ABSTRACT
Human cytomegalovirus (HCMV) infection and periodic reactivation are generally well controlled by the HCMV-specific T cell response in healthy people. While the CD8+ T cell response to HCMV has been extensively studied, the HCMV-specific CD4+ T cell effector response is not as well understood, especially in the context of direct interactions with HCMV-infected cells. We screened the gamma interferon (IFN-γ) and interleukin-10 (IL-10) responses to 6 HCMV peptide pools (pp65, pp71, IE1, IE2, gB, and US3, selected because they were the peptides most frequently responded to in our previous studies) in 84 donors aged 23 to 74 years. The HCMV-specific CD4+ T cell response to pp65, IE1, IE2, and gB was predominantly Th1 biased, with neither the loss nor the accumulation of these responses occurring with increasing age. A larger proportion of donors produced an IL-10 response to pp71 and US3, but the IFN-γ response was still dominant. CD4+ T cells specific to the HCMV proteins studied were predominantly effector memory cells and produced both cytotoxic (CD107a expression) and cytokine (macrophage inflammatory protein 1β secretion) effector responses. Importantly, when we measured the CD4+ T cell response to cytomegalovirus (CMV)-infected dendritic cells in vitro, we observed that the CD4+ T cells produced a range of cytotoxic and secretory effector functions, despite the presence of CMV-encoded immune evasion molecules. CD4+ T cell responses to HCMV-infected dendritic cells were sufficient to control the dissemination of virus in an in vitro assay. Together, the results show that HCMV-specific CD4+ T cell responses, even those from elderly individuals, are highly functional and are directly antiviral.
IMPORTANCE Human cytomegalovirus (HCMV) infection is carried for a lifetime and in healthy people is kept under control by the immune system. HCMV has evolved many mechanisms to evade the immune response, possibly explaining why the virus is never eliminated during the host's lifetime. The dysfunction of immune cells associated with the long-term carriage of HCMV has been linked with poor responses to new pathogens and vaccines when people are older. In this study, we investigated the response of a subset of immune cells (CD4+ T cells) to HCMV proteins in healthy donors of all ages, and we demonstrate that the functionality of CD4+ T cells is maintained. We also show that CD4+ T cells produce effector functions in response to HCMV-infected cells and can prevent virus spread. Our work demonstrates that these HCMV-specific immune cells retain many important functions and help to prevent deleterious HCMV disease in healthy older people.
KEYWORDS: ageing, antiviral, CD4+ T cells, human cytomegalovirus (HCMV), polyfunctional
INTRODUCTION
Human cytomegalovirus (HCMV), a betaherpesvirus, is a ubiquitous pathogen found worldwide (1). Infection with this virus is characterized by the establishment of lifelong persistence, in part because HCMV can establish a latent infection in bone marrow stem cells and cells of the myeloid lineage (2). Infection with HCMV is asymptomatic for most individuals; however, when the immune system is compromised by other infections or treatments (such as in patients with HIV infection or AIDS or transplant patients) or is immature (such as the fetus in utero), it can cause significant morbidity and mortality (3, 4). During primary infection with HCMV, both the innate and the adaptive branches of the immune system respond (1, 3, 4), and evidence from mouse studies has shown the important role that CD4+ T cells play in controlling cytomegalovirus (CMV) infection (reviewed in reference 5). Studies in humans undergoing bone marrow, stem cell, and solid organ transplantations have confirmed the role that CMV-specific CD4+ T cells have in abrogating the reactivation of infection (6–9), and studies in adults with primary infections have also clearly shown the requirement for functional CD4+ T cells in the resolution of symptomatic disease (10–12). In healthy subjects, persistent shedding of the virus into urine and saliva is associated with a lack of a CD4+ T cell response directed toward CMV, which is particularly observed in CMV infection in young children (13).
Identification of HCMV-specific CD4+ T cells has mainly been by measurement of intracellular cytokine production, predominantly gamma interferon (IFN-γ) production in response to stimulation; these studies have shown strong responses to both the pp65 and IE proteins of the virus (see the work summarized in references 3, 4, and 14). CD4+ T cell responses specific to the gB (UL55) protein (15, 16) have also been described. Analysis of the CD4+ T cell response to the whole HCMV proteome also identified numerous responses toward many different open reading frames (ORFs) (17). That study suggested that an individual donor has, on average, CD4+ T cells specific to 12 different HCMV ORFs (17). A meta-analysis of published studies has identified the 10 HCMV ORFs most frequently recognized by CD4+ T cells; these were TRL14, UL16, UL55, UL83, UL85, US3, UL25, US18, UL45, and UL32 (3). Many more studies have investigated the frequency, phenotype, and function of CD4+ T cells specific to HCMV using whole viral lysate stimulation (10, 12, 18–22). It was estimated that up to 5% (19) of the CD4+ T cell peripheral blood compartment can be directed toward the virus. This dominance of the CD4+ T cell compartment has also been observed to be as high as 10% when the whole virus proteome was used (17). Additionally, the application of major histocompatibility complex (MHC) class II tetramers also showed that as much as 5% of the CD4+ T cell pool responds to one HCMV gB protein class II epitope (23).
