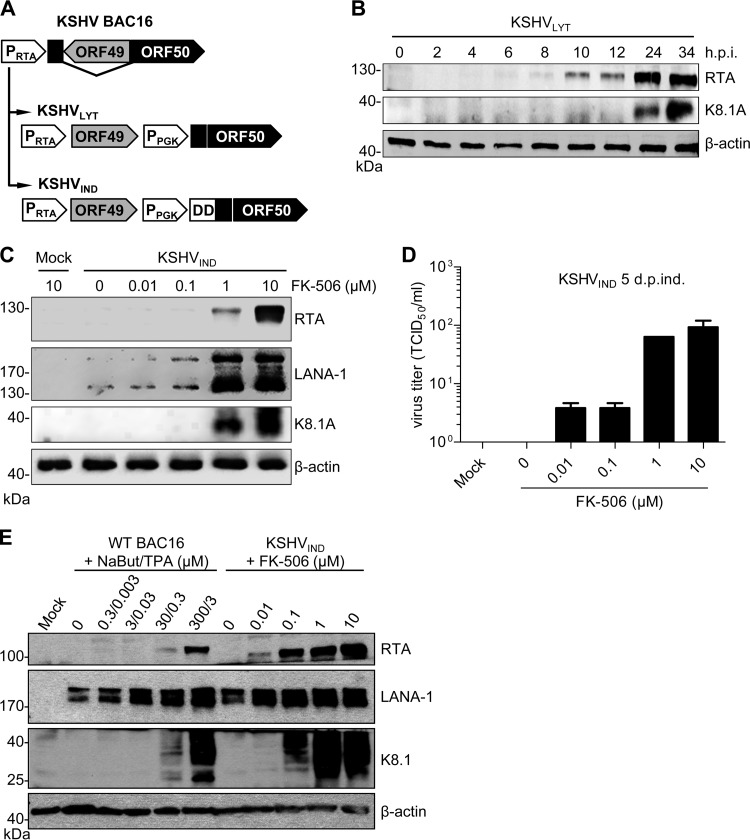
We previously constructed a lytically replicating KSHV strain, KSHVLYT (34), on the basis of the first published KSHV bacterial artificial chromosome (BAC) clone, BAC36 (37). As we and others have encountered difficulties with BAC36 related to genetic instability (34, 38), we decided to use the more recently published KSHV BAC clone, BAC16 (39), for our investigations. We modified the ORF49/ORF50 locus within BAC16 in the same way as that previously done with BAC36 (34) and as depicted in Fig. 1A. Expression of ORF50 is driven by a cellular phosphoglycerate kinase promoter (PPGK) resulting in constitutive expression of the lytic switch protein, RTA. In addition, we constructed an inducible lytic virus, KSHVIND, in which the constitutively expressed RTA protein is fused to the destabilizing domain (DD) of FKBP12 (Fig. 1A). Fusion to DD-FKBP12 has been shown to result in rapid protein degradation upon translation. The fusion protein can be stabilized by addition of an FKBP12 ligand, such as FK-506 or Shield1 (40, 41).
FIG 1.
Construction of KSHVLYT and KSHVIND. (A) The ORF49/ORF50 locus of KSHV was rearranged, and a PGK promoter was inserted for the expression of ORF50 (RTA) as described previously (34). In KSHVIND, the destabilizing domain (DD) of FKBP12 was fused to constitutively expressed RTA. (B) RPE-1 cells were infected with KSHVLYT at an MOI of 0.2 TCID50/cell. Expression of RTA and the late glycoprotein K8.1A was detected by immunoblotting. (C) 293A cells carrying KSHVIND genomes were treated with different concentrations of FK-506. Expression of RTA, LANA-1, and K8.1A was determined by immunoblotting at 5 days postinduction. (D) Virus release by the cells described for panel C was determined by titration. (E) 293A cells carrying WT KSHV BAC16 were treated with sodium butyrate (NaBut) and TPA, and 293A cells carrying KSHVIND were treated with FK-506 to induce lytic gene expression. KSHV protein expression was analyzed by immunoblotting at 5 days postinduction.
Human RPE-1 cells were transfected with the KSHVLYT BAC to reconstitute infectious KSHV. In replication kinetics experiments, the BAC16-based KSHVLYT strain replicated to peak titers of approximately 105 focus-forming units per ml (as determined by a 50% tissue culture infective dose [TCID50] assay) in RPE-1 cells (data not shown), similar to the titers previously obtained with the BAC36-based KSHVLYT strain (34). Expression of RTA and the late viral protein K8.1A were detected by Western blotting at different times postinfection (Fig. 1B).
For an inducible lytic system, 293A cells were transfected with the KSHVIND BAC and selected with hygromycin. RPE-1 cells could not be used for this purpose because these cells already carry a hygromycin resistance marker (42). Lytic replication was induced with different amounts of FK-506 (Fig. 1C). RTA expression depended on the amount of FK-506 added to cells. Latency-associated nuclear antigen 1 (LANA-1) was detected in uninduced cells, as it is expressed during latency. LANA-1 levels increased with RTA levels, as RTA is a known inducer of LANA-1 transcription (43). The amount of the late protein K8.1A (Fig. 1C) and production of virus progeny (Fig. 1D) also increased with FK-506 induction. Similar results were obtained when the synthetic ligand Shield1 was used instead of FK-506 (not shown). We also compared the inducible RTA, LANA-1, and K8.1A expression in 293A cells carrying KSHVIND with the commonly used induction of KSHV with sodium butyrate and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) in the same cell type. As shown in Fig. 1E, induction of KSHVIND with FK-506 was at least as effective as induction of wild-type (WT) BAC16 with TPA and sodium butyrate. These data indicated that lytic replication of KSHVIND can be induced in a dose-dependent manner, making KSHVIND a useful tool for the analysis of KSHV lytic replication.
KS-Bcl-2 is essential for lytic replication and cannot be replaced by other potent antiapoptotic or antiautophagic proteins.
