ABSTRACT
The baculovirus VP39 protein is a major nucleocapsid protein essential for viral propagation. However, the critical domains or residues of the VP39 protein have not yet been identified. Here, we performed mutagenesis experiments with Bombyx mori nucleopolyhedrovirus (BmNPV) using 5-bromo-2′-deoxyuridine and isolated a BmNPV mutant that produced fewer occlusion bodies than the wild-type virus. This mutant also produced fewer infectious budded viruses (BVs) than the wild-type virus in both cultured cells and B. mori larvae. Marker rescue experiments using genomic libraries identified a single nucleotide mutation in the vp39 gene. This mutation resulted in an amino acid substitution at glycine 276 (Gly-276) to serine, which was required for all the defective phenotypes observed in the mutant. Sequence comparison revealed that this residue is completely conserved among the VP39 proteins of the sequenced alphabaculoviruses, betabaculoviruses, and gammabaculoviruses. Although early viral gene expression was not significantly affected, the level of expression of a late gene, vcath, was reduced. In addition, two of the very late genes were markedly downregulated in cells infected with this mutant. Western blot and quantitative PCR analyses revealed that the BVs produced from cells infected with this mutant contained smaller amounts of the VP39 protein and viral genomic DNA than those produced from wild-type virus-infected cells. Combined with the results of transmission electron microscopy, VP39 Gly-276 can be concluded to be essential for correct nucleocapsid assembly, viral DNA packaging, and viral gene expression, especially of very late genes.
IMPORTANCE The major nucleocapsid protein gene vp39 is one of the most well-known baculovirus genes. Although several viral and host proteins that interact with the VP39 protein have been identified, the functionally important domains or residues of this protein remain unknown. The present study revealed that the glycine residue at residue 276, which is completely conserved among sequenced alphabaculoviruses, betabaculoviruses, and gammabaculoviruses, is important for the VP39 function, i.e., structural assembly of nucleocapsids and viral DNA packaging. Moreover, our results provide evidence for the link between nucleocapsid formation and the transcription of viral very late genes.
KEYWORDS: baculovirus, nucleocapsid, BmNPV, VP39, few polyhedra
INTRODUCTION
The Baculoviridae are a large family of viruses that infect insects, particularly those of the order Lepidoptera. Baculoviruses have a large circular, supercoiled, and double-stranded DNA genome packaged into rod-shaped virions (1, 2). They are divided into four genera, i.e., Alphabaculovirus, Betabaculovirus, Gammabaculovirus, and Deltabaculovirus (3). On the basis of molecular phylogenetic studies using genome sequences, alphabaculoviruses can be further subdivided into group I and II nucleopolyhedroviruses (NPVs) (4). NPVs produce two types of virions during their infection cycle. Occlusion-derived viruses (ODVs), which are occluded in occlusion bodies (OBs), play an essential role in transmitting the virus from insect to insect through oral infection, whereas budded viruses (BVs) spread infection to neighboring cells (5, 6). Using these two types of virions, NPVs can establish efficient viral propagation within infected larvae and spread among insects.
BVs and ODVs possess different envelope structures, each of which contains proteins unique to each structure, whereas the nucleocapsid components of both types of virions are believed to be common (7). In the nucleocapsids, the viral genome is condensed by the protamine-like basic protein P6.9 and the capsid sheath is mainly composed of the VP39 protein (8). Homologs of both proteins are present in all baculoviruses that have been sequenced (9) and are known to be two of the three most abundant proteins in BVs of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) (10). P6.9 is a small arginine-rich DNA-binding protein and localizes to the nuclear matrix during infection (11–14). Biochemical analysis and bacmid-based experiments have revealed in detail the properties and roles of the P6.9 protein in baculovirus-infected cells (15, 16). On the other hand, VP39 is a 39-kDa structural protein that is a major nucleocapsid component of both BVs and ODVs (17, 18). A recent study showed that VP39 interacts with a host kinesin 1 and that this interaction might be important for nucleocapsid transport (19). Although several VP39-interacting host and viral proteins have been identified (19–21), studies of VP39 itself are extremely limited, and to date, the functional domains or essential residues of this protein have not yet been identified.
For more than 20 years, we have screened Bombyx mori nucleopolyhedrovirus (BmNPV) mutants generated by treatment with 5-bromo-2′-deoxyuridine (BrdU) and have isolated numerous OB morphology mutants (22–24). Among them, some mutants have been found to possess mutations in the fp25K gene, resulting in lower levels of OB production. Such mutants have been termed “few polyhedra (FP) viruses” (25, 26). In the present study, we isolated a new BmNPV FP mutant, named #2080, which produced many fewer OBs in both cultured cells and B. mori larvae. Unexpectedly, we identified a single amino acid substitution in the VP39 protein which resulted in all the defective phenotypes. A subsequent detailed analysis revealed that the glycine at residue 276 of the VP39 protein is required for correct nucleocapsid assembly, viral DNA packaging, and very late viral gene expression.
RESULTS
Isolation and phenotypic characterization of a novel BmNPV FP mutant.
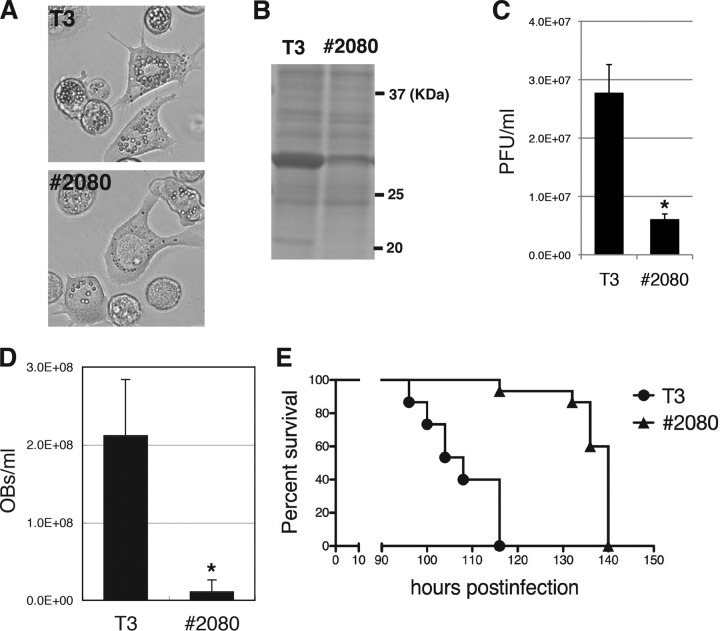
The OB morphology mutants were screened from the medium of BmNPV-infected BmN-4 cells treated with BrdU. Subsequently, about 200 clones were plaque purified; and one mutant, named #2080, which exhibited a typical FP phenotype, i.e., lower levels of OB production than the wild-type virus (T3) in BmN-4 cells, was found (Fig. 1A). SDS-PAGE analysis revealed a marked reduction in polyhedrin (POLH) expression in #2080-infected BmN-4 cells (Fig. 1B), suggesting that the FP phenotype was caused by low levels of POLH expression. In addition, BV production was reduced in #2080-infected BmN-4 cells (Fig. 1C). Next, the phenotypic defects in #2080-infected B. mori larvae were investigated, and it was found that #2080 produced fewer OBs in the hemolymph of the larvae than T3 did in the hemolymph of T3-infected larvae (Fig. 1D). The median (50%) lethal time (LT50) of #2080 was 32 h, which was longer than that of T3 when fifth-instar B. mori larvae were used (Fig. 1E). These results indicate that #2080 produces fewer OBs and infectious BVs in cultured cells and exhibits reduced pathogenicity in the host larvae than the wild type.
FIG 1.