CMV-specific CD4+ T cells, mainly identified by IFN-γ secretion following stimulation with whole CMV lysate, have been shown to be enriched for phenotypes linked to terminal differentiation and dysfunctional responses characterized by CD45RA reexpression (11, 21, 24) and the loss of expression of the costimulatory molecules CD28 and CD27 (10, 20, 22, 25, 26). These cells have also been associated with the loss of the cytokine secretion ability and a limited proliferation capacity (19, 22, 27). These previous studies have led to the hypothesis that enlarged dysfunctional HCMV-specific CD4+ T cell populations accumulate with age and that these HCMV-induced changes may become detrimental to individuals (27–29). However, it is noteworthy that in other studies, HCMV-specific CD4+ T cells have also been shown to produce a range of antiviral effector functions, including production of multiple antiviral cytokines, and to have cytolytic effector functions (30–32). Functional CMV-specific CD4+ T cells were also confirmed in the rhesus macaque model of ageing (33) and murine models of ageing looking at latent murine cytomegalovirus (MCMV) infection (34, 35). The polyfunctional capacity of CMV-specific CD4+ T cells was also maintained in the more differentiated memory cell phenotypes seen in older CMV-seropositive donors (20, 21, 26, 30, 36). A number of longitudinal studies have linked HCMV seropositivity and the associated changes to the T cell repertoire, with older individuals being more susceptible to infections, responding more poorly to vaccinations, and being at an increased risk of mortality compared to age-matched HCMV-seronegative individuals (systematically reviewed in reference 37). However, a poor response to influenza vaccination in CMV-seropositive older people is not seen in all studies, and there is evidence that, certainly in the young, being CMV positive can be beneficial in mediating responses to vaccination (34). There is a body of evidence that in individuals over the age of 65 years there are changes to the immune response that increase the rates of morbidity and mortality in response to infection and autoimmune disease (38, 39). Analysis of a number of large population cohorts recruited for cancer, dementia, and nutritional studies in the UK and the United States has shown a significant association between CMV seropositivity and mortality from cardiovascular-related disease (40–43). Despite these observations, older CMV-seropositive individuals do not appear to suffer from overt HCMV disease from reactivating virus or superinfection, suggesting that the HCMV-specific T cells retain the ability to control the virus (44). There is also evidence that does not support the role of CMV seropositivity in causing a decline in immune responses to novel infections in the elderly (45, 46).
Many studies investigating the functionality of CD4+ T cell responses to HCMV infection have relied on using whole viral lysate or have focused only on the seemingly immune-dominant viral pp65 or gB protein as a stimulus. However, few studies have interrogated the different contributions of the many HCMV proteins that CD4+ T cells respond to (17) to the functional activity of the CMV-specific CD4+ T cell response. Previously, we have shown that CD4+ T cell responses to the limited number of proteins expressed during HCMV latency result in the production of the immunosuppressive cytokine interleukin-10 (IL-10), which differed from the CD4+ T cell response to HCMV proteins expressed only during lytic infection (47). The use of only peptide pools or viral lysate also ignores the impact of the large number of immune evasion molecules encoded by the virus genome during active lytic infection on the immune response and the effector functions of CD4+ T cells. We have measured the effect of donor age on CD4+ T cell responses to 6 HCMV ORF-encoded proteins (UL83 [pp65], UL82 [pp71], UL123 [IE1], UL122 [IE2], UL55 [gB], and US3), measuring IFN-γ and IL-10 responses by a FluoroSpot assay. We did not observe an accumulation of CD4+ T cell IFN-γ responses to the 6 HCMV proteins with increasing donor age, and there were limited IL-10 responses to pp65, gB, IE1, and IE2 stimulation within this donor cohort. The IL-10 response to pp71 and US3 stimulation was more frequently observed; however, the magnitude of the response was maintained regardless of donor age. CD4+ T cells responding to the 6 HCMV proteins examined had both cytotoxic and inflammatory effector functions and were mostly effector memory T cells, with pp65-specific CD4+ T cells exhibiting a more differentiated phenotype than the other HCMV-specific CD4+ T cells. We next assessed the effector capacity of CD4+ T cells when stimulated by HCMV-infected monocyte-derived dendritic cells (moDCs). CMV-specific CD4+ T cells isolated directly ex vivo produced both cytotoxic and secretory effector functions. Using an in vitro model of lytic CMV infection where moDCs were infected with CMV for 7 days prior to coincubation with CD4+ T cells, we demonstrated that CMV-specific CD4+ T cells are able to prevent viral dissemination. This study shows that healthy people of all ages can maintain highly functional HCMV-specific CD4+ T cell responses that can respond to HCMV-infected cells.
RESULTS
The magnitude of the HCMV-specific CD4+ T cell response to 6 different HCMV ORF-encoded proteins is maintained in older donors.
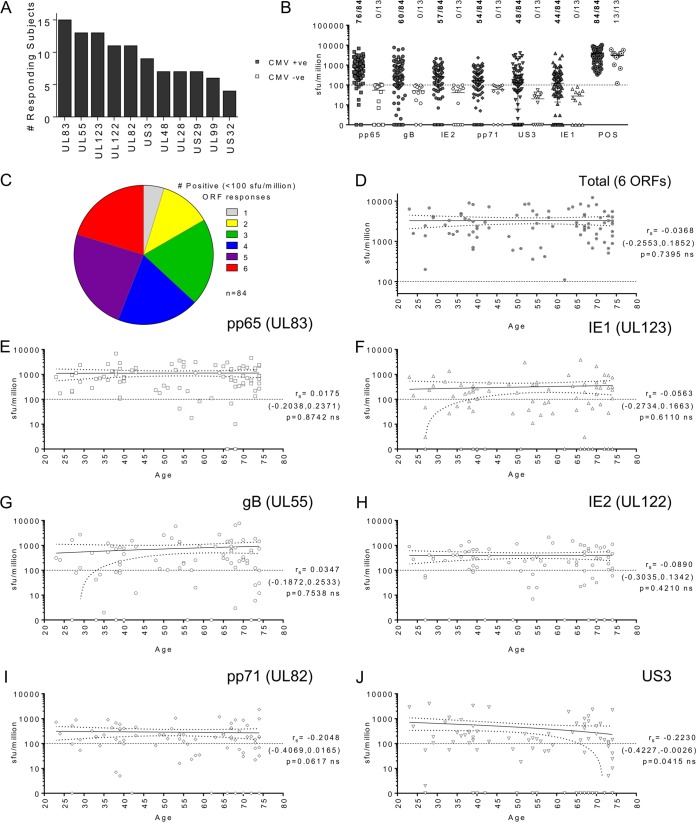
Previous work investigating the HCMV-specific CD4+ T cell response by measuring IFN-γ production by intracellular flow cytometry methods and using whole viral lysate as the stimulus has shown that the frequency of the HCMV-specific CD4+ T cell response increases with donor age (19, 21, 22). In order to measure the CD4+ T cell response to individual HCMV proteins, we performed an initial screen of the CD4+ T cell response to 11 HCMV proteins in a small cohort of 18 HCMV-seropositive and 4 HCMV-seronegative donors using pools of overlapping peptides to each HCMV protein. The 11 selected HCMV protein peptide pools included those to which CD4+ T cells responded at the highest frequency in a whole-proteome screen (17). Measurement of the frequency of the CD4+ T cell response to the selected HCMV proteins was performed by an IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay. Using 100 spot-forming units (sfu) per million cells as the cutoff for positive CD4+ T cell responses, we ranked the HCMV proteins according to the number of responding donors (Fig. 1A). This ranking enabled the identification of HCMV proteins gB, pp71, pp65, IE1, IE2, and US3 to be the peptide pools to which our donor cohort most commonly responded.