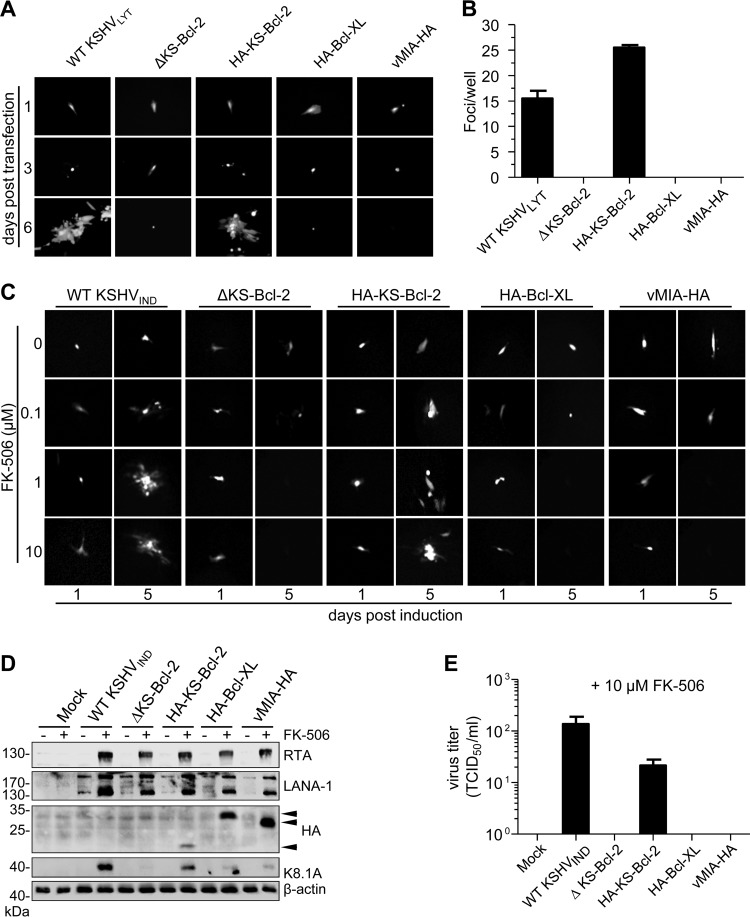
In order to determine the importance of individual KSHV genes, we constructed 45 KSHVLYT deletion mutants by BAC mutagenesis. Individual open reading frames (ORFs) were deleted and replaced by a galK-kan selection marker. The mutant BACs were transfected into RPE-1 cells to reconstitute infectious virus. Of the 45 deletion mutants, 13 did not replicate and spread, suggesting that the deleted ORFs are essential for viral replication (Table 1). To our surprise, ORF16 (which encodes KS-Bcl-2) was among the ORFs classified as essential in this screening assay. To confirm this unexpected result, we constructed a revertant of the ORF16 deletion mutant (ΔKS-Bcl-2) by reinserting the coding sequence of a hemagglutinin (HA)-tagged KS-Bcl-2 construct (HA-KS-Bcl-2). We also tried to rescue the ΔKS-Bcl-2 mutant by inserting the coding sequence of Bcl-XL, a potent cellular apoptosis and autophagy inhibitor (17), or the human cytomegalovirus (HCMV) mitochondrion-localized inhibitor of apoptosis vMIA, a potent viral apoptosis inhibitor (44, 45). An analogous set of mutants was also constructed on the KSHVIND backbone. RPE-1 cells were transfected with BAC DNAs of WT and mutant KSHVLYT and monitored for expression of virus-encoded green fluorescent protein (GFP). Although numerous individual GFP-expressing cells were observed in all dishes with BAC-transfected cells, the formation of GFP-positive cell clusters (foci) was found only in cells transfected with WT KSHVLYT or the revertant (Fig. 2A). Small foci expanded to larger foci and plaques over time (not shown). In contrast, the KS-Bcl-2 deletion mutant and the Bcl-XL and vMIA substitution mutants did not form foci (Fig. 2A). The transfected cells were kept in culture for 11 days, and the total number of foci was determined for each transfection (Fig. 2B). These results strongly suggest that KS-Bcl-2 is essential for KSHV replication and that Bcl-XL and vMIA cannot substitute for KS-Bcl-2.
TABLE 1.
KSHVLYT gene knockout screen
| Deleted ORF | KSHVLYT replication |
|---|---|
| K1 | Yes |
| K2 | Yes |
| K3 | Yes |
| K4 | Yes |
| K4.1 | Yes |
| K4.2 | Yes |
| K5 | Yes |
| K6 | Yes |
| K7 | Yes |
| K8 | Yes |
| K8.1 | Yes |
| K9 | Yes |
| K10 | Yes |
| K10.5 | Yes |
| K11 | Yes |
| K12 | No |
| K14 | Yes |
| K15 | Yes |
| ORF2 | Yes |
| ORF4 | Yes |
| ORF8 | No |
| ORF10 | No |
| ORF11 | Yes |
| ORF16 | No |
| ORF23 | Yes |
| ORF27 | No |
| ORF28 | Yes |
| ORF40 | No |
| ORF42 | No |
| ORF45 | No |
| ORF46 | No |
| ORF47 | Yes |
| ORF48 | Yes |
| ORF52 | Yes |
| ORF58 | No |
| ORF59 | No |
| ORF60 | Yes |
| ORF61 | Yes |
| ORF64 | No |
| ORF69 | No |
| ORF70 | Yes |
| ORF72 | Yes |
| ORF73 | Yes |
| ORF74 | Yes |
| ORF75 | Yes |
FIG 2.
KS-Bcl-2 is essential for KSHV lytic replication. (A) WT and mutant KSHVLYT BACs were transfected into RPE-1 cells. GFP expression and focus formation were observed by fluorescence microscopy on days 1, 3, and 6 posttransfection. (B) The total number of replication foci per dish was counted on day 11 posttransfection. (C) WT and mutant KSHVIND BACs were transfected into RPE-1 cells. Cells were cultured in the presence of increasing concentrations of FK-506, and focus formation was observed for 5 days. (D) 293A cells carrying WT or mutant KSHVIND genomes were cultured in the presence of 10 μM FK-506 for 48 h. Expression of RTA, LANA-1, K8.1A, and HA-tagged KS-Bcl-2, Bcl-XL, and vMIA (arrowheads) was detected by immunoblotting. (E) Virus release by the cells described for panel D was determined by titration.
Since KSHVLYT expresses high levels of RTA (Fig. 1B) and RTA can be cytotoxic (46), we tested whether KS-Bcl-2 is also required under conditions of lower RTA expression. To this end, we transfected RPE-1 cells with WT and mutant KSHVIND BACs and induced RTA expression with different concentrations of FK-506. Treatment with 1 or 10 μM FK-506 was sufficient to induce focus formation in cells transfected with WT KSHVIND and the revertant, whereas the KS-Bcl-2 deletion mutant and the Bcl-XL and vMIA substitution mutants did not form foci under these conditions (Fig. 2C). Next, we transfected 293A cells with the same set of KSHVIND BACs and performed selection with hygromycin in order to obtain a cell population stably carrying these KSHV genomes. Upon induction with FK-506, these cells expressed increased levels of RTA and LANA-1 (Fig. 2D). The inserted HA-tagged proteins were also expressed in these cells. However, expression of the viral late protein K8.1A was very low in cells harboring the KS-Bcl-2 deletion mutant or the Bcl-XL or vMIA substitution mutant (Fig. 2D), and release of infectious virus was not detected (Fig. 2E).
KS-Bcl-2 is needed in the late phase of KSHV replication.
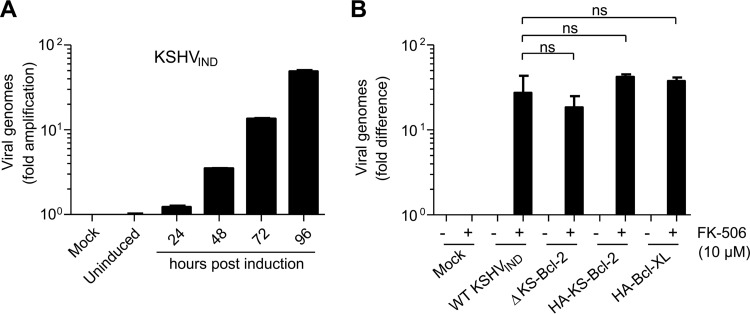
The experiments shown in Fig. 2D and E suggested that the block of KSHV replication in the absence of KS-Bcl-2 occurred in the late phases of the lytic cycle. To verify this hypothesis, we analyzed viral DNA replication in the presence or absence of KS-Bcl-2. First, we induced RTA expression in 293A cells harboring KSHVIND and measured viral DNA amplification by quantitative real-time PCR (qPCR) at different times after induction (Fig. 3A). Because the number of viral DNA genome copies increased continuously, we decided to use the latest time point (i.e., 96 h postinduction) for subsequent experiments. We measured viral DNA amplification in FK-506-treated 293A cells relative to that in untreated control cells by qPCR. As shown in Fig. 3B, viral DNA replication of the ΔKS-Bcl-2 mutant was slightly reduced, but the difference was not statistically significant. Hence, KS-Bcl-2 is not essential for viral DNA replication but is required for efficient synthesis of viral late proteins and release of infectious particles.