Characterization of a novel BmNPV FP mutant. (A) Light microscopic observations at 3 dpi of BmN-4 cells infected with T3 or #2080. (B) POLH expression. The cell lysate of BmNPV-infected cells was subjected to SDS-PAGE at 3 dpi, and a gel stained with Coomassie brilliant blue (CBB) is shown. (C) Cellular BV production. The BV titer at 3 dpi was determined by plaque assay. Data are presented as the mean ± standard deviation (SD) from triplicate experiments. *, P < 0.05, Student's t test. (D) Larval OB production. The numbers of OBs released at 4 dpi in the hemolymph of BmNPV-infected B. mori larvae were counted (n = 5). Data are presented as the mean ± SD. *, P < 0.05, Student's t test. (E) Survival curves for B. mori larvae infected with T3 or #2080. The LT50s of T3 and #2080 were 108 and 140 h, respectively (n = 15).
Identification of a mutation responsible for the defective phenotypes of #2080.
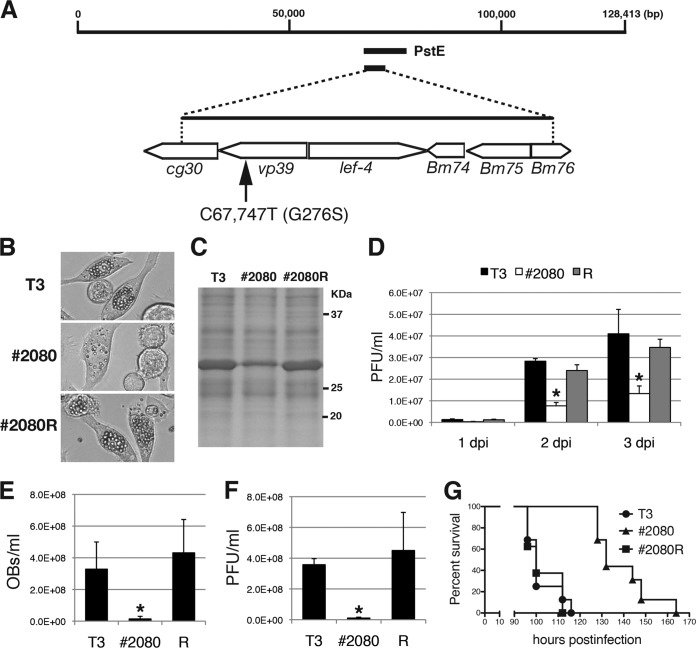
To identify the region required for the FP phenotype of #2080, marker rescue experiments with #2080 were performed using a series of plasmid libraries covering the whole genome of T3. BmN-4 cells were transfected with #2080 genomic DNA and each plasmid library containing different BmNPV genomic fragments. Subsequently, plaque assays were performed using the medium of the transfected cells, and the morphologies of the resulting plaques were examined. Many OB-containing plaques were found only when the PstI-E (PstE) fragment was cotransfected with #2080 genomic DNA (Fig. 2A). To further narrow the responsible region, subcloned plasmids covering the PstE fragment were used. Transfection experiments revealed that a 5-kbp-long fragment derived from the PstE region was sufficient to rescue the FP phenotype of #2080. This region of the BmNPV genome could potentially contain six protein-coding genes, cg30, vp39, lef-4, Bm74, Bm75, and Bm76 (Fig. 2A). Then, a virus clone that produced many OBs was plaque purified from the transfection supernatant and named #2080R. To identify the mutation(s) associated with the FP phenotype, the 5-kbp-long fragments of #2080 and #2080R were cloned, sequenced, and compared, and a single nucleotide mutation of C to T at nucleotide (nt) 67,747 in the #2080 genome was found. This nucleotide mutation resulted in an amino acid substitution of glycine (Gly) to serine (Ser) at residue 276 of the major nucleocapsid protein VP39 (Fig. 2A). The repair virus #2080R produced many OBs (Fig. 2B) and expressed a similar amount of POLH in BmN-4 cells as T3 (Fig. 2C). In addition, production of infectious BVs was restored in #2080R-infected cells (Fig. 2D). Furthermore, #2080R produced similar amounts of OBs (Fig. 2E) and infectious BVs (Fig. 2F) in the hemolymph of B. mori larvae as T3, and the LT50 of #2080R was comparable to that of T3 (Fig. 2G). These results indicate that the phenotypic defects observed in #2080 are likely due to a single amino acid mutation in the VP39 protein.
FIG 2.
Marker rescue experiments for #2080. (A) Marker rescue of #2080. BmN-4 cells were transfected with #2080 genomic DNA and BmNPV genomic libraries. One genomic library, PstE, rescued the FP phenotype of #2080. Transfection with subcloned genomic fragments revealed that the responsible mutation(s) existed within the 5-kbp-long fragment. DNA sequencing identified a single nucleotide mutation at nt 67,747 (C to T), resulting in an amino acid substitution at Gly-276 of the major capsid protein VP39. (B) Light microscopic observations at 3 dpi of BmN-4 cells infected with T3, #2080, or a repair virus (#2080R). (C) POLH expression. At 3 dpi, the cell lysate of BmNPV-infected cells was subjected to SDS-PAGE, and a gel stained with Coomassie brilliant blue is shown. (D) Cellular BV production. The BV titers in BmN-4 cells at 1, 2, or 3 dpi were determined by plaque assay. Data are presented as the mean ± SD from triplicate experiments. R, #2080R. *, P < 0.05, one-way analysis of variance with Tukey's posttest in comparison to T3. (E) Larval OB release. The numbers of OBs released in the hemolymph of BmNPV-infected B. mori larvae were quantified at 4 dpi (n = 4). Data are presented as the mean ± SD. *, P < 0.05, one-way analysis of variance with Tukey's posttest in comparison to T3. (F) Larval BV production. The BV titer in the hemolymph of BmNPV-infected B. mori larvae at 4 dpi was determined by plaque assay (n = 4). Data are presented as the mean ± SD. *, P < 0.05, one-way analysis of variance with Tukey's posttest in comparison to T3. (G) Survival curves for B. mori larvae infected with T3, #2080, or #2080R. The LT50s of T3, #2080, and #2080R were 100, 132, and 100 h, respectively (n = 16).
An amino acid substitution in #2080 is localized in the highly conserved residue among baculoviral VP39 proteins.
The VP39 protein is a major nucleocapsid protein that is conserved among all the baculoviruses (9). The sequences of the VP39 protein from 38 alphabaculoviruses, 14 betabaculoviruses, and 3 gammabaculoviruses were aligned, and it was found that the Gly residue at 276, which is mutated to Ser in #2080, was completely conserved among the VP39 proteins of all the baculoviruses examined (Fig. 3), strongly suggesting that this residue is important for the functions of the VP39 protein.
FIG 3.
