ABSTRACT
Necroptosis, a regulated form of necrotic cell death, requires the activation of the RIP3 kinase. Here, we identify that infection of host cells with reovirus can result in necroptosis. We find that necroptosis requires sensing of the genomic RNA within incoming virus particles via cytoplasmic RNA sensors to produce type I interferon (IFN). While these events that occur prior to the de novo synthesis of viral RNA are required for the induction of necroptosis, they are not sufficient. The induction of necroptosis also requires late stages of reovirus infection. Specifically, efficient synthesis of double-stranded RNA (dsRNA) within infected cells is required for necroptosis. These data indicate that viral RNA interfaces with host components at two different stages of infection to induce necroptosis. This work provides new molecular details about events in the viral replication cycle that contribute to the induction of necroptosis following infection with an RNA virus.
IMPORTANCE An appreciation of how cell death pathways are regulated following viral infection may reveal strategies to limit tissue destruction and prevent the onset of disease. Cell death following virus infection can occur by apoptosis or a regulated form of necrosis known as necroptosis. Apoptotic cells are typically disposed of without activating the immune system. In contrast, necroptotic cells alert the immune system, resulting in inflammation and tissue damage. While apoptosis following virus infection has been extensively investigated, how necroptosis is unleashed following virus infection is understood for only a small group of viruses. Here, using mammalian reovirus, we highlight the molecular mechanism by which infection with a dsRNA virus results in necroptosis.
KEYWORDS: cell death, innate immunity, necroptosis, reovirus
INTRODUCTION
Host cell death is a common outcome of virus infection (1). One form of cell death, necroptosis, has been described following infection with influenza A virus (IAV), herpes simplex virus 1 (HSV1), HSV2, murine cytomegalovirus (MCMV), and vaccinia virus (VV). In each of these cases, necroptosis protects the infected animal (2–7). There are also examples where increased necroptosis contributes to tissue injury and exacerbates viral disease (7, 8). The impact of necroptosis on these viral diseases may be due to the premature death of the infected cell or a consequence of inflammation induced by the leakage of molecules from necrotic cells (9, 10).
Necroptosis requires the activation of receptor-interacting protein 3 (RIP3) kinase (6, 11, 12). Once activated, RIP3 kinase signals via the pseudokinase mixed lineage kinase-like (MLKL) protein to promote a necrotic form of cell death that is characterized by the loss of membrane integrity and leakage of cellular contents (13–23). RIP3 contains a receptor-interacting protein homotypic interacting motif (RHIM) and is activated via interactions with other cellular RHIM-containing proteins: TRIF (TIR domain-containing adapter inducing interferon beta [IFN-β]), RIP1, or DAI (DNA-dependent activator of IFN-regulatory factors [IRFs]) (24). TRIF activation by Toll-like receptor 3 (TLR3) and TLR4 ligands can evoke necroptosis, but necroptosis by this mechanism following virus infection has not yet been demonstrated (25, 26). RIP1 activation by tumor necrosis factor alpha (TNF-α) induces RIP3-dependent necroptosis following VV infection (6). The pathogen sensor DAI is required for necroptosis in cells infected with an MCMV variant (5). The ribonucleotide reductases ICP6 and ICP10 encoded by HSV1 and HSV2, respectively, contain a RHIM-like domain. These ribonucleotide reductases interact with murine RIP1 and RIP3, promote RIP1-RIP3 or RIP3-RIP3 oligomerization, and induce necroptosis (2, 3).
In contrast to those studies on DNA viruses, mechanisms by which RNA viruses induce necroptosis are less understood. IAV induces necroptosis in the lungs of cIAP2-deficient mice (8). Because uninfected cells also undergo cell death in this model, it is thought that cell death is a consequence of an alteration of cellular homeostasis rather than being induced by viral replication events. In wild-type cells, IAV activates a RIP3-containing signaling platform that can induce either apoptosis or necroptosis (7). Recent evidence suggests that DAI, which was previously thought to be a sensor for cytoplasmic DNA, interacts with IAV components to engage RIP3 and induce necroptosis (27, 28). RNA viruses such as coxsackievirus B (CVB), coronavirus, mammalian reovirus (reovirus), Theiler's murine encephalomyelitis virus (TMEV), and West Nile virus (WNV) have also been demonstrated to evoke cell death with morphological features resembling necrosis (29–32). However, the events in viral replication that initiate pronecrotic signaling pathways have not been defined for these RNA viruses.
In this study, we investigated the mechanism by which reovirus infection culminates in necroptosis. Our results indicate that IFN-β produced by the detection of genomic RNA of incoming virus particles is required but not sufficient for eliciting necroptosis. In addition to IFN-β expression, de novo synthesis of viral double-stranded RNA (dsRNA) is also required for necroptosis induction. These results suggest that detection of viral components at two distinct stages is required for the induction of necroptosis following infection with an RNA virus.
RESULTS
Reovirus induces necroptosis.
Upon ultrastructural evaluation of L929 cells infected with prototype reovirus strain type 3 Dearing (T3D) 34 h following infection (a time point conducive for the recovery and processing of dying cells for microscopy), we observed cells with normal nuclear morphology, an absence of apoptotic blebs, swelling of the cellular cytoplasm, and early stages of disruption of the plasma membrane (Fig. 1A). These features are not characteristic of apoptosis and suggested that reovirus may elicit an alternate form of cell death such as necrosis. Cell death can be assessed by measurement of cellular ATP levels or by evaluation of the permeability of cellular nuclei to DNA-staining vital dyes. These treatments do not distinguish between cell death by apoptosis and that by necrosis and therefore need to be coupled with a pharmacologic blockade of molecules specifically involved in cell death pathways leading to apoptosis or necrosis (33). Consistent with the absence of apoptotic features, although the pancaspase inhibitors Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) and Q-VD-OPh (quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methyl ketone) abolish effector caspase activation in L929 cells infected with reovirus, they fail to block cell death (Fig. 1B and C) (30). Instead, cell death following reovirus infection of L929 cells exhibits features of necrosis and is diminished by Nec1, a RIP1 kinase inhibitor (30). The kinase activity of RIP1 can potentiate the activation of RIP3 to promote necroptosis (6). To determine if reovirus-induced cell death occurs via this mechanism, we assessed the capacity of reovirus to elicit necrosis in cells expressing reduced levels of RIP3 (Fig. 1D). We found that in comparison to cells treated with control small interfering RNA (siRNA), treatment of cells with siRNAs against RIP3 significantly decreased cell death (Fig. 1E and F). The effect of RIP3 siRNA on reovirus-induced cell death matched the effect of RIP3 siRNA on necroptosis-inducing treatment with TNF-α and Z-VAD-FMK (Fig. 1G). These data indicate a role for RIP3 in the induction of cell death following reovirus infection. RIP3 can participate in the induction of both apoptosis and necroptosis (7, 34, 35). Because cell death following reovirus infection is unaffected by a diminishment of caspase activity (Fig. 1B and C), these data suggest that reovirus induces RIP3-dependent necroptosis in L929 cells.
FIG 1.
Reovirus-induces necroptosis in L929 cells. (A) L929 cells infected with 10 PFU/cell of T3D for 34 h were fixed, stained, and imaged by using transmission electron microscopy. (B) Cell death in L929 cells 48 h following mock infection or infection with 10 PFU/cell of T3D and treatment with DMSO or Q-VD-OPh (20 μM) was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, uninfected cells was considered to represent 100% viability. (C) Caspase-3/7 activity 48 h following infection of L929 cells with 10 PFU/cell of T3D and treatment with DMSO or Q-VD-OPh was assessed by a chemiluminescent enzymatic assay. The value for caspase activity in mock-infected cells was set to 1. Data are presented as relative caspase-3/7 activity in comparison to the activity in similarly treated, uninfected cells. *, P < 0.05 compared to cells treated with DMSO. (D to G) L929 cells were transfected with nontargeting siRNAs or siRNAs specific for RIP3. (D) Efficiency of knockdown was assessed by immunoblotting for RIP3 and the PSTAIR loading control. (E) Cell death 48 h following mock infection or infection with 10 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, uninfected cells was considered to represent 100% viability. *, P < 0.05 compared to cells transfected with nontargeting siRNAs. (F) Cell death 48 h following infection with 10 PFU/cell of T3D was assessed by AOEB staining. *, P < 0.05 compared to cells transfected with nontargeting siRNAs. EtBr, ethidium bromide. (G) Cell death 3 h following treatment with TNF-α and Z-VAD-FMK was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly siRNA treated, DMSO-treated cells was considered to represent 100% viability. (H) Whole-cell extracts from L929 cells infected with 10 PFU/cell of T3D at the indicated time points were immunoblotted for phosphorylated MLKL, total MLKL, and the PSTAIR loading control.