FIG 3.
KS-Bcl-2 is not essential for KSHV DNA replication. (A) 293A cells harboring KSHVIND were treated with 10 μM FK-506 to induce the lytic replication cycle. Cells were harvested and total DNA extracted at different times postinduction. KSHV genome amplification relative to that in uninduced cells was quantified by qPCR. The number of cellular genome copies was also determined and used for normalization. (B) 293A cells carrying KSHVIND were treated with 10 μM FK-506. After 96 h of treatment, total DNA was extracted, and KSHV genome copies were quantified as described above. The experiment was done three times in triplicate. Values are displayed as mean values and standard errors of the means (SEM). Differences between WT KSHVIND and the mutants were not statistically significant (ns; P > 0.05) as determined by Student's t test.
RRV-Bcl-2 can substitute for KS-Bcl-2 and rescue KSHV replication.
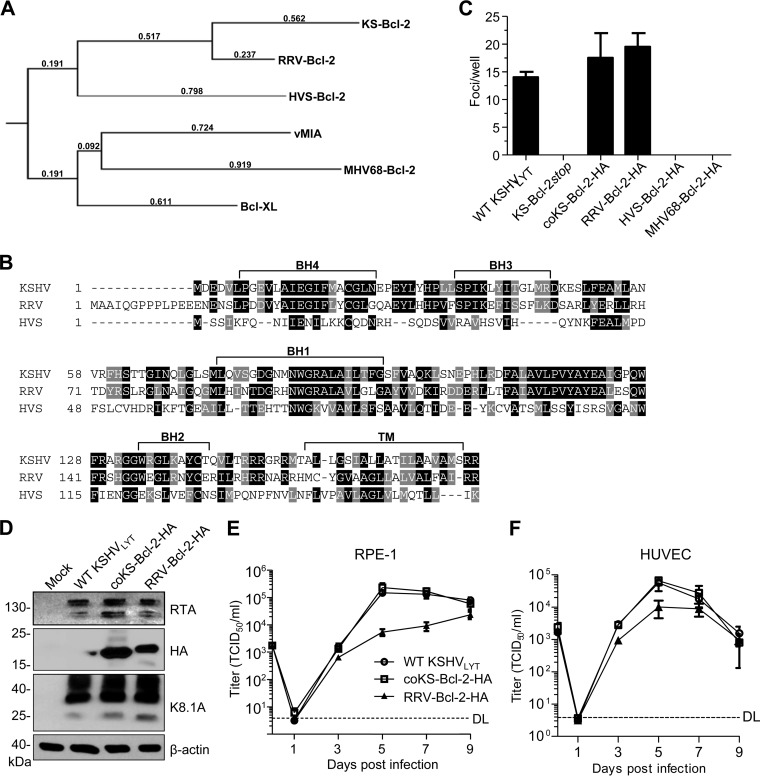
Although our experiments with deletion and substitution mutants strongly suggested that the KS-Bcl-2 protein is required for viral replication, it remained possible that the KS-Bcl-2 coding sequence, ORF16, contained hitherto unknown coding or regulatory elements that are essential for the viral life cycle. To exclude this possibility, we constructed additional KSHV mutants as follows. (i) The methionine codon at position 18 was replaced by a stop codon. The resulting KS-Bcl-2stop mutant cannot express KS-Bcl-2 but differs by only two nucleotides from the parental virus. (ii) ORF16 was replaced by a “codon-optimized” version in which every amino acid of KS-Bcl-2 is encoded by the most frequently used codon in the human genome. The resulting coKS-Bcl-2 mutant contains 125 nucleotide changes within ORF16 (76% identity) but encodes an identical KS-Bcl-2 protein. (iii) KSHV ORF16 was replaced by its presumed orthologs from related γ2-herpesviruses, i.e., the ORFs encoding the vBcl-2 proteins of RRV, HVS, and MHV-68. Phylogenetic analysis and alignment of the amino acid sequences showed that the primate rhadinovirus vBcl-2 proteins show the greatest similarity to KS-Bcl-2 (Fig. 4A and B). WT and mutant KSHVLYT BACs were transfected into RPE-1 cells, and focus formation (indicative of viral replication and spread) was analyzed. While the KS-Bcl-2stop mutant did not produce foci upon transfection of RPE-1 cells, the coKS-Bcl-2 mutant produced foci similarly to WT KSHVLYT (Fig. 4C). This result strongly argued for a requirement of the KS-Bcl-2 protein rather than the authentic ORF16 sequence. Moreover, a substitution mutant encoding RRV-Bcl-2 produced foci (Fig. 4C) and expressed amounts of viral proteins similar to those with the WT and coKS-Bcl-2 viruses (Fig. 4D) but replicated to somewhat lower titers in RPE-1 cells and human umbilical vein endothelial cells (HUVEC) (Fig. 4E and F). Surprisingly, the HVS and MHV-68 vBcl-2 substitution mutants did not replicate in RPE-1 cells (Fig. 4C), suggesting that the essential function of KS-Bcl-2 is conserved in RRV-Bcl-2 but not the phylogenetically more distant vBcl-2 proteins of HVS and MHV-68.
FIG 4.
RRV-Bcl-2 can substitute for KS-Bcl-2 and rescue KSHV replication. (A) Phylogenetic tree of viral and cellular Bcl-2 proteins used in this study. (B) The ORF16 (vBcl-2) proteins of KSHV, RRV, and HVS were aligned using T-Coffee (http://www.tcoffee.org). Identical and similar amino acids are shaded black and gray, respectively. The Bcl-2 homology (BH) and transmembrane (TM) domains are marked. (C) RPE-1 cells were transfected with WT and mutant KSHVLYT BACs. The total number of replication foci per well was counted at 11 days posttransfection. (D) RPE-1 cells were infected with the indicated KSHVLYT strains. Lysates were prepared at 72 h postinfection and subjected to immunoblot analysis. RPE-1 cells (E) and HUVEC (F) were infected with WT and mutant KSHVLYT at an MOI of 0.05 TCID50/cell. Supernatants were harvested at the indicated time points, and virus production was quantified by titration. DL, detection limit.
RRV-Bcl-2 is essential for RRV replication.