FIG 1.
The magnitude of IFN-γ-secreting CD4+ T cell responses to 6 HCMV proteins is maintained with increasing donor age. (A) The frequency of the CD4+ T cell responses to 11 HCMV protein peptide pools in 18 donors was determined by an IFN-γ ELISPOT assay. The number of donors with a positive response (<100 sfu/million cells after correction for the background count) to each protein is tallied and ranked. The IFN-γ-secreting CD4+ T cell response to 6 HCMV proteins (pp65, gB, IE2, pp71, US3, and IE1) in a cohort of 84 HCMV-seropositive and 13-seronegative donors was measured using an IFN-γ FluoroSpot technique. (B) The results were converted to the number of sfu per million T cells, from which the background counts were subtracted, and the response to each protein and the positive control by the entire cohort is summarized for both CMV-seropositive donors (CMV +ve) and CMV-seronegative donors (CMV −ve). The distribution of the CMV-seronegative donors' responses to each HCMV protein peptide pool and the response to the positive control was used to determine the cutoff for a positive response to the HCMV peptide pool, which was 100 sfu/million cells (dashed line). The proportion of donors responding to each protein and the positive control at a level above the threshold is shown. (C) The proportion of the 84 seropositive donors producing a positive response to 1 or more of the 6 HCMV protein peptide pools is summarized. (D to J) Within the seropositive cohort, the correlation of donor age with the size of the total IFN-γ donor response to all six proteins (D) and the donor response to each protein, pp65 (E), IE1 (F), gB (G), IE2 (H), pp71 (I), and US3 (J), is shown. The correlation of the CMV protein response with age was analyzed using the Spearman rank correlation (Spearman rs values, 95% confidence intervals [in parentheses], and P values are indicated on each graph); a line of best fit (solid) and the 95% confidence intervals (dotted lines) are also shown. Due to the repeated analyses performed, results were considered significant only if P was ≤0.01. ns, not significant.
In order to assess whether the frequency of CD4+ T cell responses to different HCMV proteins changes with increasing donor age, we recruited a large CMV-seropositive donor cohort (n = 84) aged 23 to 74 years and also 13 CMV-seronegative donors aged 37 to 72 years to act as controls for the background response. We measured the frequency of the CD4+ T cell IFN-γ response to the 6 highest-ranked HCMV genome-encoded proteins detailed in Fig. 1A using an IFN-γ FluoroSpot assay. The results from the entire donor cohort for all 6 HCMV proteins and the positive control are summarized in Fig. 1B. A positive response threshold of 100 sfu/million cells was determined to be a response above the distribution of any of the responses to each HCMV peptide pool measured in the seronegative cohort but below the response of both seropositive and seronegative donors to the positive control; the threshold is indicated with a line in Fig. 1B, with negative responses falling below the line. The proportion of the donor cohort responding to each protein is shown in Fig. 1C. A majority of the seropositive donor cohort responded to all six proteins studied, with 91.9% of donors generating a positive response to pp65 and 70.6%, 69.8%, 65.1%, 56.5%, and 52.3% of the donors responding to the gB, IE2, pp71, US3, and IE1 CMV peptide pools, respectively. Analysis of the frequency with which the seropositive cohort responded to 1 or more CMV proteins revealed that all donors responded to at least 1 of 6 HCMV proteins and 63.1% of donors examined produced an IFN-γ response to 4 or more proteins (Fig. 1C).
To assess whether donor age had an impact on the frequency of the CD4+ T cell response to HCMV within this cohort, the sum of the IFN-γ responses to all 6 HCMV proteins for each donor was analyzed with respect to age (Fig. 1D). Overall, there was no significant change in the magnitude of the response as donor age increased (Spearman rank correlation [Spearman rs]). The magnitude of the donor response to each of the 6 HCMV proteins is also illustrated individually for seropositive donors (Fig. 1E to J). Spearman rank correlation tests of the data showed that the relationship between the magnitude of the HCMV protein response and age was not significant, and all Spearman r values for each ORF (indicated on each graph in Fig. 1E to J) were close to zero. To correct for repeated measures, results were considered significant only if P was ≤0.01. These results suggest that there is no obvious change in the size of the response to these HCMV proteins with increasing age.
CD4+ T cells specific for HCMV ORFs expressed during lytic infection predominantly have a Th1 cytokine profile.