RIP3-dependent necroptosis requires the activation of the effector protein MLKL (13–23). MLKL is directly phosphorylated by RIP3, and MLKL phosphorylation is considered to be a hallmark of the activation of the necroptosis signaling cascade (13, 36). To determine if reovirus infection leads to the activation of MLKL, we immunoblotted extracts from reovirus-infected cells using a phospho-MLKL antibody (Ab) (Fig. 1H). Our results indicate that MLKL is activated within 24 h following reovirus infection and remains activated until 48 h postinfection, when a significant proportion of cells are undergoing cell death. The detection of this biochemical marker along with data from the genetic and pharmacologic experiments described above indicating that cell death is blocked by a loss of RIP3 function but not of caspase function meet the criteria to demonstrate that reovirus infection of L929 cells results in necroptosis (15).
Reovirus infects cells in a variety of tissues in newborn mice. Previous work on reovirus-induced apoptosis utilized primary neurons or mouse embryo fibroblasts (MEFs) to evaluate cell death pathways in primary cells. Since both neurons and MEFs succumb to reovirus via apoptosis (37–44), we used bone marrow-derived macrophages (BMDMs) to determine whether reovirus can induce necroptosis in primary cells. While it is not known if cells within bone marrow are infected in reovirus-infected animals, the identification of primary cells that undergo necroptosis following reovirus infection would allow us to complement our siRNA studies with work using cells from mice genetically deficient in important regulators of necroptosis. We found that cell death following reovirus infection of BMDMs occurred in the absence of caspase activity (Z-VAD-FMK-treated cells) or RIP1 kinase activity (Nec1-treated cells) but was diminished when the activities of caspases and RIP1 kinase were simultaneously blocked (Fig. 2A). Consistent with this, cell death was not blocked by the genetic absence of RIP3 but was reduced by the blockade of caspases in the absence of RIP3 (Fig. 2B and C). Cells lacking both caspase-8 and RIP3 were also resistant to death following reovirus infection (Fig. 2D). These data indicate that reovirus can induce necroptosis in BMDMs when apoptosis is blocked. These findings match previous work in other systems where necroptosis is evident when caspases have been rendered nonfunctional (45–47).
FIG 2.
Reovirus can induce necroptosis in primary BMDMs. (A) BMDMs from wild-type mice were mock infected or infected with 50 PFU/cell of T3D in the presence of DMSO, Z-VAD-FMK (25 μM), Nec1 (50 μM), or both inhibitors. Cell death 48 h following infection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, uninfected cells was considered to represent 100% viability. *, P < 0.05 compared to DMSO-treated cells. (B) BMDMs from wild-type (left) or RIP3−/− (right) mice were mock infected or infected with 50 PFU/cell of T3D in the presence of DMSO or Z-VAD-FMK (25 μM). Cell death 48 h following infection was assessed by using the Cell Titer Glo system. Luminescence measurement in uninfected cells of the same genotype that were similarly treated was considered to represent 100% viability. *, P < 0.05 compared to DMSO-treated cells of the same genotype. (C) BMDMs were infected with 50 PFU/cell of T3D in the presence of DMSO or Z-VAD-FMK (25 μM). Cell viability was assessed by Sytox green staining. *, P < 0.05 compared to DMSO-treated cells of the same genotype. (D) BMDMs from wild-type, RIP3−/−, or Casp8−/− × RIP3−/− mice were infected with 50 PFU/cell of T3D. Cell death 48 h following infection was assessed by using the Cell Titer Glo system. Luminescence measurement in mock-infected cells of the same genotype was considered to represent 100% viability. *, P < 0.05 compared to wild-type cells. (E) BMDMs from wild-type or RIP3−/− mice were infected with 50 PFU/cell of T3D in the presence or absence of Z-VAD-FMK (25 μM). The virus yield 24 h following infection was measured by using a plaque assay. WT, wild type.
In the context of infection by other viruses, necroptosis is antiviral (2, 4–7). To determine if necroptosis affects the replication of reovirus, we measured virus yields over 24 h of infection in wild-type and RIP3-deficient BMDMs in the presence and absence of Z-VAD-FMK. Virus yields in wild-type cells treated with dimethyl sulfoxide (DMSO) or Z-VAD-FMK were ∼1 log10 unit (Fig. 2E). The genetic absence of RIP3 enhanced the virus yield to ∼1.7 log10 units. Importantly, the virus yield did not change in RIP3-deficient BMDMs under conditions where apoptosis was blocked by using Z-VAD-FMK. While the basis for the slight increase in virus yields in the absence of RIP3 is unclear and was not further investigated, our data suggest that the capacity of cells to undergo necroptosis does not influence virus yields in cell culture. These data are reminiscent of previous evidence indicating that a blockade of apoptosis does not influence reovirus replication in cell culture (37, 38). The absence of an effect of cell death on reovirus replication in cell culture may be due to differences in the timing of the reovirus replication cycle and the induction of cell death. Whereas reovirus completes its replication cycle in 18 h, cell death following infection is not detected until 36 to 48 h following infection.
Transfection of reovirus RNA can elicit necroptosis.
Reovirus strains that exhibit a higher level of gene expression are more potent inducers of necrosis (48). A blockade of reovirus positive-strand RNA synthesis using ribavirin blocks necrosis, suggesting a possible role for viral RNA in the induction of necrosis (48). Transfection of the dsRNA mimic poly(I·C) in L929 cells treated with either type I or type II IFNs results in cell death by necrosis (25, 26, 49). Because our data suggested a role for reovirus RNA in the induction of necroptosis in infected cells, we sought to determine if viral RNA was sufficient for the induction of necroptosis. For these experiments, we purified total RNA from mock- or reovirus-infected cells 24 h following infection. We found that in comparison to RNA from mock-infected cells, RNA extracted from T3D-infected cells induced a significantly larger amount of cell death following transfection into cells (Fig. 3A and B). Cell death by transfected RNA was diminished by treatment with Nec1 but not Q-VD-OPh (Fig. 3C), analogous to what we reported previously for L929 cells infected with reovirus (30). These data are also consistent with previous work indicating that poly(I·C)-induced cell death is blocked by Nec1 (26). Our results presented above suggest that RNA isolated from reovirus-infected cells elicits necroptosis following introduction into L929 cells. Interestingly, unlike previous work with transfection of dsRNA into cells (25, 49), cell death following transfection of RNA extracted from reovirus-infected cells did not require priming of the cells with exogenous IFN.
FIG 3.