Sequence alignment of the VP39 proteins from 55 baculoviruses: 38 alphabaculoviruses, 14 betabaculoviruses, and 3 gammabaculoviruses. In the sequences shown, black shading denotes identical residues and gray shading indicates similarities among the VP39 proteins. The Gly residue at 276, which is mutated to Ser in #2080 (BmNPVmut), is completely conserved among the VP39 proteins. The sequences of the following viruses were used: AcMNPV (GenBank accession number NP_054119), Adoxophyes honmai nucleopolyhedrovirus (AdhoNPV; GenBank accession number NP_818714), Adoxophyes orana nucleopolyhedrovirus (AdorNPV; GenBank accession number YP_002300582), Adoxophyes orana granulovirus (AdorGV; GenBank accession number NP_872535), Agrotis ipsilon multiple nucleopolyhedrovirus (AgipMNPV; GenBank accession number YP_002268122), Agrotis segetum granulovirus (AgseGV; GenBank accession number YP_006258), Agrotis segetum nucleopolyhedrovirus (AgseNPV; GenBank accession number YP_529756), Andraca bipunctata granulovirus (AnbiGV; GenBank accession number ACF20365), Antheraea pernyi nucleopolyhedrovirus (AnpeNPV; GenBank accession number YP_611036), Anticarsia gemmatalis nucleopolyhedrovirus (AngeNPV; GenBank accession number YP_803480), Apocheima cinerarium nucleopolyhedrovirus (ApciNPV; GenBank accession number YP_006607825), BmNPV (BmNPVwt; GenBank accession number NP_047489), Bombyx mandarina nucleopolyhedrovirus (BomaNPV; GenBank accession number ACQ57266), Choristoneura fumiferana multiple nucleopolyhedrovirus (CfMNPV; GenBank accession number NP_848392), Choristoneura fumiferana DEF multiple nucleopolyhedrovirus (CfDEFMNPV; GenBank accession number NP_932691), Chrysodeixis chalcites nucleopolyhedrovirus (ChchNPV; GenBank accession number YP_249686), Clanis bilineata nucleopolyhedrovirus (ClbiNPV; GenBank accession number YP_717616), Choristoneura occidentalis granulovirus (CoGV; GenBank accession number YP_654497), Clostera anachoreta granulovirus (ClanGV; GenBank accession number YP_004376289), Cryptophlebia leucotreta granulovirus (CrleGV; GenBank accession number NP_891934), Cydia pomonella granulovirus (CpGV; GenBank accession number NP_148880), Ectropis obliqua nucleopolyhedrovirus (EcobNPV; GenBank accession number YP_874261), Epiphyas postvittana nucleopolyhedrovirus (EppoNPV; GenBank accession number NP_203246), Euproctis pseudoconspersa nucleopolyhedrovirus (EupsNPV; GenBank accession number YP_002854686), Helicoverpa armigera granulovirus (HearGV; GenBank accession number YP_001649095), Helicoverpa armigera multiple nucleopolyhedrovirus (HearMNPV; GenBank accession number ACH88613), Helicoverpa armigera multiple nucleopolyhedrovirus-G4 (HearMNPV-G4; GenBank accession number NP_203633), Helicoverpa zea single nucleopolyhedrovirus (HzSNPV; GenBank accession number AF334030.1.), Hyphantria cunea nucleopolyhedrovirus (HycuNPV; GenBank accession number YP_473253), Leucania separata nucleopolyhedrovirus (LeseNPV; GenBank accession number YP_758393), Lymantria dispar multiple nucleopolyhedrovirus (LdMNPV; GenBank accession number NP_047729), Lymantria xylina multiple nucleopolyhedrovirus (LxMNPV; GenBank accession number YP_003517828), Mamestra brassicae nucleopolyhedrovirus (MabrNPV; GenBank accession number YP_009011154), Mamestra configurata nucleopolyhedrovirus A (MacoNPV-A; GenBank accession number NP_613182), Mamestra configurata nucleopolyhedrovirus B (MacoNPV-B; GenBank accession number NP_689273), Maruca vitrata multiple nucleopolyhedrovirus (MvMNPV; GenBank accession number YP_950797), Neodiprion abietis nucleopolyhedrovirus (NeabNPV; GenBank accession number YP_667940), Neodiprion lecontei nucleopolyhedrovirus (NeleNPV; GenBank accession number YP_025289), Neodiprion sertifer nucleopolyhedrovirus (NeseNPV; GenBank accession number YP_025196), Orgyia leucostigma nucleopolyhedrovirus (OrleNPV; GenBank accession number YP_001650987), Orgyia pseudotsugata multiple nucleopolyhedrovirus (OpMNPV; GenBank accession number NP_046246), Plutella xylostella granulovirus (PlxyGV; GenBank accession number NP_068298), Plutella xylostella multiple nucleopolyhedrovirus (PlxyMNPV; GenBank accession number ABE68474), Phthorimaea operculella granulovirus (PoGV; GenBank accession number NP_663253), Pieris rapae granulovirus (PrGV; GenBank accession number YP_003429405), Pseudaletia unipuncta granulovirus (PsunGV; GenBank accession number YP_003422454), Rachiplusia ou multiple nucleopolyhedrovirus (RaouMNPV; GenBank accession number AAN28053), Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV; GenBank accession number NP_037835), Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV; GenBank accession number YP_001036369), Spodoptera litura granulovirus (SpliGV; GenBank accession number YP_001257038), Spodoptera litura multiple nucleopolyhedrovirus II (SpltMNPV; GenBank accession number YP_002332778), Spodoptera litura multiple nucleopolyhedrovirus (SpliMNPV; GenBank accession number NP_258349), Trichoplusia ni single nucleopolyhedrovirus (TnSNPV; GenBank accession number YP_308966), and Xestia c-nigrum granulovirus (XcGV; GenBank accession number NP_059259).
Effects of mutation at Gly-276 in the VP39 protein on viral DNA replication and gene expression.
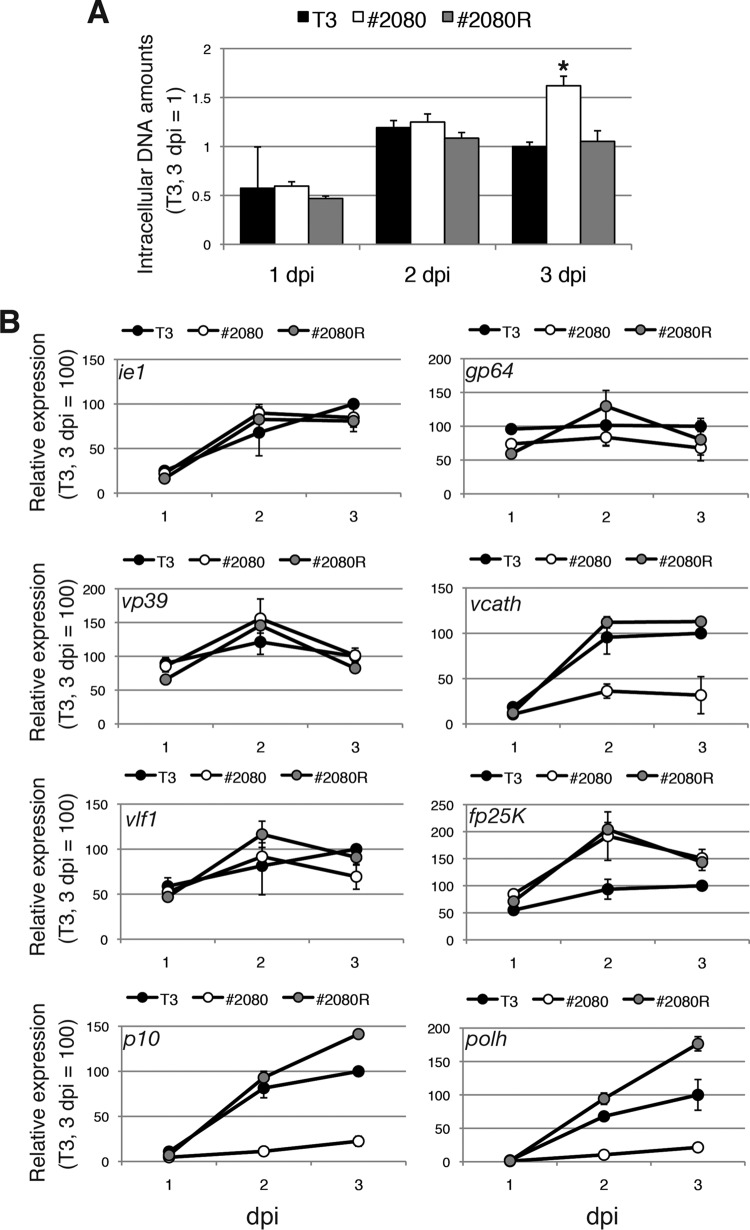
Viral DNA accumulation in T3-, #2080-, and #2080R-infected BmN-4 cells was examined by quantitative PCR (qPCR). The results revealed that the quantities of viral DNA in the infected cells at 1 and 2 days postinfection (dpi) were comparable among the viruses. At 3 dpi, the accumulation of viral DNA was significantly higher in #2080-infected cells than in T3- or #2080R-infected cells (Fig. 4A). These results indicate that the mutation in the VP39 protein does not reduce the level of viral DNA replication in BmN-4 cells.