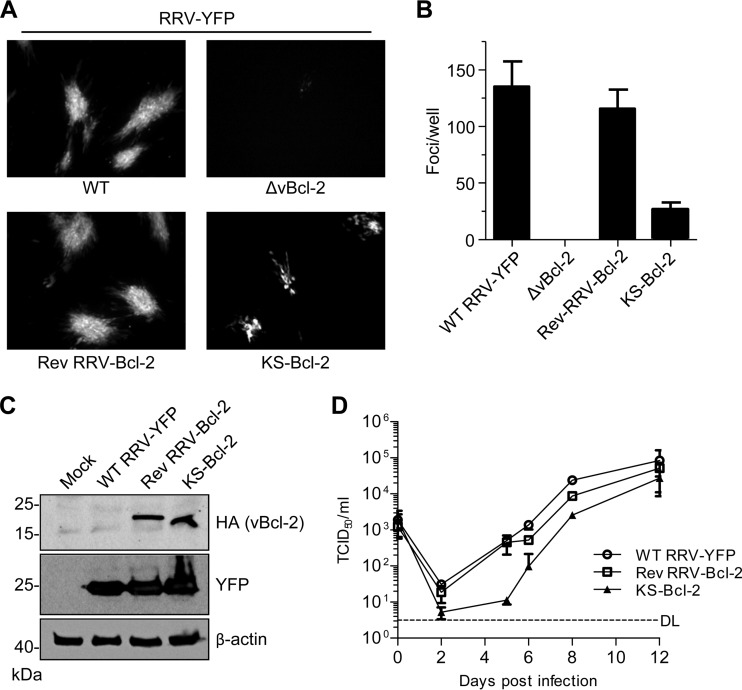
To verify the hypothesis that RRV-Bcl-2 exerts an essential function during viral replication, we used a yellow fluorescent protein (YFP)-expressing RRV BAC (47) to construct an RRV ORF16 deletion mutant, a revertant, and a substitution mutant expressing KS-Bcl-2. Transfection of rhesus fibroblasts with WT and revertant RRV BACs resulted in a large number of YFP-positive foci, indicating successful virus reconstitution (Fig. 5A and B). The foci increased in size and number until the entire cell monolayer was infected. In contrast, transfection of the RRV ΔvBcl-2 BAC did not result in focus formation, suggesting that RRV-Bcl-2 is essential for RRV replication. The KS-Bcl-2 substitution mutant produced foci in transfected rhesus fibroblasts, although they were smaller and fewer (Fig. 5A and B). However, comparable KS-Bcl-2 and RRV-Bcl-2 protein levels were detected in infected fibroblasts (Fig. 5C). The viruses also reached similar titers in rhesus fibroblasts at day 12 postinfection, but progeny production by the KS-Bcl-2 substitution mutant was delayed (Fig. 5D), consistent with the observed smaller focus size (Fig. 5A). These results confirmed that the vBcl-2 proteins of KSHV and RRV have a conserved function that is essential for viral replication.
FIG 5.
RRV-Bcl-2 is essential for RRV replication and can be replaced by KS-Bcl-2. (A) Telomerase-immortalized rhesus fibroblasts were transfected with WT and mutant RRV-YFP BACs. Replication foci were visualized by fluorescence microscopy on day 10 after transfection. (B) The number of replication foci per well was determined 10 days after transfection. Mean values and SEM for four replicates are shown. (C) Lysates of rhesus fibroblasts infected with WT and mutant RRV-YFP were prepared on day 6 after infection and subjected to SDS-PAGE. Virus-encoded YFP and HA-tagged vBcl-2 were detected by immunoblotting with specific antibodies. (D) Rhesus fibroblasts were infected with WT and mutant RRV-YFP at an MOI of 0.01 TCID50/cell. Supernatants were harvested at the indicated time points, and virus production was quantified by titration. DL, detection limit.
KS-Bcl-2 and RRV-Bcl-2 localize to the nucleus.
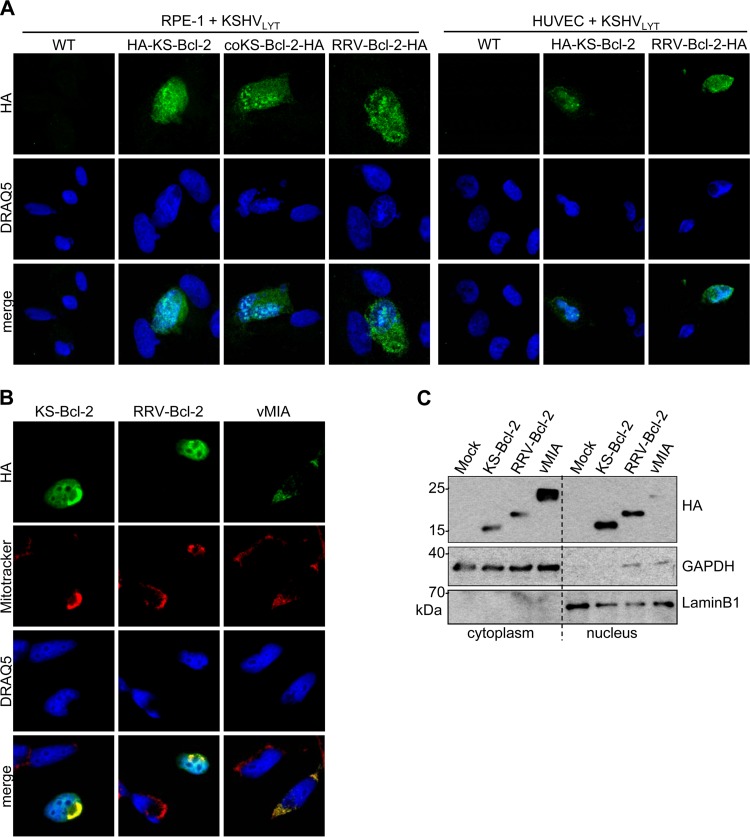
Antiapoptotic Bcl-2 family proteins usually localize to the outer mitochondrial membrane (48). Interestingly, KS-Bcl-2 has also been detected within the nuclei of transfected HEK 293 cells (33). Therefore, we investigated the subcellular localization of KS-Bcl-2 and RRV-Bcl-2 during infection. RPE-1 cells and HUVEC were infected with KSHVLYT mutants expressing HA-tagged KS-Bcl-2, coKS-Bcl-2, or RRV-Bcl-2, and the localization of the HA-tagged vBcl-2 proteins was analyzed by immunofluorescence staining and confocal laser scanning microscopy (CLSM). Despite the weak fluorescence signals, consistent with the previously reported very low expression of KS-Bcl-2 during infection (35), we could detect KS-Bcl-2 and RRV-Bcl-2 in the cytoplasm as well as the nucleus for both infected cell types (Fig. 6A). To better characterize the subcellular distribution, we expressed vBcl-2 proteins in HeLa cells by transfection with expression plasmids. Protein localization was analyzed by immunofluorescence assay and by immunoblotting upon separation of the nuclear and cytoplasmic fractions. By immunofluorescence staining, KS-Bcl-2 and RRV-Bcl-2 were detected at mitochondria (colocalizing with MitoTracker Red) and within the nuclei of transfected cells (Fig. 6B). Consistent with this observation, substantial amounts of the two proteins were detected in the cytoplasmic as well as nuclear fractions of transfected cells (Fig. 6C). In contrast, the HCMV vMIA protein (Fig. 6B), MHV-68 vBcl-2, and cellular Bcl-XL (data not shown) were not detected in the nucleus by immunofluorescence assay. The trace amount of vMIA detected in the nuclear fraction (Fig. 6C) was probably the consequence of an incomplete separation of the nuclear and cytoplasmic fractions.
FIG 6.
KS-Bcl-2 and RRV-Bcl-2 localize to the nuclei of infected and transfected cells. (A) RPE-1 cells and HUVEC were infected with WT and mutant KSHVLYT at an MOI of 0.1 TCID50/cell. Cells were fixed and stained at 48 h postinfection. HA-tagged proteins were detected by CLSM using a rat anti-HA antibody and an Alexa Fluor 555-coupled goat anti-rat antibody (shown in green). Nuclei were stained with DRAQ5. (B) HeLa cells were transfected with plasmids expressing HA-tagged KS-Bcl-2, RRV-Bcl-2, or vMIA. Mitochondria were stained with MitoTracker Red prior to fixation. HA-tagged vBcl-2 proteins were stained with a rat anti-HA antibody and a goat anti-rat–Alexa Fluor 488 antibody. (C) RPE-1 cells were transfected as described above for HeLa cells. Nuclear and cytoplasmic fractions were separated and subjected to immunoblot analysis. GAPDH and lamin B1 served as markers for the cytoplasmic and nuclear fractions, respectively.