Reovirus RNA is sufficient for the induction of necroptosis. (A and B) L929 cells were transfected with 100 ng of RNA extracted from mock-infected or reovirus-infected cells. (A) Cell death 24 h following transfection was assessed by using the Cell Titer Glo system. Luminescence measurement in untransfected cells was considered to represent 100% viability. *, P < 0.05 compared to cells transfected with RNA extracted from mock-infected cells. (B) Cell death 24 h following transfection of RNA was assessed by AOEB staining. *, P < 0.05 compared to cells transfected with RNA extracted from mock-infected cells. (C) L929 cells were transfected with 100 ng of RNA extracted from mock-infected or reovirus-infected cells in the presence of DMSO, Q-VD-OPh (25 μM), or Nec1 (50 μM). Cell death 24 h following transfection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated cells transfected with RNA from mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to cells transfected with DMSO-treated cells transfected with the same type of RNA. (D) L929 cells were transfected with 100 ng of RNA extracted from mock-infected or reovirus-infected cells. Levels of IFN-β mRNA were assessed by RT-qPCR 18 h following transfection. The IFN-β/GAPDH ratio for cells transfected with RNA from mock-infected cells was considered to have a value of 1. *, P < 0.05 compared to cells transfected with RNA from mock-infected cells. (E) L929 cells were transfected with 100 ng of RNA extracted from mock-infected or reovirus-infected cells in the presence and absence of 0.1 μg/ml anti-IFNAR Ab. Cell death 24 h following transfection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated cells transfected with RNA from mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to cells transfected with same type of RNA without anti-IFNAR Ab. (F) L929 cells were transfected with 100 ng of untreated or CIP-treated RNA extracted from reovirus-infected cells. Levels of IFN-β mRNA were assessed by RT-qPCR 18 h following transfection. The IFN-β/GAPDH ratio for cells transfected with untreated RNA from reovirus-infected cells was considered to have a value of 1. *, P < 0.05 compared to cells transfected with untreated RNA from reovirus-infected cells. (G) Cells treated with 0 or 100 U/ml IFN-β were transfected with 100 ng of untreated RNA from mock-infected cells or untreated or with CIP-treated RNA from T3D-infected cells. Cell death 24 h following transfection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated cells transfected with RNA from mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to similarly treated cells transfected with untreated RNA from T3D-infected cells; **, P < 0.05 compared to cells transfected with similarly treated RNA in the presence of 0 U/ml of IFN-β. (H) Cells treated with 0 or 100 U/ml IFN-β were transfected with 100 ng of untreated or CIP-treated RNA from T3D-infected cells. Cell death 24 h following transfection of RNA was assessed by AOEB staining. *, P < 0.05 compared to cells transfected with similarly treated RNA in the presence of 0 U/ml of IFN-β. (I) Cells pretreated with 0 or 20 mM AC were transfected with RNA from T3D-infected cells. Levels of IFN-β mRNA were assessed by RT-qPCR 18 h following transfection. The IFN-β/GAPDH ratio for 0 mM AC-treated cells transfected with RNA from T3D-infected cells was considered to have a value of 1. (J) Cells pretreated with 0 or 20 mM AC were transfected with 100 ng RNA from mock-infected or T3D-infected cells. Cell death 24 h following transfection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated cells transfected with RNA from mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to transfection of cells treated with 0 mM AC.
We reasoned that necroptosis was induced without the addition of exogenous IFN because transfection of reovirus RNA obtained from infected cells can induce the expression of IFN-β (50–52) (Fig. 3D). Indeed, treatment with an IFN-α/β receptor (IFNAR)-blocking antibody, MAR1-5A3 (53), resulted in a reduction in cell death (Fig. 3E). IFN-β production following transfection of reovirus RNA occurs via the retinoic acid inducible gene I (RIG-I)-mediated detection of the RNA (50). Consistent with this, the removal of the 5′ phosphates using calf intestinal alkaline phosphatase (CIP) resulted in a reduction in the expression of IFN-β (Fig. 3F) and the induction of cell death (Fig. 3G and H). Interestingly, if cells were primed with exogenous IFN-β before transfection, the capacity of CIP-treated RNA to elicit necroptosis was restored (Fig. 3G and H). These data suggest that although RIG-I-mediated detection of viral RNA is required for IFN-β production, it is not sufficient for the induction of cell death. Thus, cell death induction occurs by the sensing of viral RNA via an alternate pathway.
Based on the evidence that poly(I·C) elicits necroptosis by TLR3 detection and signaling to RIP3 via TRIF (25, 26), we examined whether reovirus RNA-induced necroptosis could be blocked by the treatment of cells with ammonium chloride (AC), an agent that blocks the TLR3-mediated detection of dsRNA (54). We found that although AC did not negatively impact IFN-β expression following RNA transfection (Fig. 3I), it blocked cell death induction by transfected RNA (Fig. 3J). Consistent with previous work, these data indicate that the detection of reovirus RNA via RIG-I results in IFN-β expression. In addition, these data suggest that IFN-β primes reovirus RNA-transfected cells to undergo TLR3-dependent necroptosis. Thus, the IFN- and TLR3-dependent pathway for the induction of necroptosis following transfection of reovirus RNA into L929 cells is similar to that described previously for the transfection of synthetic dsRNA (25, 49).
Sensing of reovirus RNA during infection is required for necroptosis.
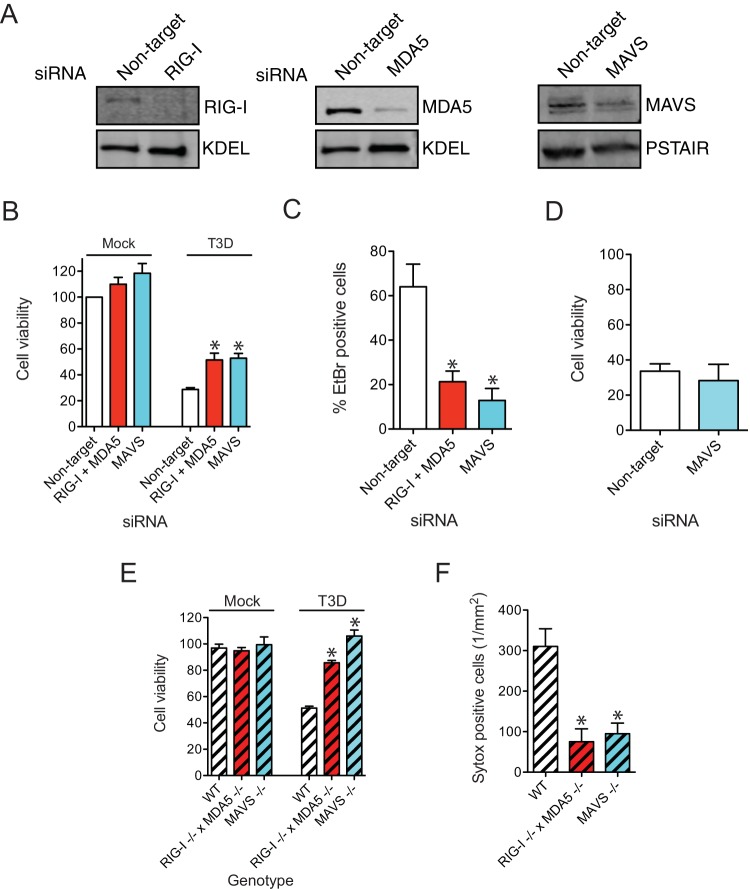
We next sought to determine if the detection of viral RNA in infected cells contributes to cell death induction in reovirus-infected cells. During infection, reovirus RNA can be detected by both the RIG-I-like receptors (RLRs) RIG-I and myeloma differentiation antigen 5 (MDA5) (55). The simultaneous reduction of the levels of both RLRs, or their common downstream signaling adaptor, mitochondrial antiviral signaling protein (MAVS), led to a significant reduction in cell death following reovirus infection (Fig. 4A to C). The susceptibility of cells to TNF-α- and Z-VAD-FMK-induced necroptosis did not change following MAVS knockdown, indicating that MAVS is not required for the function of the core necroptosis machinery (Fig. 4D). BMDMs deficient in either both RLRs or MAVS were also protected from reovirus-induced necroptosis (Fig. 4E and F). These data suggest that necroptosis following reovirus infection requires detection and signaling by RLRs.
FIG 4.