FIG 4.
Effects of mutation in the VP39 protein on DNA replication and viral gene expression. (A) Viral DNA accumulation. BmN-4 cells were infected with T3, #2080, and #2080R at an MOI of 5. Total DNA was prepared at 1, 2, or 3 dpi and subjected to qPCR experiments. The data shown are the mean ± SD from triplicate experiments. *, P < 0.05, one-way analysis of variance with Tukey's posttest in comparison to T3. (B) Viral gene expression. BmN-4 cells were infected with T3, #2080, and #2080R at an MOI of 5. Total RNA was reverse transcribed, and RT-qPCR of BmNPV ie1, gp64, vp39, vcath, vlf1, fp25K, p10, and polh was performed. The data shown are the mean ± SD from triplicate experiments.
Subsequently, viral gene expression in T3-, #2080-, and #2080R-infected BmN-4 cells was examined. Reverse transcription-qPCR (RT-qPCR) of the ie1 and gp64 genes revealed that the expression levels of the early genes were not significantly different among the viruses (Fig. 4B). Furthermore, among the four late genes examined, the expression levels of vp39 and vlf1 observed in #2080-infected cells were comparable to those observed in T3- or #2080R-infected cells (Fig. 4B). However, the expression of vcath, which encodes a cysteine protease involved in the postmortem degradation of host caterpillars (27), was markedly lower in #2080-infected cells than in T3- or #2080R-infected cells (Fig. 4B). Moreover, although #2080 expressed a higher level of fp25K mRNA than T3 at 2 and 3 dpi, this pattern of increased levels of expression was quite similar to that noted in #2080R-infected cells (Fig. 4B). This finding suggests that both #2080 and #2080R possess a common mutation(s) other than vp39 in the genome which elevated the expression level of fp25K. Notably, the levels of expression of two very late genes, polh and p10, were markedly lower in #2080-infected cells than in T3- or #2080R-infected cells (Fig. 4B). The decline in the level of polh mRNA could be responsible for the reduction in POLH expression and OB production, leading to the FP phenotype in #2080-infected cells. Collectively, #2080 exhibited a defect in the expression of vcath and both of the very late viral genes.
Effects of mutation in the VP39 protein on BV assembly.
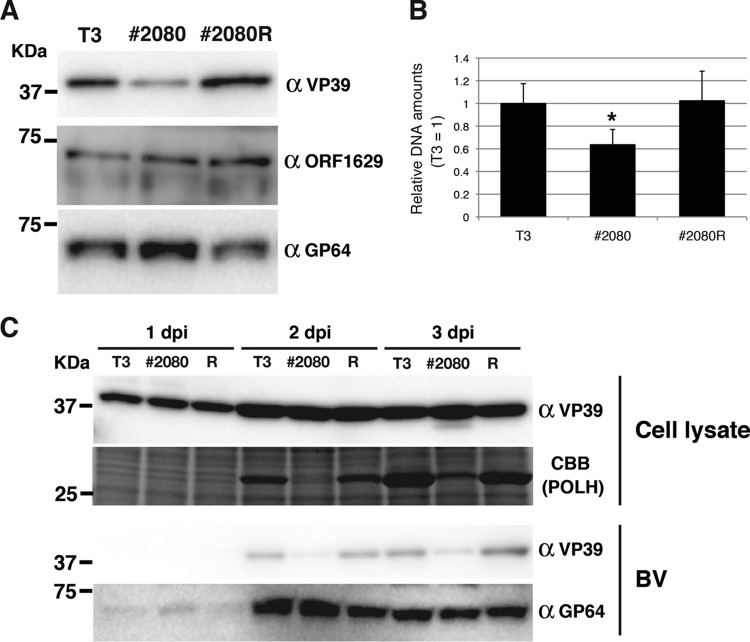
As VP39 is a major component of the capsid sheath, the properties of BV produced from BmN-4 cells infected with T3, #2080, and #2080R were compared. First, Western blot analysis of BV was performed using a VP39 monoclonal antibody, and it was found that BVs derived from #2080-infected cells contained a smaller amount of VP39 protein than those derived from T3- or #2080R-infected cells (Fig. 5A). In contrast, the quantity of a minor capsid protein, ORF1629 (also known as p78/83) (28–30), was not affected, and the level of accumulation of a major envelope protein, GP64 (31), was higher in the BVs derived from #2080-infected cells (Fig. 5A). These results demonstrate that BVs released from #2080-infected cells contain a relatively smaller amount of the VP39 protein. Subsequently, the amounts of viral genomic DNA packaged within the BVs were measured by qPCR. The results showed that the BVs derived from #2080-infected cells contained a smaller amount of viral DNA per virion than those derived from T3- or #2080R-infected cells (Fig. 5B). This result was consistent with the observation that more viral DNA accumulated in #2080-infected cells than in T3-infected cells. These results show that VP39 Gly-276 is required for the correct packaging of DNA into nucleocapsids. Moreover, expression of the VP39 protein in #2080-infected cells was comparable to that in T3- or #2080R-infected cells during infection (Fig. 5C). Furthermore, the BVs released from #2080-infected cells contained less VP39 protein than those released from T3- or #2080R-infected cells throughout the infection (Fig. 5C). These results demonstrate that the mutation at Gly-276 of the VP39 protein does not affect its expression level but reduces its efficiency of incorporation into the BVs.
FIG 5.
Effects of mutation in the VP39 protein on BV properties. (A) Composition of BV-associated proteins. Amounts of BVs equivalent to 100 ng of protein were subjected to Western blot analysis using anti-VP39, anti-ORF1629, and anti-GP64 antibodies. (B) Viral DNAs packaged in BV. The viral DNA from the same amounts of BVs from T3-, #2080-, and #2080R-infected BmN-4 cells was examined by qPCR. *, P < 0.05, one-way analysis of variance with Tukey's posttest in comparison to T3. (C) Expression of VP39 protein in BmNPV-infected cells. BmN-4 cells were infected with T3, #2080, and #2080R at an MOI of 5. The cells were prepared at 1, 2, and 3 dpi and subjected to Western blot analysis using anti-VP39 antibody. A gel stained with Coomassie brilliant blue (CBB) is also shown. The results of Western blotting for the released BVs with anti-VP39 and anti-GP64 antibodies are also shown.
Characterization of a T3-based mutant virus expressing the #2080-type vp39 gene.