The N terminus of KS-Bcl-2 is essential for nuclear localization and viral replication.
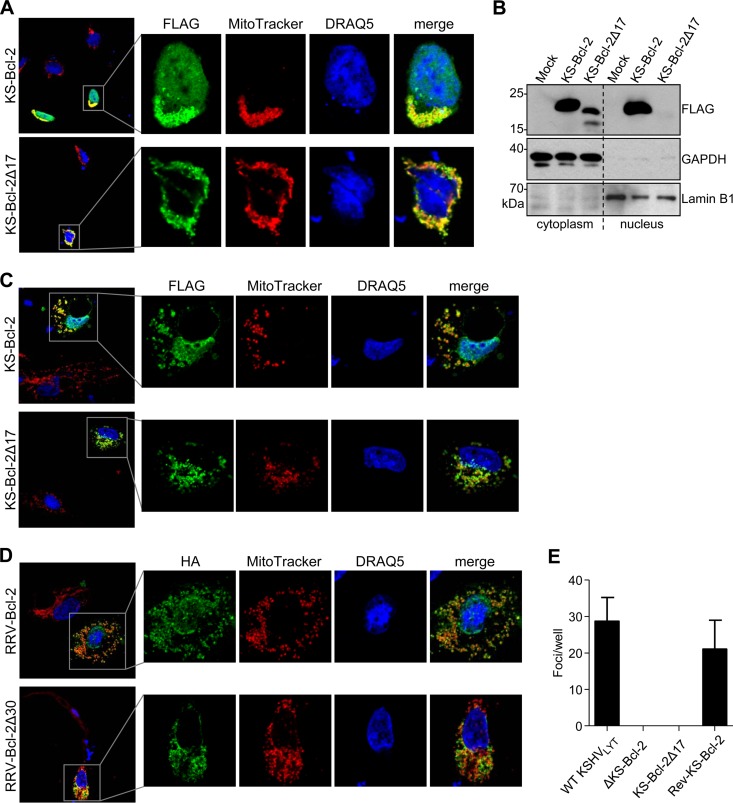
It was previously shown by Kalt et al. (33) that the first 17 amino acids of KS-Bcl-2 are required for interaction of KS-Bcl-2 with the cellular protein GLTSCR2/PICT1 and for translocation to the nucleus. When GLTSCR2 was overexpressed, KS-Bcl-2 accumulated in nucleoli (33). When we expressed full-length KS-Bcl-2 and a truncated KS-Bcl-2 protein lacking the first 17 amino acids (Δ17) in 293A cells, we confirmed the requirement of the first 17 amino acids for nuclear localization by immunofluorescence analysis and cell fractionation (Fig. 7A and B). A similar subcellular distribution was observed in transfected HUVEC (Fig. 7C). Moreover, deletion of the first 30 amino acids of RRV-Bcl-2 (which are homologous to the N-terminal 17 amino acids of KS-Bcl-2) (Fig. 4B) also resulted in a loss of nuclear localization (Fig. 7D), indicating that the requirement of the N terminus for nuclear localization is conserved between KSHV and RRV.
FIG 7.
The N terminus of KS-Bcl-2 is essential for nuclear localization and viral replication. (A) 293A cells were transfected with plasmids expressing 3×FLAG-tagged full-length or N-terminally truncated (Δ17) KS-Bcl-2. Mitochondria were stained with MitoTracker Red prior to fixation. FLAG-tagged proteins were stained with mouse anti-FLAG and an Alexa Fluor 488-coupled secondary antibody. Nuclei were stained with DRAQ5. (B) RPE-1 cells were transfected with the same plasmids as those described above. Nuclear and cytoplasmic fractions were separated and subjected to immunoblot analysis. GAPDH and lamin B1 served as markers for the cytoplasmic and nuclear fractions, respectively. (C) HUVEC were transfected with plasmids and stained as described for panel A. (D) HUVEC were transfected with plasmids expressing HA-tagged full-length or N-terminally truncated (Δ30) RRV-Bcl-2 and stained as described for panel A. (E) WT and mutant KSHVLYT BACs were transfected into RPE-1 cells, and the total number of replication foci per well was determined at 10 days posttransfection. Mean values and SEM for triplicates are shown.
Next, we tested whether the N-terminal domain of KS-Bcl-2, which is required for nuclear localization, is also required for viral replication. To do this, we used the KSHVLYT ΔKS-Bcl-2 BAC to reinsert the coding sequences of either full-length or Δ17 KS-Bcl-2. The BACs were transfected into RPE-1 cells, and replication foci were counted at 10 days posttransfection. While expression of full-length KS-Bcl-2 rescued viral replication, expression of the Δ17 mutant did not (Fig. 7C), suggesting that the KS-Bcl-2 N terminus is required for both nuclear localization and viral replication.
DISCUSSION
Several viruses encode proteins with structural and functional similarity to Bcl-2. These include members of the Herpesviridae, Poxviridae, and Adenoviridae families (49, 50). The viral Bcl-2 homologs and analogs inhibit apoptosis by interacting with proapoptotic proteins of the Bcl-2 family. Some of them also inhibit autophagy by interacting with Beclin-1. The viral Bcl-2 proteins are usually nonessential for viral replication, although they can augment viral productivity, presumably by prolonging the survival of infected cells. Hence, our observation that KS-Bcl-2 is essential for KSHV replication, which we first reported at the KSHV conference in 2012 (51), was surprising. Recently, observations similar to ours were published by others (35, 36). While the study by Gelgor et al. (35) showed that KSHV reactivation in iSLK cells is dramatically reduced in the absence of KS-Bcl-2, the study by Liang et al. (36) showed that KS-Bcl-2 is essential for KSHV lytic replication. Moreover, the latter study also showed by a comprehensive mutational analysis that the antiapoptotic and antiautophagic functions of vBcl-2 are not required for KSHV reactivation in iSLK cells but that a glutamic acid residue at position 14 is essential. Our results are largely consistent with the published data but go substantially beyond them. We show that cellular Bcl-XL and viral Bcl-2 homologs of related viruses cannot compensate for the loss of KS-Bcl-2, indicating that KS-Bcl-2 fulfills an additional function not shared by these proteins. However, the essential function of KS-Bcl-2 is conserved in the vBcl-2 protein of a closely related Old World primate γ2-herpesvirus, RRV. Replacement of KSHV ORF16 by RRV ORF16 rescued KSHV replication, and vice versa (Fig. 4 and 5). Interestingly, ORF16 of a New World primate rhadinovirus, HVS, and the vBcl-2 gene of a rodent rhadinovirus, MHV-68, could not substitute for KSHV ORF16 and rescue KSHV replication. Moreover, it is known that the MHV-68 vBcl-2 protein exerts important functions in vivo but is not essential for viral replication (52–54), and unpublished observations indicate that HVS ORF16 is also nonessential (Armin Ensser, personal communication). Apparently, the essential function was acquired more recently in evolution by a common ancestor of RRV and KSHV. As herpesviruses are thought to have coevolved with their respective host species (55), this probably happened after the divergence of Old and New World primates 35 million years ago (56).
KS-Bcl-2 and RRV-Bcl-2 show an unexpected subcellular distribution: they localize to mitochondria and the nucleus (Fig. 6). While mitochondrial localization is typical of antiapoptotic Bcl-2 family proteins, nuclear localization is unusual. Only one cellular Bcl-2 family protein, Mcl-1, has been reported to translocate to the nucleus upon DNA damage, where it is involved in regulating the DNA damage response by interacting with checkpoint kinase 1 (57–59). Thus, it might be worthwhile to check whether Mcl-1 can substitute for KS-Bcl-2.