Detection of viral RNA by cytoplasmic sensors is required for necroptosis. (A) L929 cells were transfected with nontargeting siRNAs or siRNAs specific for RIG-I, MDA5, or MAVS. The efficiency of knockdown was assessed by immunoblotting for RIG-I, MDA5, MAVS, and the KDEL or PSTAIR loading control. (B to D) L929 cells were transfected with nontargeting siRNAs or siRNAs specific for both RIG-I and MDA5 or MAVS. (B) Cell death 48 h following mock infection or infection with 10 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in uninfected cells transfected with the same siRNA was considered to represent 100% viability. *, P < 0.05 compared to cells transfected with nontargeting siRNAs. (C) Cell death 48 h following infection with 10 PFU/cell of T3D was assessed by AOEB staining. *, P < 0.05 compared to cells transfected with nontargeting siRNAs. (D) Cell death 3 h following treatment with TNF-α and Z-VAD-FMK was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly siRNA-treated, DMSO-treated cells was considered to represent 100% viability. (E) Cell death in wild-type, RIG-I−/− × MDA5−/−, or MAVS−/− BMDMs treated with Z-VAD-FMK following mock infection or infection with 50 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in mock-infected cells of the same genotype was considered to represent 100% viability. *, P < 0.05 compared to wild-type cells. (F) Cell death in wild-type, RIG-I−/− × MDA5−/−, or MAVS−/− BMDMs treated with Z-VAD-FMK (25 μM) following infection with 50 PFU/cell of T3D was assessed by Sytox green staining. *, P < 0.05 compared to wild-type cells.
IFN signaling is required for necroptosis.
To determine whether type I IFNs produced by RLR-MAVS signaling are required for reovirus-induced necroptosis, we quantified the capacity of reovirus to induce necroptosis in the presence of an IFNAR-blocking antibody (53). We found that this antibody diminished the expression of a representative interferon-stimulated gene (ISG), ZBP1, which is potently induced following reovirus infection (56), and diminished the capacity of reovirus to induce necroptosis (Fig. 5A to C). This reduction in necroptosis was not due to a deleterious effect of the antibody on the capacity of reovirus to establish infection (Fig. 5D). Blocking IFNAR signaling did not alter the capacity of cotreatment with TNF-α and Z-VAD-FMK to induce necroptosis, suggesting that the IFNAR-blocking Ab did not affect the function of the core necroptosis machinery (Fig. 5E). IFNAR-deficient BMDMs treated with Z-VAD-FMK were also resistant to reovirus-induced necroptosis (Fig. 5F and G), indicating a role for IFN signaling in the induction of necroptosis following reovirus infection.
FIG 5.
Signaling via IFNAR is required for necroptosis (A to D) L929 cells were infected with 10 PFU/cell of T3D in the presence of 0.1 μg/ml of anti-IFNAR Ab. (A) Levels of ZBP1 mRNA at 24 h postinfection were assessed by using RT-qPCR. The ZBP1/GAPDH ratio at 0 h postinfection was set to a value of 1. *, P < 0.05 compared to cells infected without IFNAR antibody. (B) Cell death 48 h following mock infection or infection with 10 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to cells infected without IFNAR antibody. (C) Cell death 48 h following infection with 10 PFU/cell of T3D was assessed by AOEB staining. *, P < 0.05 compared to cells infected without IFNAR antibody. (D) Viral infectivity 18 h following infection with 2 PFU/cell T3D was assessed by indirect immunofluorescence. (E) Cell death 3 h following treatment with TNF-α and Z-VAD-FMK was assessed by using the Cell Titer Glo system. Luminescence measurement in cells treated without IFNAR antibody was considered to represent 100% viability. (F) Cell death in wild-type and IFNAR-deficient BMDMs treated with Z-VAD-FMK following mock infection or infection with 50 PFU/cell of T3D. Cell viability was assessed by using the Cell Titer Glo system. Luminescence measurement in mock-infected cells of the same genotype was considered to represent 100% viability. *, P < 0.05 compared to wild-type cells. (G) Cell death in wild-type and IFNAR-deficient BMDMs treated with Z-VAD-FMK following infection with 50 PFU/cell of T3D was assessed by Sytox green staining. *, P < 0.05 compared to wild-type cells. (H) L929 cells were mock infected or infected with 100 PFU/cell of T3D ISVPs in the presence or absence of 20 mM AC. Cell death 48 h following infection was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, mock-infected cells was considered to represent 100% viability. *, P < 0.05 compared to cells infected without AC. (H and I) L929 cells were transfected with nontargeting siRNAs or siRNAs specific for TRIF. (H) Efficiency of knockdown was assessed by immunoblotting for TRIF and the PSTAIR loading control. (I) Cell death 48 h following mock infection or infection with 10 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in uninfected cells transfected with the same siRNA was considered to represent 100% viability.
Based on the role for TLR3 in necroptosis following the transfection of RNA obtained from reovirus-infected cells (Fig. 3), we next sought to evaluate whether TLR3 is also required for cell death in reovirus-infected cells. Toward this goal, we tested the effect of AC on cell death induction in reovirus-infected cells. Because treatment of cells with AC prevents reovirus infection by blocking capsid disassembly, we initiated infection of AC-treated cells with infectious subvirion particles (ISVPs), a virus entry intermediate that bypasses the inhibitory effect of AC (57). We found that necroptosis following infection by ISVPs was unaffected by treatment with AC (Fig. 5H). A parallel transfection of cells with reovirus RNA in control and AC-treated cells yielded results that matched those shown in Fig. 3I (data not shown), indicating that AC treatment was effective. The siRNA-mediated reduction in the expression of TRIF, the TLR3 adaptor, also did not block cell death following reovirus infection (Fig. 5I and J). These data indicate that although RLR-mediated IFN-β expression and signaling are required for necroptosis following both RNA transfection and viral infection, TLR3-mediated signaling is required only for cell death after viral RNA transfection.
Two-stage detection of reovirus infection is required for necroptosis.
We next sought to determine the stage of infection required for the induction of necroptosis. Blockade of viral plus-strand RNA synthesis by using ribavirin diminishes reovirus-induced necrosis (48). The reovirus positive-sense RNA can direct protein synthesis or can be packaged into progeny core particles and serve as the template for minus-strand RNA synthesis to generate viral genomic dsRNA (58). Progeny cores containing genomic dsRNA undergo secondary transcription to produce additional viral mRNAs. Thus, the diminishment of necroptosis by ribavirin treatment may be due to a blockade of any of these steps in reovirus replication. To further define the stage of infection required for necroptosis, we used guanidine hydrochloride (GuHCl). GuHCl does not affect reovirus plus-strand RNA synthesis but prevents the generation of genomic dsRNA within infected cells (Fig. 6A and C) (59). Under the conditions used, perhaps because sufficient translation occurs from primary transcripts, we did not observe a diminishment in viral protein synthesis in the presence of GuHCl (Fig. 6B). Treatment of reovirus-infected cells with GuHCl led to a diminishment in necroptosis (Fig. 6D and E). Because GuHCl does not affect necroptosis induced by treatment with TNF-α and Z-VAD-FMK (Fig. 6F), our results point to the importance of the synthesis of viral genomic dsRNA for the induction of necroptosis following reovirus infection.
FIG 6.
Synthesis of genomic dsRNA is required for necroptosis. L929 cells were infected with 10 PFU/cell of T3D in the presence of ribavirin (200 μM) or GuHCl (15 mM). (A) Levels of reovirus plus-strand RNA corresponding to the viral S1 gene segment were measured by RT-qPCR at 24 h postinfection. The ratio of the reovirus T3D S1 plus strand to GAPDH in untreated cells infected for 0 h was considered to have a value of 1.*, P < 0.05 compared to cells infected with T3D in the absence of the inhibitor. (B) Levels of the reovirus μ1C protein and the PSTAIR loading control 24 h following infection with 10 PFU/cell of T3D were assessed by immunoblotting. (C) Generation of reovirus genomic dsRNA 24 h following infection was evaluated by electropherotyping. Positions of the reovirus large (L), medium (M), and small (S) gene segments are indicated. (D) Cell death 48 h following mock infection or infection with 10 PFU/cell of T3D was assessed by using the Cell Titer Glo system. Luminescence measurement in similarly treated, uninfected cells was considered to represent 100% viability. *, P < 0.05 compared to untreated cells. (E) Cell death 48 h following infection with 10 PFU/cell of T3D was assessed by AOEB staining. *, P < 0.05 compared to untreated cells. (F) Cell death 4 h following treatment with TNF-α and Z-VAD-FMK treatment was assessed by using the Cell Titer Glo system. Cell viability in similarly treated cells in the absence of TNF-α and Z-VAD-FMK was considered 100%.