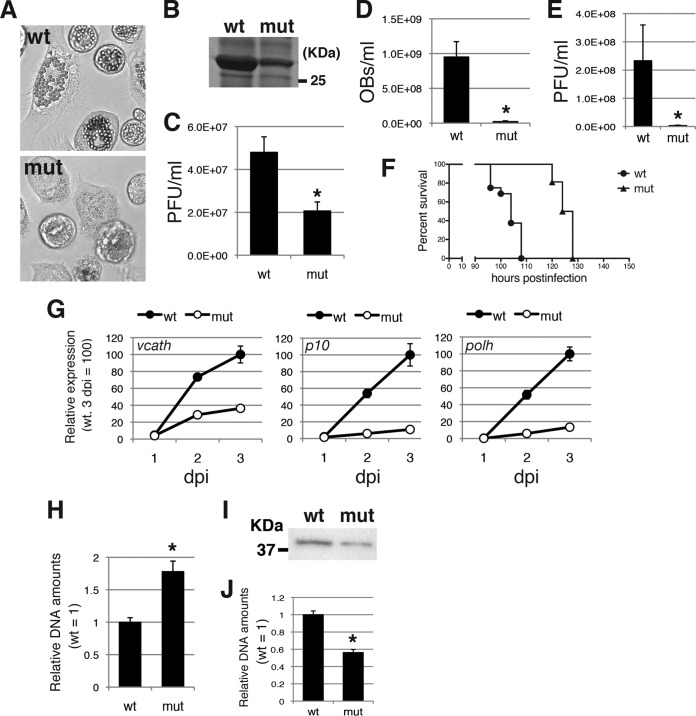
To provide evidence that a single amino acid substitution at Gly-276 of the VP39 protein is sufficient for all the defective phenotypes observed in #2080, we generated two T3-based viruses, one expressing wild-type VP39 (VP39-wt) and one expressing a mutant VP39 (VP39-mut), by transfecting the BmCG30D-1 genome and the T3- or #2080-derived vp39 fragment. BmCG30D-1 is a BmNPV mutant in which cg30, the gene located adjacent to the vp39 gene (Fig. 2), was disrupted by insertion of a lacZ gene cassette (32). VP39-mut produced fewer OBs than VP39-wt in BmN-4 cells (Fig. 6A). SDS-PAGE analysis revealed a marked reduction in the level of POLH expression in VP39-mut-infected BmN-4 cells (Fig. 6B). BV production was significantly reduced in VP39-mut-infected BmN-4 cells (Fig. 6C). Production of OBs and BVs was also reduced in the hemolymph of VP39-mut-infected larvae (Fig. 6D and E). The LT50 of VP39-mut was significantly longer than that of VP39-wt (Fig. 6F). The expression of vcath, polh, and p10 was markedly reduced in VP39-mut-infected cells (Fig. 6G). The accumulation of viral DNA was significantly higher in VP39-mut-infected cells (Fig. 6H). Furthermore, the BVs of VP39-mut contained less VP39 protein and viral genomic DNA (Fig. 6I and J). These results confirm that a single mutation at Gly-276 of the VP39 protein is sufficient to recapitulate all defective phenotypes observed in #2080.
FIG 6.
Characterization of T3-based mutant viruses expressing the #2080 VP39 protein (Ser-276) or the wild-type VP39 protein (Gly-276). (A) Light microscopic observations of BmN-4 cells infected with VP39-wt (wt) or VP39-mut (mut) at 3 dpi. (B) POLH expression. The cell lysate of BmNPV-infected cells was subjected to SDS-PAGE at 3 dpi, and a gel stained with Coomassie brilliant blue is shown. (C) Cellular BV production. The BV titer at 3 dpi was determined by plaque assay. Data are presented as the mean ± SD from triplicate experiments. *, P < 0.05, Student's t test. (D) Larval OB production. The numbers of OBs released at 4 dpi in the hemolymph of BmNPV-infected larvae were counted (n = 6). Data are presented as the mean ± SD. *, P < 0.05, Student's t test. (E) Larval BV production. The BV titer at 4 dpi in the hemolymph of BmNPV-infected larvae was determined by plaque assay (n = 6). Data are presented as the mean ± SD. *, P < 0.05, Student's t test. (F) Survival curves for B. mori larvae infected with VP39-wt or VP39-mut. The LT50s of VP39-wt and VP39-mut were 104 and 126 h, respectively (n = 16). (G) Viral gene expression. BmN-4 cells were infected with VP39-wt or VP39-mut at an MOI of 5. Total RNA was reverse transcribed, and RT-qPCR of p10 and polh was performed. The data shown are the mean ± SD from triplicate experiments. (H) Viral DNA accumulation. BmN-4 cells were infected with VP39-wt or VP39-mut at an MOI of 5. Total DNA was prepared at 3 dpi and subjected to qPCR experiments. The data shown are the mean ± SD from triplicate experiments. *, P < 0.05, Student's t test. (I) BV-associated VP39 protein. A total of 100 ng of BV was subjected to Western blot analysis using anti-VP39 antibody. (J) Viral DNAs packaged in BV. The viral DNA from the same amounts of BV from VP39-wt- or VP39-mut-infected BmN-4 cells was examined by qPCR. *, P < 0.05, Student's t test.
TEM of vp39 mutant-infected cells.
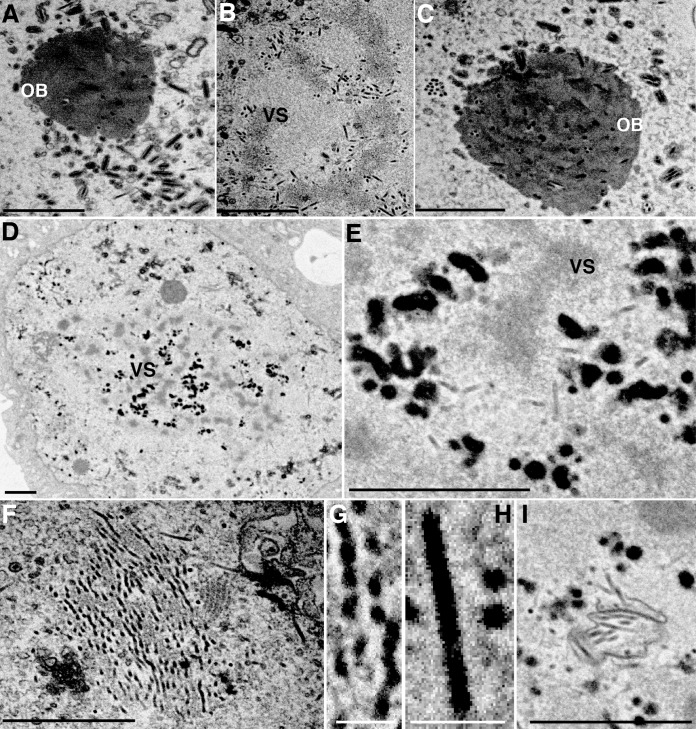
Finally, T3-, #2080-, and #2080R-infected BmN-4 cells were observed by transmission electron microscopy (TEM). In T3- or #2080R-infected cells at 2 dpi, a large quantity of nucleocapsids was normally assembled at the virogenic stroma (VS) (Fig. 7B) and mature ODVs were incorporated into the developing OBs (Fig. 7A and C). In #2080-infected cells, few electron-translucent, rod-shaped nucleocapsid-like structures were observed around the VS (Fig. 7E). Instead, morphologically aberrant, large aggregated electron-dense structures accumulated in massive amounts (Fig. 7D and E). On the basis of their electron density, these structures were considered abnormally assembled nucleocapsids filled with viral genomic DNA. Moreover, we also observed nucleocapsid-like structures accumulated in the ring zone (RZ) of #2080-infected cells, which were phenotypically different from those observed in the VS of #2080R-infected cells (Fig. 7F and G). These structures were not aggregated and contained electron-dense contents, probably viral DNA. Unlike the nucleocapsids assembled in T3-infected cells (Fig. 7H), DNA-like contents were not evenly distributed within individual nucleocapsids (Fig. 7F and G). Furthermore, aberrant ODV formation was frequently observed in #2080-infected cells (Fig. 7I). Taken together, it can be concluded that Gly-276 of the VP39 protein is critical for the correct assembly of nucleocapsids in BmNPV-infected cells.
FIG 7.