It has been reported that KS-Bcl-2 translocates to the nucleus and accumulates in nucleoli upon overexpression in HEK 293 cells (33). In our experiments, we detected KS-Bcl-2 in the nucleus but did not see an accumulation in nucleoli in transfected HeLa and 293A cells (Fig. 6 and 7). This might be due to the fact that we did not overexpress GLTSCR2 as done by Kalt et al. (33). However, in KSHV-infected cells, we did see an accumulation of KS-Bcl-2 and RRV-Bcl-2 in dot-like structures within the nucleus (Fig. 6A). These structures may indeed be nucleoli. However, further experiments would be necessary to confirm this assumption.
Nuclear localization of KS-Bcl-2 and RRV-Bcl-2 requires the N termini of these proteins. Deletion of the first 17 amino acids of KS-Bcl-2 abrogates the nuclear localization but preserves the mitochondrial localization (33). We showed that the homologous N-terminal part of RRV-Bcl-2 is also required for nuclear localization (Fig. 7D). The N-terminal part of KS-Bcl-2 is also essential for KSHV replication (Fig. 7C), suggesting that KS-Bcl-2 might execute its essential function in the nucleus. However, we cannot exclude the possibility that the N-terminal part is also involved in an essential function or interaction independent of the cell nucleus. For instance, the truncated KS-Bcl-2Δ17 protein was expressed at lower levels than the full-length KS-Bcl-2 protein in transfected cells (Fig. 7B). Hence, the N terminus might also be necessary to ensure adequate KS-Bcl-2 levels.
KS-Bcl-2 does not have its own nuclear localization signal. Instead, its nuclear localization depends on its interaction with other proteins, as in the case of GLTSCR2 (33, 60). Which function KS-Bcl-2 and RRV-Bcl-2 fulfill in the nucleus remains unknown and needs to be addressed by future studies. RRV is probably a more manageable system for such studies, as RRV naturally enters the lytic cycle and replicates to useful titers in cell culture. Interestingly, a recent KSHV-host protein interaction screen identified 8 cellular proteins as potential interaction partners of KS-Bcl-2 (61). Four of these 8 proteins have functions within the nucleus: Cullin 3 and Kelch-like proteins 9 and 22 (both of which interact with Cullin 3) are involved in regulation of the cell cycle and mitosis (62, 63), and zinc finger protein 622 (also known as zinc finger-like protein 9) enhances B-MYB transcriptional activity (64). Thus, it should be worthwhile to explore whether these putative interactions can be verified and how they affect the viral life cycle.
MATERIALS AND METHODS
Cells and plasmids.
Telomerase-immortalized human retinal pigment epithelial cells (hTERT-RPE-1; ATCC CRL-4000), telomerase-immortalized rhesus dermal fibroblasts (65), human embryonic kidney 293A cells (Invitrogen), HeLa cells, and rhesus macaque fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Human umbilical vein endothelial cells (HUVEC; Lonza) were cultured in EGM-2 medium supplemented with growth factors, cytokines, and other supplements (Lonza).
Expression plasmids encoding HA-tagged KS-Bcl-2, MHV-68 vBcl-2 (M11), Bcl-XL, and vMIA (UL37x1) have been described previously (66). HVS ORF16 was PCR amplified from HVS BAC DNA (kindly provided by Armin Ensser, University of Erlangen, Germany) and cloned into pcDNA3 (Invitrogen). RRV ORF16 and codon-optimized KSHV ORF16 were purchased as synthetic DNA molecules from Thermo Fisher and cloned with an HA tag into pcDNA3. pcDNA3 plasmids expressing full-length and truncated versions of KS-Bcl-2 or RRV-Bcl-2, with C-terminal 3×FLAG or HA tags, respectively, were generated by PCR cloning.
BAC mutagenesis.
KSHV BAC16 (39) was kindly provided by Jae U. Jung (University of Southern California, Los Angeles, CA). The RRV-YFP BAC (47) was kindly provided by Alexander Hahn (German Primate Center, Göttingen, Germany). KSHV BAC16 was modified to enforce lytic replication by inserting a phosphoglycerate kinase 1 (PGK) promoter in front of ORF50 in the same way as that previously described for another KSHV clone, BAC36 (34). The resulting virus was named KSHVLYT. KSHVIND was constructed on the basis of KSHVLYT by inserting the sequence of the FKBP12 destabilizing domain (40, 41) in front of ORF50 as described in Results. The integrity of the KSHVLYT and KSHVIND BACs was verified by Illumina sequencing of the entire BACs.
KSHV and RRV mutants were constructed using the galK-kan system of BAC mutagenesis (67). Briefly, KSHV and RRV BACs were first introduced into Escherichia coli strain SW102. The gene to be deleted was replaced by a cassette encoding galactokinase (galK) and carrying a kanamycin resistance gene (kan) for positive selection. The KSHV and RRV ORF16 deletion mutants were used to construct revertants and substitution mutants. The sequences to be inserted were amplified by PCR with oligonucleotide primers containing 50-bp homologies to sequences upstream or downstream of ORF16. Recombinant BACs were obtained by homologous recombination, using 2-deoxygalactose (Sigma-Aldrich) for negative selection. The integrity of the mutant BACs was verified by digestion with two different restriction enzymes and gel electrophoresis and by sequence analysis of the mutated region. Two independent clones of each mutant were used for initial phenotypic analysis. The results were accepted only if the two independent clones showed the same phenotype.
Virus propagation and titration.
For virus reconstitution, KSHV BAC DNA was transfected into RPE-1 cells by use of PolyFect (Qiagen). RRV BAC DNA was transfected into rhesus fibroblasts by use of Lipofectamine 2000 (Thermo Fisher). KSHVLYT was grown on RPE-1 cells and titrated using the TCID50 method as previously described (34). RRV was grown and titrated on rhesus fibroblasts. KSHVIND BAC DNA was transfected into 293A cells by use of PolyFect, and cells carrying the viral genome were selected with 100 μg/ml hygromycin B (Roth). For comparison of KSHVIND with WT BAC16, 293A cells were transfected with BAC DNA. After selection with 100 μg/ml hygromycin B, lytic replication was induced with different concentrations of FK-506 in the case of KSHVIND or of a combination of sodium butyrate (Sigma) and 12-O-tetradecanoyl-phorbol-13-acetate (TPA; Applichem) in the case of WT KSHV. Samples were then analyzed by SDS-PAGE and immunoblotting.
For assays of KSHV and RRV replication kinetics, RPE-1 cells, HUVEC, or rhesus fibroblasts were infected in 6-well plates in triplicate. Supernatant samples for titration were collected at different times, and fresh medium was added to cells. KSHV titers were determined by titration on RPE-1 cells, and RRV titers were determined by titration on rhesus fibroblasts.
Infectious focus assay.
RPE-1 cells or rhesus fibroblasts were seeded in 12-well plates (4 × 104 cells/well) and transfected on the following day with a KSHV or RRV BAC. For transfection, 1.5 μg BAC DNA was combined with 60 μl DMEM and 8 μl PolyFect. Cells were rinsed with phosphate-buffered saline (PBS), and the DNA-PolyFect mixture combined with 1.2 ml of fresh medium was added. All transfections were done in triplicate. After 10 or 11 days, the number of KSHV replication foci (defined as clusters of 3 or more GFP-positive cells) was counted for each well. Alternatively, the transfected cells were analyzed each day by use of a fluorescence microscope. Transfected, GFP-positive cells were marked on the plate and monitored for 11 days. Foci usually started to appear 5 to 6 days after transfection.