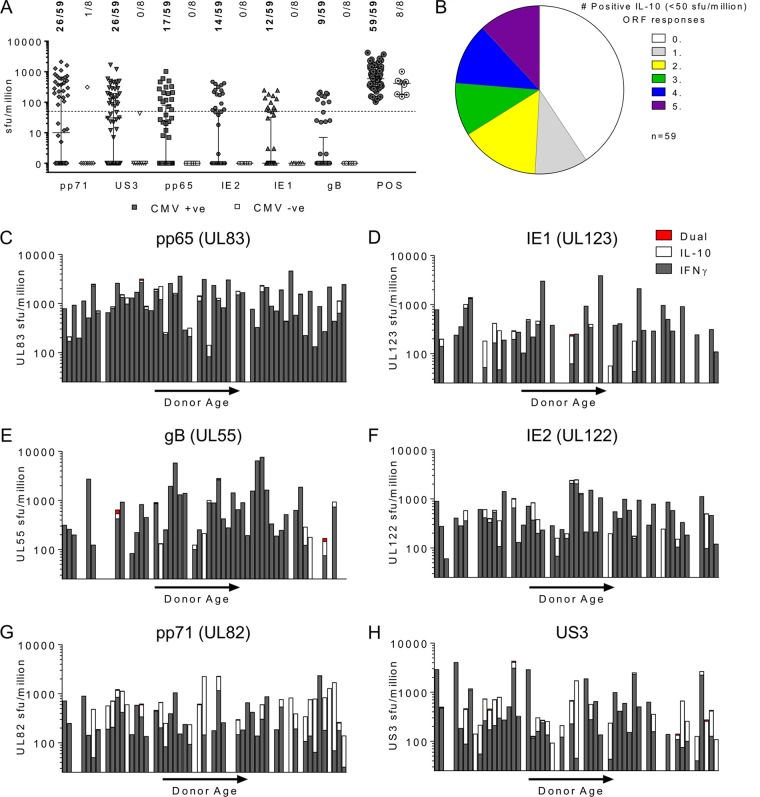
CD4+ T cells can be characterized by the expression of certain transcription factors and the cytokines that they secrete into different T helper cell populations (48). We have previously shown that CD4+ T cells specific to HCMV ORF-encoded proteins UL138 and LUNA have the capacity to secrete the immunomodulatory cytokine IL-10, as well as have distinct UL138- and LUNA-specific CD4+ T cell populations able to secrete IFN-γ, a Th1-defined cytokine (47). Others have identified CMV-specific CD4+ T cells which secrete IL-10, and their experiments suggested that the generation of inducible T regulatory (iTreg) cells specific for HCMV (pp65 and IE ORFs) was related to frequent exposure to the CMV antigen (49). This suggests that an older CMV-seropositive donor may have increased numbers of CD4+ T cells secreting IL-10 following CMV stimulation because the individual was potentially exposed to viral antigens for a longer period of time. We measured the ability of CD4+ T cells from 59 seropositive donors and 8 seronegative donors to secrete IL-10 and/or IFN-γ in response to the 6 HCMV proteins using a dual FluoroSpot method. We assessed the IL-10 responses of the donor cohort to the 6 HCMV proteins analyzed and the positive control alone and used the distribution of the response to the 6 HCMV protein peptide pools and the positive control for the seronegative cohort to derive a positive response threshold for IL-10 responses, which was 50 sfu/million cells (the line shown on the graph in Fig. 2A). The proportion of donors responding above the positive cutoff for each HCMV protein is also shown. The US3 and pp71 proteins produced an IL-10 response for 44.1% of the cohort, 28.8% of the cohort responded to pp65, 25.4% responded to IE2, 20.3% responded to IE1, and 15.3% responded to gB, confirming the reduced number of donors in this cohort with IL-10 responses to the 6 HCMV proteins. The number of proteins that triggered an IL-10 response above the positive threshold for each donor was analyzed, and it was found that 40.7% of the cohort did not make an IL-10 response to any of these 6 HCMV proteins (Fig. 2B). No donor produced an IL-10 response to all 6 proteins, whereas 20.2% of donors produced an IFN-γ response to all 6 proteins analyzed (Fig. 1C).
FIG 2.
The HCMV-specific CD4+ T cell response is predominantly Th1. The frequency of IL-10-secreting CD4+ T cells in response to 6 HCMV proteins and positive-control stimulation in 59 seropositive and 8 seronegative donors is shown. IL-10 secretion was measured using a FluoroSpot method. The results were converted to the number of sfu per million cells, and background counts were subtracted. (A) The response to each protein and the positive control by the entire cohort (both CMV-seropositive donors and CMV-seronegative donors) is summarized. The distribution of the CMV-seronegative donors' responses to each protein and the response to the positive control were used to determine the cutoff for a positive response to the HCMV proteins of 50 sfu/million cells (dashed line). The proportion of donors responding to each protein and the positive control at a level above the threshold is indicated (range, 44% seropositive donors responding to pp71 and US3 stimulation to 15% responding to gB stimulation). (B) The frequency with which seropositive donors produced a positive response to none, 1, or 1 or more of the 6 proteins is summarized. The frequency of CD4+ T cells that secreted IFN-γ or IL-10 in response to the 6 HCMV proteins was measured simultaneously using a dual IFN-γ and IL-10 FluoroSpot assay. (C to H) The response of the 59 donors to 6 HCMV proteins, pp65 (UL83) (C), IE1 (UL123) (D), gB (UL55) (E), IE2 (UL122) (F), pp71 (UL82) (G), and US3 (H), is summarized. The results are arranged along the x axis by age (donor age range, 23 to 74 years). In the graphs for each HCMV protein, only the results for donors with positive responses above the threshold for either IFN-γ (>100 sfu/million cells) or IL-10 (>50 sfu/million cells) are shown; the results for donors with cells secreting both IFN-γ and IL-10 are also indicated when present. No significant change in the magnitude of the IFN-γ response with donor age was measured using the Spearman rank correlation. There was a significant decrease in the number of IE1-specific cells secreting IL-10 with donor age (Spearman rs = −0.4185 [confidence interval = −0.6595, −0.0994], P = 0.01; n = 37), there was no significant change in the levels of IL-10 secretion with donor age for the other 5 proteins.
The FluoroSpot technology used allowed the simultaneous assessment of the IFN-γ and IL-10 responses to each HCMV protein, enabling the contribution of IFN-γ and IL-10 secretion to the overall response for each donor to be assessed. The data are summarized in Fig. 2C to H for the entire seropositive cohort. Only the results for donors with responses above the positive threshold cutoff for either IFN-γ or IL-10 are shown. For each donor, the size of the IFN-γ (gray bars) and IL-10 (white bars) response (in number of sfu per million cells) is shown in order of increasing donor age along the x axis. The rarely observed cell population secreting both IFN-γ and IL-10 is indicated by a red bar. The data show that, overall, the majority of the T cells produced IFN-γ and that for pp65, IE1, gB, and IE2, the IL-10 responses were limited. The combined IFN-γ and IL-10 results for pp71 and US3 clearly show a greater IL-10 response for each donor, with no impact on the frequency with increasing donor age being seen; however, the IFN-γ response still predominated for the majority of the donors analyzed. Spearman rank correlation analysis of the correlation between age and the magnitude of the IL-10 response to the 6 HCMV ORFs for the donor cohort revealed that there was a significant decline in the level of IL-10 production in response to IE1 stimulation (Spearman rs = −0.4185, P = 0.01). The IL-10 response to the other 5 ORFs did not reveal any significant changes in the magnitude of the response with increasing donor age.