TEM of vp39 mutant-infected cells. TEM of BmN-4 cells infected with T3 (A, B, H), #2080 (D to G, I), and #2080R (C) at 2 dpi. (A to C) Normally assembled nucleocapsids at the VS and ODV formation in T3- or #2080R-infected cells; (D) morphologically aberrant, aggregated structures observed in #2080-infected cells; (E) an enlargement of the VS shown in panel D; (F) aberrant nucleocapsid-like structures observed in the RZ of #2080-infected cells; (G) an enlargement of the nucleocapsid-like structures shown in panel F; (H) nucleocapsids observed in T3-infected cells; (I) aberrant ODV formation in #2080-infected cells. Bars, 1 μm (A to F, I) and 100 nm (G, H).
DISCUSSION
We previously characterized five BrdU-induced BmNPV FP mutants and found that all of them possessed mutations in the fp25K gene (22), which is required for efficient transcription of polh (33, 34). In the present study, we analyzed a new BmNPV FP mutant, #2080, which produced fewer OBs and infectious BVs in both cultured cells and larvae (Fig. 1A to D). These phenotypes are very similar to those of Bm25KD, a BmNPV mutant in which fp25K is disrupted by insertion of a lacZ cassette (22, 34). However, marker rescue experiments and DNA sequencing revealed that the defective phenotypes observed in #2080 were likely caused by a single amino acid substitution in the major nucleocapsid protein VP39 (Fig. 2). Together with further experiments using VP39-mut, a T3-based BmNPV expressing a mutant vp39 gene, we concluded that a single mutation at Gly-276 of the VP39 protein is necessary and sufficient for all the defective phenotypes observed in #2080 (Fig. 6). Although VP39 is one of the most well-known baculovirus proteins, only one study had reported on a vp39 mutant. As expected, disruption of vp39 in the BmNPV genome resulted in a complete loss of viral growth in cultured cells (35), but that study simply revealed the essential role of VP39 in baculovirus infection and did not identify the functional domains or essential residues of the protein. Thus, to the best of our knowledge, the present study is the first to identify one essential residue for the functions of the VP39 protein.
Gene disruption analyses using AcMNPV bacmids showed that minor capsid proteins, such as VP1054, BV/ODV-C42, VLF1, and 38K, are required for correct nucleocapsid assembly (15, 21, 36–38). Most of these knockout bacmids produced electron-lucent, elongated tube-like structures, i.e., nucleocapsids lacking viral DNA, in the VS of the transfected cells, indicating that these minor capsid proteins play essential roles in viral DNA packaging (15, 21, 36–38). Interestingly, the nucleocapsid-like structures, which probably contained an aberrant VP39 protein, that formed in the nuclei of #2080-infected cells were phenotypically different from other capsid mutants. As shown in Fig. 7, most of the structures were likely filled with DNA-like contents, and notably, two different forms were observed in the VS and RZ of #2080-infected cells. Aggregated, large structures were observed mainly in the VS, whereas elongated tube-like structures that were irregularly occupied with DNA-like contents accumulated in the RZ. The former likely develop into ODVs, and the latter likely egress from the nucleus and bud from the cells as BVs. qPCR experiments revealed that more viral DNA accumulated in #2080-infected cells than in T3- or #2080R-infected cells (Fig. 4A), suggesting that the aggregates that formed in the VS contained more viral DNA than the amount commonly found in T3-infected cells. In contrast, BVs released from #2080-infected cells contained less viral genomic DNA and VP39 protein than those released from T3-infected cells (Fig. 5A and B), implying that the elongated tube-like structures that accumulated in the RZ contained fewer nucleocapsid components. Despite such abnormal structures, #2080 produced infectious BVs in both cultured cells and larvae. Western blot analysis showed that the same amount of GP64 was detected in the BVs released from T3- and #2080-infected cells (Fig. 5C), suggesting that #2080 likely produces similar numbers of BVs with aberrant nucleocapsids and normal envelopes. Such BVs are considered to possess similar cell entry activity, but #2080 produces fewer infectious progeny BVs than T3. This could be supported by the observation that #2080 or VP39-mut produced much smaller plaques than wild-type viruses (data not shown).
Surprisingly, the expression of aberrant VP39 protein alone markedly reduced the levels of mRNA for the very late genes (Fig. 4B), which necessarily led to low levels of OB production, suggesting that the mutation in the VP39 protein affected the expression of the genes required for high-level expression of very late genes. We previously showed that disruption of Bm34 severely decreased the level of POLH expression, presumably owing to the downregulation of vlf1 and fp25K (39), both of which are required for the efficient expression of two very late genes (33, 40). However, in the present study, the RT-qPCR results clearly showed that the levels of expression of vlf1 and fp25K were not decreased in #2080-infected cells (Fig. 4B), indicating that a marked decrease in p10 and polh expression did not result from the reduced levels of expression of vlf1 and fp25K. Another hypothesis is the link between nucleocapsid formation and the transcription of very late genes. A diagram for the roles of two types of baculovirus genomic DNAs, namely, the DNA destined for packaging into the nucleocapsids and the DNA utilized for hyperexpression of two very late genes, polh and p10, has been proposed (9). The former is produced through DNA replication in the partitioned area within the nuclei of the infected cells and is subsequently packaged into the preformed capsids. On the other hand, much of the viral DNA in the nuclei might be used as the template for gene expression. As hyperexpression of very late genes is initiated at the stage after packaging of viral genome DNA, transcription of polh and p10 largely depends on the number of copies of free viral DNA. The TEM data obtained in the present study clearly showed that the aberrant VP39 protein induced the formation of numerous abnormal aggregates filled with viral DNA-like contents, suggesting the possibility that abnormal aggregates in the VS tend to incorporate more viral DNA into them and leave less free DNA for very late gene transcription, resulting in extremely low levels of transcription of very late genes. In conclusion, the study of the vp39 mutant provided evidence for the relationship between nucleocapsid assembly and viral very late gene expression in baculovirus-infected cells.
MATERIALS AND METHODS
Insects, cell lines, and viruses.
B. mori larvae were reared as described previously (41). BmN-4 cells were cultured at 27°C in TC-100 medium (AppliChem) supplemented with 10% fetal bovine serum. BmNPV T3 (42) was used as the wild-type virus. The virus titers were determined by plaque assay on BmN-4 cells (42). BmN-4 cells were infected with BmNPV at a multiplicity of infection (MOI) of 5.
Mutagenesis experiments.
BmN-4 cells (4 × 106 per 60-mm-diameter dish) were infected with T3 at an MOI of 5. Five milliliters of TC-100 medium containing 5-bromo-2′-deoxyuridine (BrdU) at a concentration of 100 μg/ml was added following a 1-h virus adsorption period. At 5 days postinfection (dpi), the culture supernatant was collected, centrifuged (3,000 × g for 5 min) to remove the cell debris, and stored at 4°C prior to the isolation of mutants.
Isolation of a novel BmNPV FP mutant.
The culture medium of BmNPV-infected BmN-4 cells treated with BrdU was subjected to plaque assay, and approximately 200 clones from individual plaques were isolated. These clones were used to infect BmN-4 cells and screened according to their levels of OB production or their OB morphology. A virus (#2080) producing fewer OBs in the nuclei of the infected cells than the parent virus (T3) was isolated.
Marker rescue experiments.
BmN-4 cells were transfected with #2080 genomic DNA and each plasmid library containing the BmNPV genomic fragment (43) using the Cellfectin II reagent (Invitrogen). At 5 days after transfection, the medium was collected and stored at 4°C until use. The transfection supernatant was used for plaque assays to identify the genomic clones that rescued the FP phenotype of #2080.
Generation of T3-based BmNPVs possessing the wild-type or mutant vp39 gene.