KSHV genome quantification.
Genomic DNA was extracted from 293A cells harboring WT or mutant KSHVIND by use of an InnuPREP DNA minikit (Analytik Jena). Samples were subjected to quantitative real-time PCR (qPCR) in order to quantify KSHV genome replication. Primers GGTCCACCCCTTCTTTGATT and GCGAGCGGTTGTGGTATATT were used for PCR amplification of the KSHV genome, and primers GCTGAGGCCCAGTTCTAAAT and TTCAAGTCCCATCCCAGAAAG were used for amplification of the cellular β-actin gene. qPCR was performed on a model 7900HT Fast real-time PCR system (Thermo Fisher), and data were processed using the ΔΔCT method (68). Statistical analysis was done using Student's t test.
Immunoblot and immunofluorescence analyses.
For immunoblot analysis, cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% NP-40; 0.25% sodium deoxycholate; 1 mM EDTA) supplemented with protease inhibitors (Roche) and subjected to SDS-PAGE followed by transfer to a nitrocellulose membrane. Cell fractionation experiments were carried out using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher) according to the manufacturer's protocol. Mouse monoclonal antibodies against RTA (69) (kindly provided by Keiji Ueda, Osaka University, Japan), LANA-1 (clone AT4C11; Acris), K8.1A/B (clone 4A4; Santa Cruz), β-actin (clone AC-15; Sigma-Aldrich), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (clone 14C10; Cell Signaling), lamin B1 (clone A-11; Santa Cruz), GFP (clones 13.1/7.1; Roche), HA (clone 16B12; Covance), and FLAG (clone M2; Sigma-Aldrich) were used for protein detection.
For immunofluorescence assay, cells were plated onto glass coverslips coated with 0.4% gelatin in PBS for 30 min. RPE-1 cells or HUVEC were infected with KSHVLYT at a multiplicity of infection (MOI) of 0.1 TCID50/cell. HeLa or 293A cells or HUVEC were transfected with pcDNA3 expression plasmids by use of Lipofectamine 2000. Mitochondria were stained with 300 nM MitoTracker Red CMXRos (Thermo Fisher) for 30 min at 37°C. Cells were fixed at −20°C in methanol-acetone for 10 min, washed with PBS, and blocked with PBS containing 1% gelatin. HA- and FLAG-tagged proteins were detected with rat anti-HA (3F10; Roche) and mouse anti-FLAG (M2; Sigma-Aldrich) primary antibodies and with secondary antibodies coupled to Alexa Fluor 488 and 555 (Thermo Fisher), respectively. Incubations with the antibodies were carried out for 30 min at room temperature in 1% gelatin-PBS. Nuclei were stained with DRAQ5 (BioStatus) diluted 1:1,000 in PBS for 10 min at room temperature. Coverslips were mounted on microscope slides and subjected to confocal laser scanning microscopy (CLSM).
Accession number(s).
The complete sequences of the KSHVLYT and KSHVIND BACs were deposited in the GenBank database under accession numbers KY246443 and KY246444, respectively.
ACKNOWLEDGMENTS
We thank Kevin Brulois and Jae U. Jung for KSHV BAC16, Alexander Hahn and Shou-Jiang Gao for the RRV-YFP BAC, and Armin Ensser for the HVS BAC.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (grant BR 1730/3-2 to W.B.). The Heinrich Pette Institute is supported by the Free and Hanseatic City of Hamburg and the Federal Ministry of Health.
REFERENCES
- 1.Mesri EA, Cesarman E, Boshoff C. 2010. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer 10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 305:163–174. doi: 10.1016/j.canlet.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittmer DP, Damania B. 2013. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)—an update. Curr Opin Virol 3:238–244. doi: 10.1016/j.coviro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gramolelli S, Schulz TF. 2015. The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma. J Pathol 235:368–380. doi: 10.1002/path.4441. [DOI] [PubMed] [Google Scholar]
- 5.Barbera AJ, Ballestas ME, Kaye KM. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J Virol 78:294–301. doi: 10.1128/JVI.78.1.294-301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J Virol 72:8309–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye F, Lei X, Gao SJ. 2011. Mechanisms of Kaposi's sarcoma-associated herpesvirus latency and reactivation. Adv Virol 2011:93860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol 73:2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Biggar RJ, Marshall VA, Walters MA, Gamache CJ, Whitby D, Goedert JJ. 2003. Detection and quantification of Kaposi's sarcoma-associated herpesvirus to predict AIDS-associated Kaposi's sarcoma. AIDS 17:1847–1851. doi: 10.1097/00002030-200308150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukac DM, Kirshner JR, Ganem D. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol 73:9348–9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol 74:6207–6212. doi: 10.1128/JVI.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardwick JM, Youle RJ. 2009. SnapShot: BCL-2 proteins. Cell 138:404, 404.e1. doi: 10.1016/j.cell.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkmann N, Marassi FM, Newmeyer DD, Hanein D. 2014. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ 21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. 2011. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. 2005. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Sinha S, Kroemer G. 2008. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. 2007. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germain M, Slack RS. 2011. MCL-1 regulates the balance between autophagy and apoptosis. Autophagy 7:549–551. doi: 10.4161/auto.7.5.15098. [DOI] [PubMed] [Google Scholar]
- 20.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A 90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WL, Yim C, Gustafson E, Graf T, Sage DR, Hanify K, Williams L, Fingeroth J, Finberg RW. 1999. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol 73:5181–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med 3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 23.Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, Reitz MS, Hardwick JM. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci U S A 94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J Virol 74:3388–3398. doi: 10.1128/JVI.74.7.3388-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nava VE, Cheng EH, Veliuona M, Zou S, Clem RJ, Mayer ML, Hardwick JM. 1997. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol 71:4118–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang GH, Garvey TL, Cohen JI. 1999. The murine gammaherpesvirus-68 M11 protein inhibits Fas- and TNF-induced apoptosis. J Gen Virol 80:2737–2740. doi: 10.1099/0022-1317-80-10-2737. [DOI] [PubMed] [Google Scholar]
- 27.Ku B, Woo JS, Liang C, Lee KH, Hong HS, Xiaofei E, Kim KS, Jung JU, Oh BH. 2008. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog 4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha S, Colbert CL, Becker N, Wei Y, Levine B. 2008. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy 4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo JY, Yamada A, Kajino T, Wu JQ, Tang W, Freel CD, Feng J, Chau BN, Wang MZ, Margolis SS, Yoo HY, Wang XF, Dunphy WG, Irusta PM, Hardwick JM, Kornbluth S. 2008. Aven-dependent activation of ATM following DNA damage. Curr Biol 18:933–942. doi: 10.1016/j.cub.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau BN, Cheng EH, Kerr DA, Hardwick JM. 2000. Aven, a novel inhibitor of caspase activation, binds Bcl-xL and Apaf-1. Mol Cell 6:31–40. doi: 10.1016/S1097-2765(05)00021-3. [DOI] [PubMed] [Google Scholar]