HCMV-specific CD4+ T cells have a predominantly effector memory cell phenotype and cytotoxic capacity.
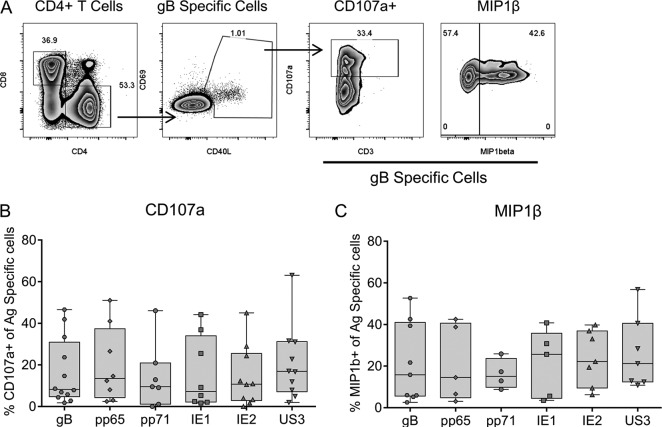
We and others have previously reported that CMV-specific CD4+ T cells can have direct effector functions through both their cytotoxic capacity and their secretion of inflammatory cytokines (16, 24, 26, 30, 47). To examine the functional capacity of CD4+ T cells specific to the 6 different HCMV proteins studied here, we determined the expression of CD107a, a well-defined marker of degranulation that is indicative of the cytotoxic capacity in CD4+ T cells (30, 50), and the secretion of the proinflammatory chemokine macrophage inflammatory protein 1β (MIP-1β), which has been shown to be secreted by CD4+ T cells (30) in response to stimulation by HCMV protein peptide pools. CMV-specific CD4+ T cells were identified to be CD40L+ and CD69high by their level of expression above the background level of expression observed in the unstimulated control (51), and the proportion of CD107a- or MIP-1β-positive cells was measured. The results of a representative analysis of the gB-specific CD4+ T cell response are shown in Fig. 3A. The results of CD107a expression by CMV-specific CD4+ T cells for 12 donors (Fig. 3B) and MIP-1β secretion by CMV-specific CD4+ T cells for 9 donors (Fig. 3C) are summarized for each of the 6 HCMV ORFs. CD4+ T cells stimulated directly ex vivo are capable of degranulation, as shown by the proportion of antigen-specific CD107a-expressing cells present (Fig. 3B). The ability of gB-specific CD4+ T cells to produce a cytotoxic function has previously been established (16, 47, 52); however, this analysis indicates that CD4+ T cells specific for the other 5 proteins also possess this capacity. CMV-specific CD4+ T cells from all the donors examined expressed a CD107a response to at least 1 of the 6 HCMV proteins. A proportion of CD4+ T cells specific to all 6 HCMV proteins studied was also able to secrete MIP-1β following stimulation, extending previous reports of this phenomenon in pp65-specific CD4+ T cells (30).
FIG 3.
A portion of the HCMV-specific CD4+ T cells has a cytotoxic capacity and can secrete MIP-1β. PBMCs were stimulated overnight with HCMV peptide pools in the presence of an anti-CD107a antibody, brefeldin A, and monensin to measure the degranulation and production of MIP-1β. (A) HCMV-specific CD4+ T cell responses were identified as described in Materials and Methods; antigen-specific CD4+ populations were identified as CD40L+ and CD69high by their level of expression above the background level of expression observed in the unstimulated control, and the proportion of antigen-specific CD4+ T cells upregulating CD107a or producing MIP-1β was measured (a representative example of the response to gB is shown). (B and C) The results from all the donors examined are summarized for CD107a expression (n = 12) and MIP-1β production (n = 9). There were no significant differences in the proportion of CMV protein-specific CD4+ T cells upregulating CD107a or producing MIP-1β (Kruskal-Wallis 1-way ANOVA with Dunn's post hoc multiple-comparison test).
In previous studies, the memory cell phenotype of CMV-specific CD4+ T cells has been shown to be a differentiated memory cell phenotype, characterized by the downregulation of the costimulatory molecule CD27 (21, 24), reexpression of CD45RA (11, 21, 24), and the loss of CD28 expression and expression of CD57 (20, 22, 27, 30). To assess whether the memory T cell phenotype differs between the 6 HCMV protein-specific CD4+ T cell populations, the proportion of antigen-specific cells with 1 of 4 memory cell populations, defined by the expression of CD27 and CD45RA molecules, and the proportion that lost the expression of CD28 and that had upregulated CD57 expression were measured.