We generated T3-based viruses possessing the wild-type or #2080 vp39 gene using BmCG30D-1 (32) as a backbone virus. PCR fragments from T3 and #2080 genomic DNAs about 5 kbp long (nt 65,496 to 70,456; GenBank accession number L33180) were cloned into pcDNA3.1(−) (Invitrogen). These plasmids were cotransfected with Bsu36I-digested BmCG30D-1 genomic DNA into BmN-4 cells using the X-tremeGENE HP transfection reagent (Roche). Recombinants exhibiting white plaques were isolated by plaque assay with agarose overlays containing 5-bromo-4-chloro-3-indolyl-β-d-galactoside. Precise recombination of the cg30-vp39 region was confirmed by PCR and DNA sequencing. The BmNPVs possessing the T3 and #2080 vp39 gene were designated VP39-wt and VP39-mut, respectively.
Larval bioassays.
Fifth-instar B. mori larvae were starved for several hours, injected with 50 μl of a viral suspension containing 1 × 105 PFU, and returned to the artificial diet at 25°C. The hemolymph of the infected larvae was collected, and the released OBs were counted using a hemocytometer. The BV titers in the hemolymph of the infected larvae were determined by plaque assay. Intrahemocoelic injection of BVs into the fifth-instar B. mori larvae was performed within 12 h after molting, and the median (50%) lethal time (LT50) was calculated using Prism (version 5) software (GraphPad). A total of 15 or 16 larvae were used for each infection.
Western blot analysis.
BmN-4 cells were infected with BmNPVs at an MOI of 5. The cells and BVs were collected at the postinfection time points indicated above and below and subjected to Western blot analysis with anti-VP39 (44), anti-GP64 (catalog number AcV5; Santa Cruz Biotechnology), and anti-ORF1629 (28) antibodies as described previously (45).
RT-qPCR.
BmN-4 cells were infected with BmNPVs at an MOI of 5. The cells were collected at 1, 2, and 3 dpi, and the total RNA was prepared using the TRIzol reagent (Invitrogen). The first-strand cDNA was synthesized from 0.5 μg of total RNA, and reverse transcription-quantitative PCR (RT-qPCR) experiments with ie1, gp64, vp39, vcath, vlf1, fp25K, p10, and polh were performed using a Kapa SYBR Fast qPCR kit (Kapa Biosystems) with previously published primers (39). Amplification was detected using a StepOnePlus real-time PCR system (Applied Biosystems). The expression values were calculated using the 2−CT threshold cycle (CT) method. The value of each viral transcript in T3-infected cells at 3 dpi was considered to be 100, and the relative levels of transcripts in cells infected with mutant viruses were estimated.
Quantification of viral genomic DNA.
To assess the amount of viral DNA in BmNPV-infected BmN-4 cells, the cells were harvested with 1 ml of phosphate-buffered saline (PBS), lysed with 500 μl of cell lysis buffer (10 mM Tris, pH 8.0, 100 mM EDTA, 20 μg/ml RNase A, 0.5% SDS, 20 μg of proteinase K), and incubated overnight at 65°C. The total DNA was extracted with phenol, precipitated with ethanol, and suspended in 500 μl of water. qPCR was performed with the primers rpolhF1 and rpolhR1 as described previously (39). The amount of the intracellular viral genome was normalized to the amount of the host cell genome (46).
BVs were purified by a standard procedure (47), and their concentrations were determined with a Coomassie Plus protein assay reagent kit (Pierce Biotechnology). To determine the amount of viral DNA in the BVs, 500 ng of BVs was lysed with 500 μl of 0.025% SDS, boiled for 5 min, diluted with distilled water, and subjected to qPCR analysis with the same primer sets described above.
Transmission electron microscopy (TEM).
BmN-4 cells were infected with BmNPVs at an MOI of 3. After 48 h, the infected cells were fixed with 2% glutaraldehyde in PBS. Subsequently, the cells were postfixed with 1% osmium tetroxide in PBS overnight, dehydrated using a standard acetone series, and embedded in epoxy resin. The blocks were sectioned at 70 nm, stained with uranyl acetate and lead citrate, and viewed under a JEM-2000EX transmission electron microscope (JEOL Ltd.) operating at 200 kV.
ACKNOWLEDGMENTS
We thank the Technology Advancement Center, Graduate School of Agricultural and Life Sciences, The University of Tokyo, for TEM sample preparation and data acquisition. We also thank George Rohrmann, Motoko Ikeda, Taro Ohkawa, and Loy Volkman for providing antibodies and Munetaka Kawamoto for clerical assistance.
S.K. conceived of and designed the experiments. S.K. performed most of the experiments. R.K. performed TEM analysis and interpreted the results. S.K. drafted and revised the manuscript with intellectual input from R.K.
This work was supported by JSPS KAKENHI grant numbers JP24658047, JP25292196, and JP16H05051 to S.K.
REFERENCES
- 1.Summers MD, Anderson DL. 1972. Granulosis virus deoxyribonucleic acid: a closed, double-stranded molecule. J Virol 9:710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summers MD, Anderson DL. 1973. Characterization of nuclear polyhedrosis virus DNAs. J Virol 12:1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herniou EA, Arif BM, Becnel JJ, Blissard GW, Bonning B, Harrison R, Jehle JA, Theilmann DA, Vlak JM. 2012. Family baculoviridae, p 163–173. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 4.Herniou EA, Luque T, Chen X, Vlak JM, Winstanley D, Cory JS, O'Reilly DR. 2001. Use of whole genome sequence data to infer baculovirus phylogeny. J Virol 75:8117–8126. doi: 10.1128/JVI.75.17.8117-8126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granados RR, Lawler KA. 1981. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology 108:297–308. doi: 10.1016/0042-6822(81)90438-4. [DOI] [PubMed] [Google Scholar]
- 6.Keddie BA, Aponte GW, Volkman LE. 1989. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science 243:1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- 7.Slack J, Arif BM. 2007. The baculoviruses occlusion-derived virus: virion structure and function. Adv Virus Res 69:99–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Bosch BJ, Vlak JM, van Oers MM, Rottier PJ, van Lent JW. 2016. Budded baculovirus particle structure revisited. J Invertebr Pathol 134:15–22. doi: 10.1016/j.jip.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrmann GF. 2013. Baculovirus molecular biology, 3rd ed National Library of Medicine, National Center for Biotechnology Information, Bethesda, MD. [PubMed] [Google Scholar]
- 10.Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. 2010. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J Virol 84:7233–7242. doi: 10.1128/JVI.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tweeten KA, Bulla LA, Consigli RA. 1980. Characterization of an extremely basic protein derived from granulosis virus nucleocapsids. J Virol 33:866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson ME, Mainprize TH, Friesen PD, Miller LK. 1987. Location, transcription, and sequence of a baculovirus gene encoding a small arginine-rich polypeptide. J Virol 61:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson ME, Price KH. 1988. Association of Autographa californica nuclear polyhedrosis virus (AcMNPV) with the nuclear matrix. Virology 167:233–241. doi: 10.1016/0042-6822(88)90073-6. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheimer DI, Volkman LE. 1995. Proteolysis of p6.9 induced by cytochalasin D in Autographa californica M nuclear polyhedrosis virus-infected cells. Virology 207:1–11. doi: 10.1006/viro.1995.1046. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhao H, Fang Z, Yuan M, Yang K, Pang Y. 2012. Distribution and phosphorylation of the basic protein P6.9 of Autographa californica nucleopolyhedrovirus. J Virol 86:12217–12227. doi: 10.1128/JVI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A, Zhao H, Lai Q, Huang Z, Yuan M, Yang K. 2015. Posttranslational modifications of baculovirus protamine-like protein P6.9 and the significance of its hyperphosphorylation for viral very late gene hyperexpression. J Virol 89:7646–7659. doi: 10.1128/JVI.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson MN, Russell RL, Rohrmann GF, Beaudreau GS. 1988. p39, a major baculovirus structural protein: immunocytochemical characterization and genetic location. Virology 167:407–413. [PubMed] [Google Scholar]