- 31.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. 2000. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol 2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 32.Bellows DS, Chau BN, Lee P, Lazebnik Y, Burns WH, Hardwick JM. 2000. Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J Virol 74:5024–5031. doi: 10.1128/JVI.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalt I, Borodianskiy-Shteinberg T, Schachor A, Sarid R. 2010. GLTSCR2/PICT-1, a putative tumor suppressor gene product, induces the nucleolar targeting of the Kaposi's sarcoma-associated herpesvirus KS-Bcl-2 protein. J Virol 84:2935–2945. doi: 10.1128/JVI.00757-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budt M, Hristozova T, Hille G, Berger K, Brune W. 2011. Construction of a lytically replicating Kaposi's sarcoma-associated herpesvirus. J Virol 85:10415–10420. doi: 10.1128/JVI.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelgor A, Kalt I, Bergson S, Brulois KF, Jung JU, Sarid R. 2015. Viral Bcl-2 encoded by the Kaposi's sarcoma-associated herpesvirus is vital for virus reactivation. J Virol 89:5298–5307. doi: 10.1128/JVI.00098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Q, Chang B, Lee P, Brulois KF, Ge J, Shi M, Rodgers MA, Feng P, Oh BH, Liang C, Jung JU. 2015. Identification of the essential role of viral Bcl-2 for Kaposi's sarcoma-associated herpesvirus lytic replication. J Virol 89:5308–5317. doi: 10.1128/JVI.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J Virol 76:6185–6196. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakushko Y, Hackmann C, Gunther T, Ruckert J, Henke M, Koste L, Alkharsah K, Bohne J, Grundhoff A, Schulz TF, Henke-Gendo C. 2011. Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome contains a duplication of a long unique-region fragment within the terminal repeat region. J Virol 85:4612–4617. doi: 10.1128/JVI.02068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brulois KF, Chang H, Lee AS, Ensser A, Wong LY, Toth Z, Lee SH, Lee HR, Myoung J, Ganem D, Oh TK, Kim JF, Gao SJ, Jung JU. 2012. Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone. J Virol 86:9708–9720. doi: 10.1128/JVI.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. 2006. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass M, Busche A, Wagner K, Messerle M, Borst EM. 2009. Conditional and reversible disruption of essential herpesvirus proteins. Nat Methods 6:577–579. doi: 10.1038/nmeth.1346. [DOI] [PubMed] [Google Scholar]
- 42.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura S, Fujita Y, Gomez E, Tanese N, Wilson AC. 2005. Activation of the Kaposi's sarcoma-associated herpesvirus major latency locus by the lytic switch protein RTA (ORF50). J Virol 79:8493–8505. doi: 10.1128/JVI.79.13.8493-8505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, Han J, Lutz RJ, Watanabe S, McFarland ED, Kieff ED, Mocarski ES, Chittenden T. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A 96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauleau AL, Larochette N, Giordanetto F, Scholz SR, Poncet D, Zamzami N, Goldmacher VS, Kroemer G. 2007. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 26:7067–7080. doi: 10.1038/sj.onc.1210511. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura K, Ueda K, Sakakibara S, Do E, Ohsaki E, Okuno T, Yamanishi K. 2003. A viral transcriptional activator of Kaposi's sarcoma-associated herpesvirus (KSHV) induces apoptosis, which is blocked in KSHV-infected cells. Virology 316:64–74. doi: 10.1016/S0042-6822(03)00582-8. [DOI] [PubMed] [Google Scholar]
- 47.Hahn AS, Grosskopf AK, Jungnickl D, Scholz B, Ensser A. 2016. Viral FGARAT homolog ORF75 of rhesus monkey rhadinovirus effects proteasomal degradation of the ND10 components SP100 and PML. J Virol 90:8013–8028. doi: 10.1128/JVI.01181-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cory S, Adams JM. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 49.Polster BM, Pevsner J, Hardwick JM. 2004. Viral Bcl-2 homologs and their role in virus replication and associated diseases. Biochim Biophys Acta 1644:211–227. doi: 10.1016/j.bbamcr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Kvansakul M, Hinds MG. 2013. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis 4:e909. doi: 10.1038/cddis.2013.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallo A, Lampe M, Brulois K, Jung J, Brune W. 2012. An inducible lytic KSHV for the analysis of lytic gene function, p 89 Int Congr Oncogenic Herpesviruses Assoc Dis, Philadelphia, PA. [Google Scholar]
- 52.Gangappa S, van Dyk LF, Jewett TJ, Speck SH, Virgin HW IV. 2002. Identification of the in vivo role of a viral bcl-2. J Exp Med 195:931–940. doi: 10.1084/jem.20011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Lima BD, May JS, Marques S, Simas JP, Stevenson PG. 2005. Murine gammaherpesvirus 68 bcl-2 homologue contributes to latency establishment in vivo. J Gen Virol 86:31–40. doi: 10.1099/vir.0.80480-0. [DOI] [PubMed] [Google Scholar]
- 54.Coleman CB, McGraw JE, Feldman ER, Roth AN, Keyes LR, Grau KR, Cochran SL, Waldschmidt TJ, Liang C, Forrest JC, Tibbetts SA. 2014. A gammaherpesvirus Bcl-2 ortholog blocks B cell receptor-mediated apoptosis and promotes the survival of developing B cells in vivo. PLoS Pathog 10:e1003916. doi: 10.1371/journal.ppat.1003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davison AJ. 2007. Comparative analysis of the genomes, p 10–26. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 56.Schrago CG, Russo CA. 2003. Timing the origin of New World monkeys. Mol Biol Evol 20:1620–1625. doi: 10.1093/molbev/msg172. [DOI] [PubMed] [Google Scholar]
- 57.Pawlikowska P, Leray I, de Laval B, Guihard S, Kumar R, Rosselli F, Porteu F. 2010. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ 17:1739–1750. doi: 10.1038/cdd.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jamil S, Mojtabavi S, Hojabrpour P, Cheah S, Duronio V. 2008. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol Biol Cell 19:3212–3220. doi: 10.1091/mbc.E07-11-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamil S, Stoica C, Hackett TL, Duronio V. 2010. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell Cycle 9:2843–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalt I, Levy A, Borodianskiy-Shteinberg T, Sarid R. 2012. Nucleolar localization of GLTSCR2/PICT-1 is mediated by multiple unique nucleolar localization sequences. PLoS One 7:e30825. doi: 10.1371/journal.pone.0030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis ZH, Verschueren E, Jang GM, Kleffman K, Johnson JR, Park J, Von Dollen J, Maher MC, Johnson T, Newton W, Jager S, Shales M, Horner J, Hernandez RD, Krogan NJ, Glaunsinger BA. 2015. Global mapping of herpesvirus-host protein complexes reveals a transcription strategy for late genes. Mol Cell 57:349–360. doi: 10.1016/j.molcel.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumara I, Quadroni M, Frei C, Olma MH, Sumara G, Ricci R, Peter M. 2007. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell 12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Maerki S, Olma MH, Staubli T, Steigemann P, Gerlich DW, Quadroni M, Sumara I, Peter M. 2009. The Cul3-KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J Cell Biol 187:791–800. doi: 10.1083/jcb.200906117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seong HA, Kim KT, Ha H. 2003. Enhancement of B-MYB transcriptional activity by ZPR9, a novel zinc finger protein. J Biol Chem 278:9655–9662. doi: 10.1074/jbc.M207478200. [DOI] [PubMed] [Google Scholar]
- 65.Kirchoff V, Wong S, St JS, Pari GS. 2002. Generation of a life-expanded rhesus monkey fibroblast cell line for the growth of rhesus rhadinovirus (RRV). Arch Virol 147:321–333. doi: 10.1007/s705-002-8322-9. [DOI] [PubMed] [Google Scholar]
- 66.Jurak I, Brune W. 2006. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J 25:2634–2642. doi: 10.1038/sj.emboj.7601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, Ueda K, Sakakibara S, Okuno T, Parravicini C, Corbellino M, Yamanishi K. 2001. Activation of latent Kaposi's sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc Natl Acad Sci U S A 98:4119–4124. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]