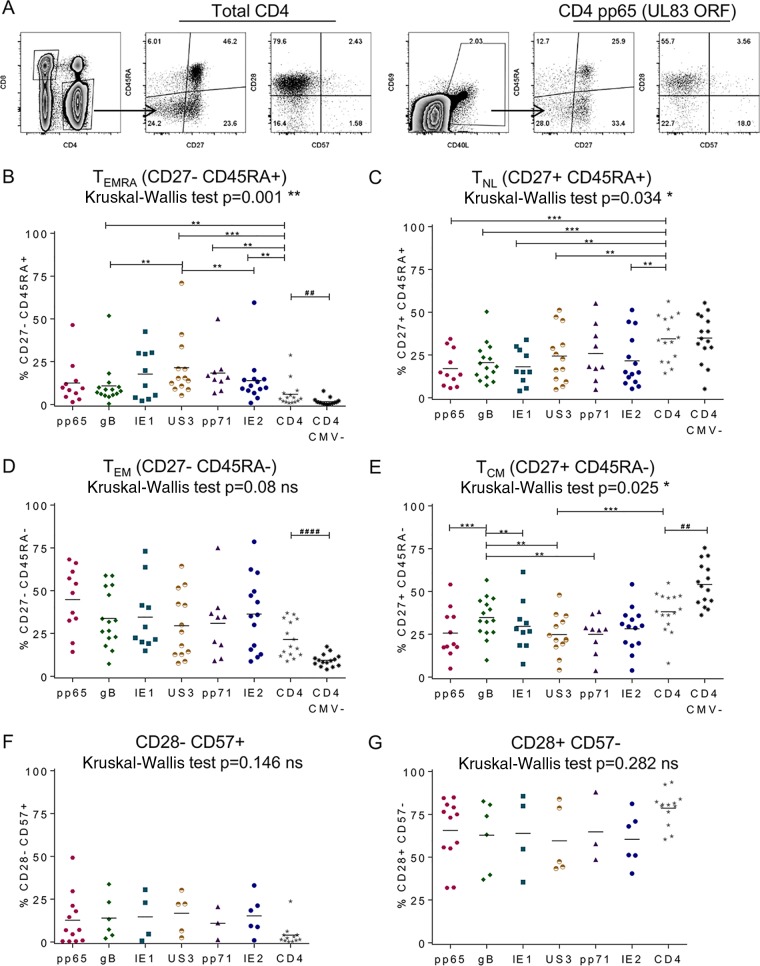
Antigen-specific CD4+ T cells were again identified by the upregulation of CD69 and CD40L (51). An example of the phenotype of the total CD4+ T cell population and an example of the HCMV-specific population from one donor are shown in Fig. 4A. The proportion of HCMV protein-specific CD4+ T cells with the different memory cell populations are compared in summary graphs for CD27− CD45RA+ effector memory CD45RA-expressing (EMRA) T cells (TEMRA) (Fig. 4B), CD27+ CD45RA+ naive-like (NL) T cells (TNL) (Fig. 4C), CD27− CD45RA− effector memory (EM) T cells (TEM) (Fig. 4D), CD27+ CD45RA− central memory (CM) T cells (TCM) (Fig. 4E), CD28− CD57+ highly differentiated memory cells (Fig. 4F), and CD28+ CD57− less differentiated memory cells (Fig. 4G). Additionally, the proportion of total CD4+ T cells from age-matched CMV-seronegative donors for the 4 memory cell populations defined by CD27 and CD45RA expression is also shown (Fig. 4B to E). The comparison of the total CD4+ populations for each of the 4 memory cell populations from CMV-seronegative and CMV-seropositive donors revealed that CMV-seropositive donors have significantly more differentiated CD4+ TCM (Fig. 4E), TEM (Fig. 4D), and TEMRA (Fig. 4B) (significant results by the Mann-Whitney U test [P < 0.01 and P < 0.0001] are shown). This confirms previous observations of the impact of CMV infection on the phenotype of CD4+ T cells (summarized in reference 37). The distribution of the CMV-seropositive donor CD4+ T cell responses to the 6 HCMV proteins and total CD4+ T cells for the 6 different memory cell phenotypes was analyzed using a nonparametric Kruskal-Wallis 1-way analysis of variance (ANOVA) test (results are shown Fig. 4B to G). Where there was a significant variance, a Wilcoxon signed-rank test was used as a posttest to compare pairwise the proportion of the antigen-specific population expressing each phenotype with the total CD4+ population and the population specific for the other HCMV proteins for each donor; significant differences with P values of ≤0.01 (to account for repeated measures) between the populations are indicated on the graphs in Fig. 4B to G. The analysis of the memory cell population phenotypes showed that CMV protein-specific CD4+ T cells have a significant decrease in the less differentiated memory cell phenotypes compared to the total CD4+ population (Fig. 4C and E). There was a corresponding significant enrichment in the differentiated effector memory cell subpopulations for all 6 HCMV protein-specific populations (Fig. 4B). Comparison of the proportion of CMV-specific CD4+ T cells exhibiting different memory cell phenotypes revealed that US3-specific CD4+ T cells had a significantly greater proportion of TEMRA than gB- and IE2-specific CD4+ T cells (Fig. 4B); gB-specific CD4+ T cells were more enriched in the TCM population than T cells specific for 4 of the other 5 HCMV proteins analyzed (Fig. 4E). There were no significant changes in the proportion of CMV-specific CD4+ T cells with highly differentiated (Fig. 4F) or less differentiated (Fig. 4G) memory cell populations, although the antigen-specific populations were enriched for the highly differentiated population compared to the total CD4 T cell population.
FIG 4.
HCMV-specific CD4+ T cells have a predominantly effector memory cell phenotype and are not highly differentiated. PBMCs were stimulated overnight with HCMV protein peptide pools in the presence of brefeldin A. HCMV-specific CD4+ T cell responses were identified to be CD40L and CD69 by their level of expression above the background level of expression observed in the unstimulated control, and 4 subsets of memory cell phenotypes were defined according to the expression of CD27 and CD45RA: CD27+ CD45RA+ naive-like (NL) T cells (TNL), CD27+ CD45RA− central memory (CM) T cells (TCM), CD27− CD45RA− effector memory (EM) T cells (TEM), and CD27− CD45RA+ effector memory CD45RA-expressing (EMRA) T cells (TEMRA). The proportions of 2 memory cell differentiation phenotype populations defined according to the expression of CD57 and CD28, less differentiated (CD28+ CD57−) and highly differentiated (CD28− CD57+), were also measured. (A) A representative example illustrating the expression of these six phenotypes by total CD4+ and pp65-specific T cells is shown. (B to E) The results are summarized for each phenotype population of interest. The responses to 6 HCMV proteins by the total CD4+ T cell population for 15 CMV-seropositive donors and the total CD4+ T cell population for 15 age-matched CMV-seronegative donors were compared for the following populations: TEMRA (B), TNL (C), TEM (D), and TCM (E). (F and G) The responses to 6 HCMV proteins by the total T cell population for 15 CMV-seropositive donors only are summarized for the CD28− CD57+ (F) and CD28+ CD57− (G) populations. A nonparametric Kruskal-Wallis 1-way ANOVA test was performed for the CMV-seropositive donors for each memory cell population (the P values are indicated on each graph). Where significant variation was observed, a Wilcoxon signed-rank posttest was performed to compare the different proportions of CMV-specific CD4+ T cells to total CD4+ T cells and each of the other CMV-specific protein populations. For each comparison, results with significant differences are indicated on the appropriate graph (**, P < 0.01; ***, P < 0.001). Lastly, the total CD4+ T cell CD27 and CD45RA defined memory cell populations for CMV-seropositive donors were compared to those for CMV-seronegative donors using a Mann-Whitney U test; significant results are indicated on the appropriate graph (##, P < 0.01; ####, P < 0.0001).