The time during infection when reovirus RNA is detected to produce IFN-β is not known. The type of reovirus RNA that activates the expression of IFN-β in the context of infection also remains undefined. Ribavirin and GuHCl may thus indirectly prevent cell death because they affect the synthesis of RNA required for IFN-β synthesis. To better understand the effect of ribavirin and GuHCl on reovirus-induced cell death, we measured the expression level of IFN-β mRNA at different times following infection of L929 cells with reovirus. We observed a ∼10-fold increase in IFN-β mRNA levels 12 h following infection (Fig. 7A). No further increase in IFN-β mRNA levels was observed 18 or 24 h following infection. We found that although IFN-β mRNA expression was diminished by the blockade of virus disassembly using AC, it was not decreased by either ribavirin or GuHCl treatment (Fig. 7B). Although UV-inactivated virus failed to produce detectable levels of reovirus S1 plus-strand RNA (>3-log10-fold reduction), it remained capable of eliciting the same level of IFN-β mRNA expression as that of the control infectious virus (Fig. 7C and D). These data suggest that genomic RNA present within incoming viral particles is sufficient for the induction of IFN-β expression. These results are consistent with previously reported data showing IFN induction by a UV-inactivated reovirus mutant and IRF3 activation following reovirus infection in the absence of RNA synthesis and recent studies on IFN expression following avian reovirus infection (39, 60, 61). We observed that the infection-induced increase in IFN-β expression levels was diminished in cells transfected with MAVS siRNA (Fig. 7E). Importantly, MAVS was also required for the efficient induction of IFN-β expression in reovirus-infected cells when viral plus-strand RNA synthesis was blocked by using ribavirin (Fig. 7E). These data suggest that genomic RNA within incoming virus particles is detected by cytoplasmically localized RLRs and signals via MAVS to produce IFN-β. Because necroptosis is blocked by GuHCl under conditions where IFN-β is produced but viral dsRNA synthesis is diminished (Fig. 6 and 7), these studies indicate that IFN-β signaling is required but not sufficient for the induction of cell death. Together, our data indicate a role for reovirus RNA at two different stages of infection in inducing necroptosis. First, viral genomic dsRNA is detected during entry to activate type I IFN signaling. Second, the generation of newly synthesized viral dsRNA is required for the induction of necroptosis.
FIG 7.

De novo synthesis of viral RNA is not required for IFN expression. (A) L929 cells were infected with 10 PFU/cell of T3D. Levels of IFN-β mRNA were assessed at the indicated time intervals by using RT-qPCR. The IFN-β/GAPDH ratio at 0 h postinfection was set to a value of 1. *, P < 0.05 compared to cells infected for 0 h. (B) L929 cells treated with AC (20 mM), ribavirin (200 μM), or GuHCl (15 mM) were infected with 10 PFU/cell of T3D. Levels of IFN-β mRNA were assessed by RT-qPCR at 24 h postinfection. The IFN-β/GAPDH ratio for untreated, T3D-infected cells was set to a value of 1. *, P < 0.05 compared to control treated, infected cells. (C and D) L929 cells were infected with 10 PFU/cell of T3D and equivalent numbers of particles of UV-treated T3D. (C) Levels of reovirus plus-strand RNA corresponding to the viral S1 gene segment were measured by RT-qPCR at 24 h postinfection. The ratio of the reovirus T3D S1 plus strand to GAPDH in cells infected for 0 h was considered to have a value of 1. UD, undetectable (value below that detected at 0 h). (D) Levels of the corresponding reovirus IFN-β RNA were measured by RT-qPCR at 24 h postinfection. The IFN-β/GAPDH ratio in cells infected with infectious T3D was considered to have a value of 1. (E) L929 cells were transfected with nontargeting siRNAs or siRNAs specific for MAVS. Levels of IFN-β mRNA in cells infected with 10 PFU/cell of T3D in the presence or absence of ribavirin (200 μM) were assessed by RT-qPCR. The IFN-β/GAPDH ratio for untreated, T3D-infected, nontargeting siRNA-treated cells was set to a value of 1. *, P < 0.05 compared to untreated, T3D-infected, nontargeting siRNA-treated cells.
DISCUSSION
In this work, we demonstrate that reovirus infection of both cultured cells and primary murine macrophages evokes necroptosis. Our results point to a role for viral components at two stages of infection in evoking necroptosis (Fig. 8). First, the detection of the incoming viral genomic RNA by host cell cytoplasmic sensors to produce IFN-β is required for necroptosis (Fig. 4 and 5). In addition, the synthesis of new viral genomic dsRNA is also required for the induction of necroptosis (Fig. 6). This work indicates that the type I IFN signaling pathway functions in the induction of necroptosis following infection by an RNA virus. These data provide evidence for a previously unknown signaling cascade by which infection with an RNA virus culminates in necroptosis.
FIG 8.
Model for reovirus-induced necroptosis. Genomic RNA from incoming viral particles is sensed by RLRs to produce type I IFN in a MAVS-dependent manner. De novo-synthesized viral genomic dsRNA or viral secondary transcripts produced from newly synthesized genomic dsRNA (GuHCl-sensitive replication events) are detected by an as-yet-unidentified ISG to elicit RIP3-dependent necrotic cell death.
IFN signaling was previously implicated in the induction of necroptosis. In Salmonella enterica serovar Typhimurium-infected mice, murine macrophages undergo necroptosis (62). In this context, the IFNAR is internalized and complexes with RIP1 and RIP3 to elicit necroptosis (62). ISGF3, a protein complex that drives the expression of ISGs following IFN signaling, is required for the sustained activation of RIP3 following the ligation of TNF receptor (TNFR) or TLRs (63). However, whether a particular ISG modulates the basal activity of RIP3 has not been defined. Multiple ISGs are implicated in the induction of necroptosis. These ISGs include ZBP1/DAI, which may sense either viral DNA, viral RNA, or viral proteins, and those that recognize viral dsRNA (TLR3 and protein kinase R [PKR]) (5, 25, 27, 28, 64). Based on the role of DAI in the induction of necroptosis following IAV infection (27, 28), we tested the contribution of DAI to reovirus-induced cell death. We found that reovirus remained capable of inducing cell death in ZBP1-deficient BMDMs (data not shown). Our results suggest that TLR3 does not participate in necroptosis induction following reovirus infection (Fig. 5). PKR can promote necroptosis in cells lacking functional Fas-associated death domain-containing protein (FADD) (64). Reovirus induces necroptosis in wild-type cells expressing FADD (Fig. 1 and 2). Moreover, because reovirus encodes a well-described PKR inhibitor, we think that it is unlikely that PKR is involved in this process (65). Thus, the identity of the ISGs that control necroptosis following reovirus infection remains to be determined. Because IAV-induced necroptosis is unaffected by the genetic absence of MAVS or IFNAR (7) and requires ZBP1 (27, 28), whereas reovirus-induced necroptosis requires MAVS and IFNAR (Fig. 4 and 5) but is not affected by the absence of ZBP1, the mechanism underlying necroptosis following reovirus infection appears distinct from that reported for IAV.