- 18.Thiem SM, Miller LK. 1989. Identification, sequence, and transcriptional mapping of the major capsid protein gene of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol 63:2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danquah JO, Botchway S, Jeshtadi A, King LA. 2012. Direct interaction of baculovirus capsid proteins VP39 and EXON0 with kinesin-1 in insect cells determined by fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy. J Virol 86:844–853. doi: 10.1128/JVI.06109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas S, Blissard GW, Theilmann DA. 2016. Trichoplusia ni kinesin-1 associates with Autographa californica multiple nucleopolyhedrovirus nucleocapsid proteins and is required for production of budded virus. J Virol 90:3480–3495. doi: 10.1128/JVI.02912-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Lin T, Pan L, Yu M, Li Z, Pang Y, Yang K. 2006. Autographa californica multiple nucleopolyhedrovirus nucleocapsid assembly is interrupted upon deletion of the 38K gene. J Virol 80:11475–11485. doi: 10.1128/JVI.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsuma S, Noguchi Y, Zhou CL, Kobayashi M, Maeda S. 1999. Characterization of the 25K FP gene of the baculovirus Bombyx mori nucleopolyhedrovirus: implications for post-mortem host degradation. J Gen Virol 80:783–791. doi: 10.1099/0022-1317-80-3-783. [DOI] [PubMed] [Google Scholar]
- 23.Katsuma S, Noguchi Y, Shimada T, Nagata M, Kobayashi M, Maeda S. 1999. Molecular characterization of baculovirus Bombyx mori nucleopolyhedrovirus polyhedron mutants. Arch Virol 144:1275–1285. doi: 10.1007/s007050050586. [DOI] [PubMed] [Google Scholar]
- 24.Katsuma S, Deng DX, Zhou CL, Iwanaga M, Noguchi Y, Kobayashi M, Maeda S. 2000. Identification of novel residues involved in nuclear localization of a baculovirus polyhedrin protein. Virus Genes 21:233–240. doi: 10.1023/A:1008151916849. [DOI] [PubMed] [Google Scholar]
- 25.Beames B, Summers MD. 1989. Location and nucleotide sequence of the 25K protein missing from baculovirus few polyhedra (FP) mutants. Virology 168:344–353. doi: 10.1016/0042-6822(89)90275-4. [DOI] [PubMed] [Google Scholar]
- 26.Harrison RL, Summers MD. 1995. Mutations in the Autographa californica multinucleocapsid nuclear polyhedrosis virus 25 kDa protein gene result in reduced virion occlusion, altered intranuclear envelopment and enhanced virus production. J Gen Virol 76:1451–1459. doi: 10.1099/0022-1317-76-6-1451. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa T, Majima K, Maeda S. 1994. A cysteine protease encoded by the baculovirus Bombyx mori nuclear polyhedrosis virus. J Virol 68:6619–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell RL, Funk CJ, Rohrmann GF. 1997. Association of a baculovirus encoded protein with the capsid basal region. Virology 227:142–152. doi: 10.1006/viro.1996.8304. [DOI] [PubMed] [Google Scholar]
- 29.Goley ED, Ohkawa T, Mancuso J, Woodruff JB, D'Alessio JA, Cande WZ, Volkman LE, Welch MD. 2006. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science 314:464–467. doi: 10.1126/science.1133348. [DOI] [PubMed] [Google Scholar]
- 30.Vialard JE, Richardson CD. 1993. The 1,629-nucleotide open reading frame located downstream of the Autographa californica nuclear polyhedrosis virus polyhedrin gene encodes a nucleocapsid-associated phosphoprotein. J Virol 67:5859–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monsma SA, Oomens AG, Blissard GW. 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol 70:4607–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara G, Shimada T, Katsuma S. 2013. Functional characterization of Bombyx mori nucleopolyhedrovirus CG30 protein. Virus Res 174:52–59. doi: 10.1016/j.virusres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Harrison RL, Jarvis DL, Summers MD. 1996. The role of the AcMNPV 25K gene, “FP25,” in baculovirus polh and p10 expression. Virology 226:34–46. doi: 10.1006/viro.1996.0625. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi T, Goto C, Kobayashi M, Kang W, Suzuki T, Dohmae N, Matsumoto S, Shimada T, Katsuma S. 2010. Comparative studies of lepidopteran baculovirus-specific protein FP25K: development of a novel Bombyx mori nucleopolyhedrovirus-based vector with a modified fp25K gene. J Virol 84:5191–5200. doi: 10.1128/JVI.00099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono C, Kamagata T, Taka H, Sahara K, Asano S, Bando H. 2012. Phenotypic grouping of 141 BmNPVs lacking viral gene sequences. Virus Res 165:197–206. doi: 10.1016/j.virusres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Marek M, Romier C, Galibert L, Merten OW, van Oers MM. 2013. Baculovirus VP1054 is an acquired cellular PURα, a nucleic acid-binding protein specific for GGN repeats. J Virol 87:8465–8480. doi: 10.1128/JVI.00068-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li K, Wang Y, Bai H, Wang Q, Song J, Zhou Y, Wu C, Chen X. 2010. The putative pocket protein binding site of Autographa californica nucleopolyhedrovirus BV/ODV-C42 is required for virus-induced nuclear actin polymerization. J Virol 84:7857–7868. doi: 10.1128/JVI.00174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanarsdall AL, Okano K, Rohrmann GF. 2006. Characterization of the role of very late expression factor 1 in baculovirus capsid structure and DNA processing. J Virol 80:1724–1733. doi: 10.1128/JVI.80.4.1724-1733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuma S, Shimada T. 2009. Bombyx mori nucleopolyhedrovirus ORF34 is required for efficient transcription of late and very late genes. Virology 392:230–237. doi: 10.1016/j.virol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Miller LK. 1999. Activation of baculovirus very late promoters by interaction with very late factor 1. J Virol 73:3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsuma S, Horie S, Daimon T, Iwanaga M, Shimada T. 2006. In vivo and in vitro analyses of a Bombyx mori nucleopolyhedrovirus mutant lacking functional vfgf. Virology 355:62–70. doi: 10.1016/j.virol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Maeda S. 1984. A plaque assay and cloning of Bombyx mori nuclear polyhedrosis virus. J Seric Sci Jpn 53:547–548. [Google Scholar]
- 43.Maeda S, Majima K. 1990. Molecular cloning and physical mapping of the genome of Bombyx mori nuclear polyhedrosis virus. J Gen Virol 71:1851–1855. doi: 10.1099/0022-1317-71-8-1851. [DOI] [PubMed] [Google Scholar]
- 44.Whitt MA, Manning JS. 1988. A phosphorylated 34-kDa protein and a subpopulation of polyhedrin are thiol-linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology 163:33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- 45.Katsuma S, Daimon T, Mita K, Shimada T. 2006. Lepidopteran ortholog of Drosophila Breathless is a receptor for the baculoviral fibroblast growth factor. J Virol 80:5474–5481. doi: 10.1128/JVI.00248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokusho R, Koh Y, Fujimoto M, Shimada T, Katsuma S. 2016. Bombyx mori nucleopolyhedrovirus BM5 protein regulates progeny virus production and viral gene expression. Virology 498:240–249. doi: 10.1016/j.virol.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 47.Maeda S. 1989. Gene transfer vectors of a baculovirus, Bombyx mori, and their use for expression of foreign genes in insect cells, p 167–181. In Mitsuhashi J. (ed), Invertebrate cell system applications. CRC Press LLC, Boca Raton, FL. [Google Scholar]