HCMV-specific CD4+ T cells produce polyfunctional responses to virus-infected cells.
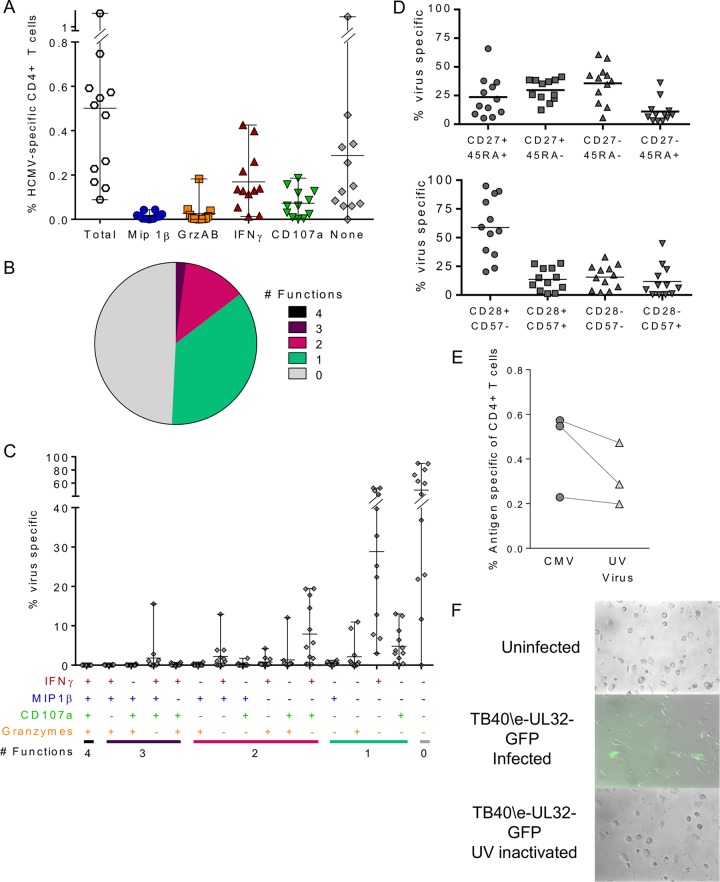
The use of a CMV lysate or overlapping peptide pools to characterize the CD4+ T cell responses to CMV does not allow the effect of CMV genome-encoded immune evasion molecules during infection, particularly the downregulation of MHC class II expression, on antigen-presenting cells to be determined (53). To assess the CD4+ T cell response in the presence of virus genome-encoded immune evasion molecules, we used in vitro infection of autologous dendritic cells with a clinical isolate of CMV. Autologous dendritic cells derived from individual donor monocytes (moDCs) were infected with HCMV strain TB40/E whose UL32 protein was tagged with green fluorescent protein (GFP) (TB40/E-UL32-GFP). The CMV-infected moDCs were incubated for 7 days prior to coincubation with autologous CD4+ T cells overnight and measurement of the functional responses in the responding CD4+ T cells by flow cytometry. In total, the functional responses of cells from 12 donors to virus-infected moDCs were analyzed. The size of the HCMV-specific response (identified by the coupregulation of the activation markers CD40L and 4-1BB) above the background response by the CD4+ T cells and the fraction of those cells producing each of 4 individual functional markers (MIP-1β, granzymes A and B, IFN-γ, and CD107a) or no marker were compared (Fig. 5A). The breakdown of the antigen-specific response equated to 49.2% ± 8.9% (standard error of the mean [SEM]) of the responding CD4+ T cells not producing any of these effector markers. Of the remaining antigen-specific CD4+ T cells, 41.7% ± 8.6% (SEM) produced IFN-γ and 16.3% ± 4.0% (SEM) expressed CD107a, with a minority of virus-specific CD4+ T cells producing MIP-1β (4.9% ± 2.4% [SEM]) or granzymes A and B (4.6% ± 2.1% [SEM]). The polyfunctionality of the virus-specific CD4+ T cell response in 12 donors was assessed, and the mean proportion of virus-specific cells producing one or more functional responses was compared (Fig. 5B); 14.6% of responding cells produced two, three, or four functional responses, and a further 36.2% produced one functional response. The proportion of virus-specific CD4+ T cells responding within the 16 different categories created by combinations of the four functional response markers for all 12 donors is shown in Fig. 5C. This breakdown analysis of the HCMV-specific CD4+ T cells that produce one or more functional responses (comprising 50.8% of the activated cells response) confirms the dominance of IFN-γ production alone (28.8% ± 5.3% [SEM]) and in combination with CD107a expression (7.9% ± 2.3% [SEM]). The other notable populations were the population expressing CD107a only (4.8% ± 1.3% [SEM]), the population producing IFN-γ and MIP-1β (2.2% ± 1.1% [SEM]), the population producing granzymes A and B only (2.1% ± 1.1% [SEM]), and the population expressing IFN-γ, MIP-1β, and CD107a (1.8% ± 1.2% [SEM]). Phenotype analysis of the virus-specific CD4+ T cells revealed an undifferentiated (CD28+ CD57−; 58.9% ± 7.5% [SEM]) effector memory cell (CD27− CD45RA−) population (35.6% ± 2.9% [SEM]) (Fig. 5D) similar to that seen in peptide-stimulated cells (Fig. 4).
FIG 5.