Investigations into reovirus-induced cell death indicate that reovirus infection can initiate cell death signaling from distinct stages of replication and elicit cell death via a variety of pathways. The precise pathway that executes cell death likely varies by cell type. One model suggests that events initiated during cell entry that occur after virus disassembly but prior to the de novo synthesis of viral RNA and proteins can elicit cell death by apoptosis (66). Apoptosis by this mechanism is thought to occur independently of the presence of viral genomic RNA but relies on the function of the μ1 capsid protein and the host transcription factor NF-κB (37, 67). Another set of studies implicates a role for viral genomic RNA, viral RNA sensors, and IRF3 in the induction of apoptosis. However, cell death by this pathway does not appear to require viral replication or type I IFN signaling (39, 68). Two BH3-only members of the Bcl-2 family, Bid and Noxa, appear to be involved in the induction of apoptosis, and their function is downstream of the transcription factors NF-κB and IRF3 (38, 68). Our studies presented here highlight an additional way in which reovirus infection leads to cell death. First, distinct from previous work on reovirus-induced apoptosis, we show that IFN signaling is required for necroptosis. Although we have not directly tested its requirement, IRF3, which is required for IFN-β expression (69), likely also plays a role in necroptosis. Thus, the requirement for IRF3 in reovirus-induced apoptosis and necroptosis is likely shared. Unlike for apoptosis, we demonstrate that the generation of viral genomic dsRNA late in infection is required for necroptosis (Fig. 5). The requirement for genomic dsRNA synthesis may be direct, similar to the detection of viral RNA during transfection (Fig. 3). Alternatively, the synthesis of genomic dsRNA may be required to produce secondary transcripts, which in turn are detected by host cells to induce necroptosis. Secondary transcripts generated following reovirus infection are qualitatively different than primary transcripts, and therefore, it is possible that secondary transcripts are detected in a manner distinct from that of primary transcripts (70). Although our studies indicate that protein synthesis in the absence of ongoing dsRNA synthesis is not sufficient for necroptosis induction (Fig. 6), it remains possible that viral proteins modulate necroptosis following reovirus infection.
Studies thus far have indicated a pathogenic role for apoptosis in reovirus-induced encephalitis and myocarditis (71). Cell death pathways in reovirus-infected animals are thought to be tissue specific, but precisely how these cell death pathways differ in a tissue-specific manner has not been defined (72, 73). It is possible that in some cases, cell death via IFN-dependent pathways that we have described in this study contributes to tissue injury. Due to its natural preference for infecting and killing transformed cells and its innocuousness to human adults, reovirus is currently in phase III clinical trials as a cancer therapeutic (74). The capacity of reovirus to elicit cell death via multiple mechanisms may therefore underlie its efficacy as an effective therapeutic.
MATERIALS AND METHODS
Cells and viruses.
Spinner-adapted L929 cells (obtained from the laboratory of T. Dermody) were maintained in Joklik's minimal essential medium (MEM) (Lonza) supplemented with 5% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml streptomycin, and 25 ng/ml of amphotericin B. Spinner-adapted L929 cells were used for cultivating and purifying viruses and for plaque assays. Prototype reovirus strain T3D was regenerated by plasmid-based reverse genetics (75, 76). Viral particles were purified by Vertrel XF extraction and CsCl gradient centrifugation (77). The viral titer was determined by a plaque assay using spinner-adapted L929 cells (78). UV-inactivated virus was generated by using a UV cross-linker (CL-1000 UV cross-linker; UVP). Virus diluted in phosphate-buffered saline (PBS) was placed into a 60-mm tissue culture dish and irradiated with short-wave (254-nm) UV on ice at a distance of 10 cm for 1 min at 120,000 μJ/cm2. Murine L929 cells (ATCC CCL-1) were maintained in Eagle's MEM (Lonza) supplemented with 10% FBS and 2 mM l-glutamine. L929 cells were used for all experiments to assess cell death, viral RNA and protein synthesis, and cell signaling. Distinct from some L929 cell lines, the L929 cells used for this study do not undergo TNF-α- or Z-VAD-mediated cell death (79, 80). Wild-type and mutant bone marrow-derived macrophages were obtained from Edward Mocarski and Mehul Suthar (Emory University) and were maintained in Dulbecco's modified Eagle's medium (DMEM) with 20% FBS, 10% filtered conditioned medium from L929 cells, 2 mM l-glutamine, 100 U/ml of penicillin, and 100 μg/ml streptomycin.
Reagents.
Z-VAD-FMK and Q-VD-OPh were purchased from Enzo Life Sciences or R&D Systems, and necrostatin-1 was purchased from Calbiochem. Ammonium chloride (AC), GuHCl, poly(I·C), and human TNF-α were purchased from Sigma-Aldrich. siRNAs were purchased from Dharmacon as SMARTpools of On-Target Plus siRNA. A nontargeting siRNA control pool or siRNA targeting β-galactosidase was used as a control. Antisera raised against reovirus were obtained from T. Dermody. Monoclonal antibody against IFNAR and rabbit antisera against RIP3 were purchased from Santa Cruz Biotechnology; those against TRIF, phospho-MLKL, and total MLKL were purchased from Abcam; and those against RIG-I and MDA5 were purchased from Cell Signaling. Mouse antiserum specific for PSTAIR was purchased from Sigma, and mouse antiserum specific for KDEL was purchased from Enzo Life Sciences. Alexa Fluor-conjugated anti-mouse IgG, anti-rabbit IgG, and anti-goat IgG secondary antibodies were purchased from Invitrogen. IRDye-conjugated anti-guinea pig IgG was purchased from Li-Cor.
Fixing, embedding, and sectioning of infected cells.
L929 cells grown on 100-mm dishes were either mock infected or infected with 10 PFU/cell of T3D for 1 h at room temperature. Following incubation for virus attachment, cells were washed twice with PBS and then overlaid with fresh medium. At 34 h postinfection, uninfected and infected cells were washed with PBS, trypsinized, pelleted for 5 min at 800 × g, and washed again with PBS. The pelleted cells were then fixed with 2.5% glutaraldehyde diluted in sodium cacodylate buffer (100 mM sodium cacodylate [pH 7.5], 2 mM MgCl2, 2 mM CaCl2, 0.5% NaCl) for 60 min at room temperature. Following fixation, the cells were washed twice with sodium cacodylate buffer. The washed cells were postfixed with 1% osmium tetroxide diluted in sodium cacodylate buffer for 60 min at room temperature. The fixed cells were washed twice with sodium cacodylate buffer, followed by one wash with 100 mM sodium acetate (pH 4.2). The cells were then stained with 0.5% uranyl acetate diluted in 100 mM sodium acetate (pH 4.2) for 60 min at room temperature. After staining, the cells were washed twice with 100 mM sodium acetate (pH 4.2). Prior to embedding, fixed and stained cells were dehydrated with sequential concentrations of ethanol (EtOH): 35% EtOH once for 5 min, 50% EtOH once for 5 min, 70% EtOH once for 5 min, 90% EtOH once for 5 min, 95% EtOH once for 5 min, and 100% EtOH four times for 5 min each. The dehydrated cells were incubated in a solution composed of 50% EMbed 812 resin and 50% EtOH for 2 h at room temperature. The cells were then incubated in 100% EMbed 812 resin overnight at room temperature. The next day, the resin was replaced with fresh EMbed 812 resin, which was allowed to harden for 18 h at 65°C. Thin sections (85 nm thick) were collected by using a diamond knife on a Leica Biosystems microtome.
Transmission electron microscopy.
Thin sections of uninfected and infected cells were applied to 300-mesh copper grids and stained with Reynolds' lead citrate and 2% uranyl acetate (81). The stained grids were analyzed by using a JEOL 1010 transmission electron microscope operating at 80 kV. Images were recorded by using a Gatan MegaScan 794 charge-coupled-device camera. Micrographs were processed and analyzed by using ImageJ software.
Infections and preparation of extracts.
Cells were adsorbed with either PBS or T3D at room temperature for 1 h, followed by incubation with medium at 37°C for the indicated time intervals. Ribavirin, GuHCl, Z-VAD-FMK, Q-VD-OPh, necrostatin-1, or anti-IFNAR Ab was added to the medium immediately after the 1-h adsorption period. For the preparation of whole-cell lysates, cells were washed in PBS and lysed with 1× radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.5], 50 mM NaCl, 1% Triton X-100 [TX-100], 1% deoxycholate [DOC], 0.1% SDS, and 1 mM EDTA) containing a protease inhibitor cocktail (Roche), 500 μM dithiothreitol (DTT), and 500 μM phenylmethylsulfonyl fluoride (PMSF), followed by centrifugation at 15,000 × g for 10 min to remove debris. For the detection of phosphorylated MLKL, cells were lysed in 1× RIPA buffer supplemented with 10 mM NaF.
RNA transfection and cell death.
L929 cells were mock infected or infected with 10 PFU/cell of T3D for 24 h. Total RNA was extracted by using Tri reagent (Molecular Research Center). When needed, the RNA was mock treated or treated with CIP for 1 h at 37°C and repurified by using Tri reagent. One hundred nanograms of RNA was introduced into cells by Lipofectamine 2000 transfection. Cell death was measured 21 to 24 h following transfection.
Immunoblot assay.
Cell lysates were resolved by electrophoresis in polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked for at least 1 h in blocking buffer (PBS containing 5% milk or 2.5% bovine serum albumin [BSA]) and incubated with antisera against MLKL (1:2,000), phospho-MLKL (1:750), RIP3 (1:1,000), MAVS (1:1,000), RIG-I (1:1,000), MDA5 (1:1,000), TRIF (1:1,000), or PSTAIR (1:1,0000) at 4°C overnight. Membranes were washed three times for 5 min each with washing buffer (Tris-buffered saline [TBS] containing 0.1% Tween 20) and incubated with a 1:20,000 dilution of Alexa Fluor-conjugated goat anti-rabbit IgG (for RIP3, RIG-I, and MDA5), donkey anti-goat IgG (for RIP3), goat anti-mouse IgG (for KDEL and PSTAIR), or IRDye-conjugated anti-guinea pig IgG (for σNS) in blocking buffer. Following three washes, membranes were scanned by using an Odyssey infrared imager (Li-Cor).
Knockdown of host proteins by siRNA.
In 96-well plates, 0.25 μl Lipofectamine 2000 was used to transfect 15 pmol of siRNA. Cells (1 × 104) were seeded on top of the siRNA-Lipofectamine mixture. In 24-well plates, 0.75 μl Lipofectamine 2000 was used to transfect 45 pmol of siRNA. Cells (5 × 104) were seeded on top of the siRNA-Lipofectamine mixture. Virus infection was performed 48 h following siRNA transfection.
Assessment of cell death by measurement of cellular ATP levels.
Cells (1 × 104) grown in black, clear-bottom, 96-well plates were mock infected with PBS or adsorbed with 10 PFU/cell of T3D at room temperature for 1 h. Following the incubation of cells at 37°C for 42 h, ATP levels were assessed by using the Cell Titer Glo assay system (Promega).
Assessment of cell death by acridine orange-ethidium bromide staining.
Cells grown in 24-well plates or 96-well plates were adsorbed with the indicated amounts of the virus. Inhibitors were added immediately following adsorption. The percentage of dead cells after 48 h of incubation was determined by using acridine orange-ethidium bromide (AOEB) staining as described previously (82). For identifying host regulators of cell death, cells were transfected with siRNA as described above and incubated for 48 h prior to infection with T3D. For each experiment, >250 cells were counted by researchers in a blind manner, and the percentage of isolated cells exhibiting orange staining (ethidium bromide [EtBr] positivity) was determined by epi-illumination fluorescence microscopy using a fluorescein filter set on an Olympus IX71 microscope. Fewer than 5% of uninfected cells were EB positive following treatment with each inhibitor or siRNA.
Assessment of cell death by IncuCyte automated cell imaging.
Cells grown in 48-well plates were mock infected with PBS or adsorbed with the indicated amounts of virus. Inhibitors were added immediately following adsorption, in addition to 500 nM Sytox green. The cells were imaged over a time course of 48 h. Numbers of Sytox-positive cells per square millimeter 48 h following infection are shown.
Assessment of caspase-3/7 activity.
L929 cells (1 × 104) were seeded into black, clear-bottom, 96-well plates and adsorbed with 10 PFU/cell of reovirus in serum-free medium at room temperature for 1 h. Following the incubation of cells at 37°C for 48 h, caspase-3/7 activity was quantified by using the Caspase-Glo-3/7 assay system (Promega).
Assessment of virus yield.
BMDMs in 24-well plates were adsorbed in triplicate with 50 PFU/cell of T3D for 1 h. Cells were washed once with PBS and incubated for 0 h or 24 h. Cells were frozen and thawed twice prior to the determination of viral titers by a plaque assay. Virus yields were calculated according to the following formula: log10 yield24 h = log10(PFU/ml)24 h − log10(PFU/ml)0 h.
RT-qPCR.
RNA was extracted from infected cells at various time intervals after infection by using Tri reagent or an RNeasy kit (Qiagen). For reverse transcription-quantitative PCR (RT-qPCR), 0.5 to 2 μg of RNA was reverse transcribed using random hexamers or gene-specific primers by using a High Capacity cDNA reverse transcription kit (Applied Biosystems). A 1:10 dilution of the cDNA was subjected to PCR using SYBR Select master mix (Applied Biosystems). ΔCT values for each cDNA sample were calculated by subtracting the threshold cycle (CT) values for T3DS1, ZBP1, or IFN-β from the CT values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The fold increase in gene expression with respect to the control sample (indicated in each figure legend) was measured by using the ΔΔCT method (83).
Statistical analysis.
Statistical significance between experimental groups was determined by using the unpaired t test function of GraphPad Prism software. Statistical analyses for differences in gene expression by RT-qPCR were done by using ΔCT values.
ACKNOWLEDGMENTS
We are grateful to Bernardo Mainou, Indiana University Virology colleagues, and members of our laboratory for helpful discussions and review of the manuscript. Transmission electron microscopy was performed in the Indiana University Bloomington Electron Microscopy Center with the assistance of Barry Stein.
REFERENCES
- 1.Danthi P. 2016. Viruses and the diversity of cell death. Annu Rev Virol 3:533–553. doi: 10.1146/annurev-virology-110615-042435. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. 2014. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A 111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. 2015. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upton JW, Kaiser WJ, Mocarski ES. 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH III, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, Verbist K, Gough PJ, Bertin J, Hartmann BM, Sealfon SC, Kaiser WJ, Mocarski ES, Lopez CB, Thomas PG, Oberst A, Green DR, Balachandran S. 2016. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe 20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigue-Gervais IG, Labbe K, Dagenais M, Dupaul-Chicoine J, Champagne C, Morizot A, Skeldon A, Brincks EL, Vidal SM, Griffith TS, Saleh M. 2014. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 15:23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Chan FK. 2012. Fueling the flames: mammalian programmed necrosis in inflammatory diseases. Cold Spring Harb Perspect Biol 4:a008805. doi: 10.1101/cshperspect.a008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, Upton JW, Daley-Bauer LP. 2014. True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J Immunol 192:2019–2026. doi: 10.4049/jimmunol.1302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 12.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. 2014. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. 2012. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, Rodriguez DA, Wakefield R, Frase S, Moldoveanu T, Green DR. 2016. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol Cell 61:589–601. doi: 10.1016/j.molcel.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. 2013. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Moujalled DM, Cook WD, Murphy JM, Vaux DL. 2014. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis 5:e1086. doi: 10.1038/cddis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, Tripaydonis A, Babon JJ, Mulcair MD, Scanlon MJ, Alexander WS, Wilks AF, Czabotar PE, Lessene G, Murphy JM, Silke J. 2014. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A 111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galluzzi L, Kepp O, Kroemer G. 2014. MLKL regulates necrotic plasma membrane permeabilization. Cell Res 24:139–140. doi: 10.1038/cr.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou JC, Bertrand MJ, Vandenabeele P. 2014. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. 2014. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton K. 2015. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol 25:347–353. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He S, Liang Y, Shao F, Wang X. 2011. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuriakose T, Man SM, Malireddi RS, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1:aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, ten Oever BR, Thomas PG, Upton JW, Balachandran S. 13 October 2016. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozym RA, Patel K, White C, Cheung KH, Bergelson JM, Morosky SA, Coyne CB. 2011. Calcium signals and calpain-dependent necrosis are essential for release of coxsackievirus B from polarized intestinal epithelial cells. Mol Biol Cell 22:3010–3021. doi: 10.1091/mbc.E11-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger AK, Danthi P. 2013. Reovirus activates a caspase-independent cell death pathway. mBio 4:e00178–13. doi: 10.1128/mBio.00178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu JJ, Ng ML. 2003. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J Gen Virol 84:3305–3314. doi: 10.1099/vir.0.19447-0. [DOI] [PubMed] [Google Scholar]
- 32.Meessen-Pinard M, Le Coupanec A, Desforges M, Talbot PJ. 16 December 2016. Pivotal role of RIP1 and MLKL in neuronal cell death induced by the human neuroinvasive coronavirus OC43. J Virol doi: 10.1128/JVI.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Berghe T, Grootjans S, Goossens V, Dondelinger Y, Krysko DV, Takahashi N, Vandenabeele P. 2013. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 61:117–129. doi: 10.1016/j.ymeth.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. 2014. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 35.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. 2014. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, Chen T, Green DR. 2016. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ 23:76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly JL, Rodgers SE, Clarke P, Ballard DW, Kerr LD, Tyler KL, Dermody TS. 2000. Reovirus-induced apoptosis requires activation of transcription factor NF-kappaB. J Virol 74:2981–2989. doi: 10.1128/JVI.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danthi P, Pruijssers AJ, Berger AK, Holm GH, Zinkel SS, Dermody TS. 2010. Bid regulates the pathogenesis of neurotropic reovirus. PLoS Pathog 6:e1000980. doi: 10.1371/journal.ppat.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holm GH, Zurney J, Tumilasci V, Leveille S, Danthi P, Hiscott J, Sherry B, Dermody TS. 2007. Retinoic acid-inducible gene-I and interferon-beta promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J Biol Chem 282:21953–21961. doi: 10.1074/jbc.M702112200. [DOI] [PubMed] [Google Scholar]
- 40.Pruijssers AJ, Hengel H, Abel TW, Dermody TS. 2013. Apoptosis induction influences reovirus replication and virulence in newborn mice. J Virol 87:12980–12989. doi: 10.1128/JVI.01931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke P, Beckham JD, Leser JS, Hoyt CC, Tyler KL. 2009. Fas-mediated apoptotic signaling in the mouse brain following reovirus infection. J Virol 83:6161–6170. doi: 10.1128/JVI.02488-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berens HM, Tyler KL. 2011. The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J Virol 85:3858–3871. doi: 10.1128/JVI.01958-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dionne KR, Zhuang Y, Leser JS, Tyler KL, Clarke P. 2013. Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis. J Virol 87:3447–3460. doi: 10.1128/JVI.02324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang Y, Berens-Norman HM, Leser JS, Clarke P, Tyler KL. 2016. Mitochondrial p53 contributes to reovirus-induced neuronal apoptosis and central nervous system injury in a mouse model of viral encephalitis. J Virol 90:7684–7691. doi: 10.1128/JVI.00583-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. 2011. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. 2011. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. 2011. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiller BE, Berger AK, Danthi P. 28 July 2015. Viral gene expression potentiates reovirus-induced necrosis. Virology doi: 10.1016/j.virol.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalai M, Van Loo G, Vanden Berghe T, Meeus A, Burm W, Saelens X, Vandenabeele P. 2002. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ 9:981–994. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- 50.Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, Schuberth C, Van der Veen AG, Fujimura T, Rehwinkel J, Iskarpatyoti JA, Barchet W, Ludwig J, Dermody TS, Hartmann G, Reis ESC. 10 August 2014. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAllister CS, Taghavi N, Samuel CE. 2012. Protein kinase PKR amplification of interferon beta induction occurs through initiation factor eIF-2alpha-mediated translational control. J Biol Chem 287:36384–36392. doi: 10.1074/jbc.M112.390039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res 26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 54.de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. 2005. Recognition of double-stranded RNA by human Toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem 280:38133–38145. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- 55.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke P, Leser JS, Bowen RA, Tyler KL. 2014. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. mBio 5:e00902–14. doi: 10.1128/mBio.00902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sturzenbecker LJ, Nibert ML, Furlong DB, Fields BN. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol 61:2351–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dermody TS, Parker JC, Sherry B. 2013. Orthoreoviruses, p 1304–1346. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 59.Murray KE, Nibert ML. 2007. Guanidine hydrochloride inhibits mammalian orthoreovirus growth by reversibly blocking the synthesis of double-stranded RNA. J Virol 81:4572–4584. doi: 10.1128/JVI.02106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson DR, Joklik WK. 1978. The mechanism of interferon induction by UV-irradiated reovirus. Virology 91:389–406. doi: 10.1016/0042-6822(78)90386-0. [DOI] [PubMed] [Google Scholar]
- 61.Lostale-Seijo I, Martinez-Costas J, Benavente J. 2016. Interferon induction by avian reovirus. Virology 487:104–111. doi: 10.1016/j.virol.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. 2012. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. 21 July 2014. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. 2013. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imani F, Jacobs BL. 1988. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc Natl Acad Sci U S A 85:7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connolly JL, Dermody TS. 2002. Virion disassembly is required for apoptosis induced by reovirus. J Virol 76:1632–1641. doi: 10.1128/JVI.76.4.1632-1641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danthi P, Coffey CM, Parker JS, Abel TW, Dermody TS. 2008. Independent regulation of reovirus membrane penetration and apoptosis by the mu1 phi domain. PLoS Pathog 4:e1000248. doi: 10.1371/journal.ppat.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knowlton JJ, Dermody TS, Holm GH. 2012. Apoptosis induced by mammalian reovirus is beta interferon (IFN) independent and enhanced by IFN regulatory factor 3- and NF-kappaB-dependent expression of Noxa. J Virol 86:1650–1660. doi: 10.1128/JVI.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fensterl V, Chattopadhyay S, Sen GC. 2015. No love lost between viruses and interferons. Annu Rev Virol 2:549–572. doi: 10.1146/annurev-virology-100114-055249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarbl H, Skup S, Millward S. 1980. Reovirus progeny subviral particles synthesize uncapped mRNA. J Virol 34:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clarke P, Tyler KL. 2009. Apoptosis in animal models of virus-induced disease. Nat Rev Microbiol 7:144–155. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Donnell SM, Hansberger MW, Connolly JL, Chappell JD, Watson MJ, Pierce JM, Wetzel JD, Han W, Barton ES, Forrest JC, Valyi-Nagy T, Yull FE, Blackwell TS, Rottman JN, Sherry B, Dermody TS. 2005. Organ-specific roles for transcription factor NF-kappaB in reovirus-induced apoptosis and disease. J Clin Invest 115:2341–2350. doi: 10.1172/JCI22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holm GH, Pruijssers AJ, Li L, Danthi P, Sherry B, Dermody TS. 2010. Interferon regulatory factor 3 attenuates reovirus myocarditis and contributes to viral clearance. J Virol 84:6900–6908. doi: 10.1128/JVI.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maitra R, Ghalib MH, Goel S. 4 October 2012. Reovirus: a targeted therapeutic—progress and potential. Mol Cancer Res doi: 10.1158/1541-7786.MCR-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi T, Antar AAR, Boehme KW, Danthi P, Eby EA, Guglielmi KM, Holm GH, Johnson EM, Maginnis MS, Naik S, Skelton WB, Wetzel JD, Wilson GJ, Chappell JD, Dermody TS. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1:147–157. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danthi P, Kobayashi T, Holm GH, Hansberger MW, Abel TW, Dermody TS. 2008. Reovirus apoptosis and virulence are regulated by host cell membrane penetration efficiency. J Virol 82:161–172. doi: 10.1128/JVI.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berard A, Coombs KM. 2009. Mammalian reoviruses: propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15:Unit 15C.1. doi: 10.1002/9780471729259.mc15c01s14. [DOI] [PubMed] [Google Scholar]
- 78.Virgin HW, Bassel-Duby IVR, Fields BN, Tyler KL. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol 62:4594–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. 2011. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ 18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 81.Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyler KL, Squier MK, Rodgers SE, Schneider BE, Oberhaus SM, Grdina TA, Cohen JJ, Dermody TS. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J Virol 69:6972–6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]