The HCMV-specific CD4+ T cell response to HCMV-infected cells is polyfunctional. (A) moDCs from each donor were prepared and then mock or lytically infected with HCMV strain TB40/E-UL32-GFP at an MOI of 0.1 for 7 days. Autologous CD4+ T cells were incubated overnight with either uninfected, HCMV-infected, or UV-irradiated HCMV-infected moDCs in the presence of an anti-CD107a antibody, monensin, and brefeldin A. CD4+ T cells were then stained with a polyfunctional flow cytometry antibody panel, acquired, and analyzed. Virus-specific CD4+ T cells were identified by the upregulation of CD40L and 4-1BB above the background response. The total specific response to CMV and the proportion of the specific response, composed of MIP-1β, granzyme A and B (GrzAB), CD107a, and IFN-γ production or the production of no functional marker, in 12 donors are shown. (B) The mean proportion of virus-specific CD4+ T cells from all donors generating polyfunctional responses is summarized as a pie chart indicating the proportion of HCMV-specific CD4+ T cells producing 4, 3, 2, 1, or no functions. (C) The composition of the HCMV-specific CD4+ T cell response as a proportion of the antigen-specific population for all donors is illustrated. (D) The proportion of virus-specific cells expressing different memory cell phenotype markers (CD27, CD45RA, CD28, and CD57) is shown. (E) A direct comparison of the size of the specific T cell response to live virus (CMV) versus UV-inactivated virus (UV Virus) in 3 donors is shown. (F) A representative example of UL32 (late gene)-tagged GFP expression in moDCs infected with live virus versus UV-inactivated virus indicating GFP expression in live virus-infected moDCs only.
For 3 donors, we compared the CD4+ T cell response to TB40/E-UL32-GFP-infected cells with that of moDCs infected with a UV-inactivated virus at an identical multiplicity of infection (MOI). The size of the corrected antigen-specific response measured by upregulation of CD40L and 4-1BB is shown for each donor in Fig. 5E. This comparison shows that for all three donors, the response to live virus was greater than that to the UV-inactivated virus. There was, however, a notable CD4+ T cell response to cells treated with UV-inactivated virus, suggesting that inactive viral particle proteins were still being presented by the moDCs 7 days after the initial infection. We confirmed that UV treatment of the virus caused the virus to be inactive by fluorescence microscopy analysis of GFP expression in the moDCs (an example of the results, those for donor CMV320, is shown in Fig. 5F), which clearly shows that the late gene UL32, which was tagged with GFP in the HCMV strain used for these studies, is expressed only in the infected cells at day 7 postinfection and is not observed in the UV-inactivated virus-treated moDCs.
HCMV-specific CD4+ T cells control the dissemination of virus in vitro.
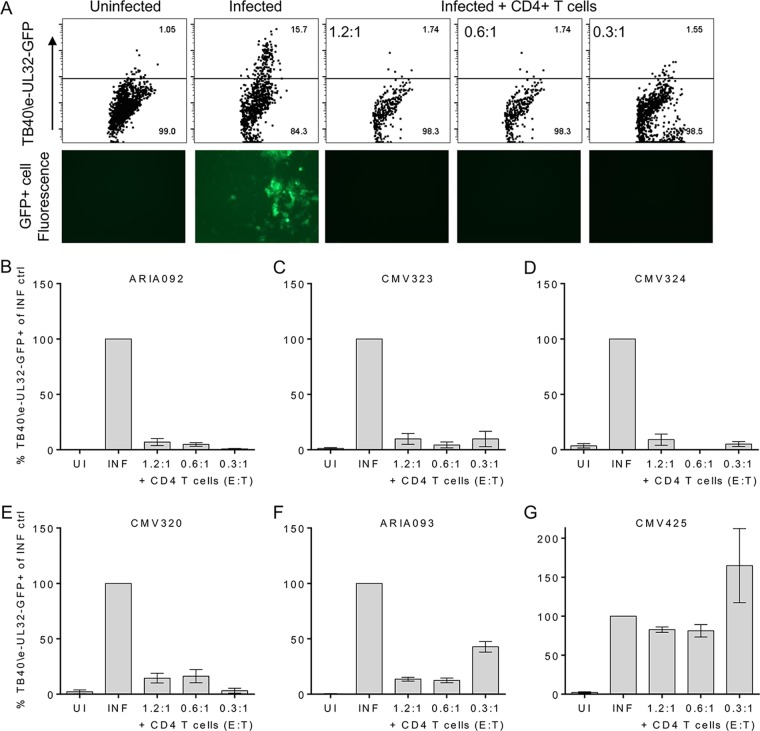
The evidence that live cytomegalovirus infection was able to stimulate functional CD4+ T cell responses to a greater extent that UV-inactivated virus led to an investigation of whether these CMV-specific CD4+ T cells could directly target an active lytic infection. We have previously established a viral dissemination assay for CD8+ T cells (54) using autologous fibroblasts infected with the TB40/E-UL32-GFP strain at a low MOI to measure the ability of the CD8+ T cell subset to abrogate viral spread. To be able to interrogate the role of CMV-specific CD4+ T cells in the same way required an adaptation of this experimental model because fibroblasts do not constitutively express MHC class II molecules (55). Autologous in vitro-differentiated moDCs were chosen, as these cells both constitutively express MHC class II (55) and are permissive for lytic CMV infection (56). Dendritic cells derived from each donor were infected with TB40/E-UL32-GFP at a low MOI. After 7 days of culture, CD4+ T cells isolated directly ex vivo were added at effector cell (CD4+ T cells)-to-target cell (moDCs) (E/T) ratios of 1.2:1, 0.6:1, and 0.3:1 in triplicate. The CD4+ T cells were coincubated with the infected moDCs for a further 7 days. Indicator fibroblasts were then added to be infected by virus released from any remaining infected dendritic cells, and the cells were coincubated for 14 to 21 days prior to analysis by flow cytometry for GFP-expressing (virus-infected) fibroblasts.
We measured viral dissemination in 5 HCMV-seropositive donors and 1 HCMV-seronegative donor as a control. The dissemination of TB40/E-UL32-GFP to fibroblast cells was assessed by fluorescence microscopy and flow cytometry. An example of the results from one donor, donor CMV320, obtained following 14 days of incubation with indicator fibroblasts is illustrated in Fig. 6A. The results clearly show a lack of GFP expression in the uninfected wells and the wells treated with CD4+ T cells at all 3 E/T ratios, whereas GFP was expressed in control wells that were only infected with HCMV. The results from the flow cytometry analysis for 5 seropositive donors (Fig. 6B to F, respectively) and 1 seronegative donor (Fig. 6G) are summarized in Fig. 6. The data for each treatment were corrected for the background response and then expressed as a proportion of the data for the infected control (which was therefore set at 100% for all 6 donors). The graphs from all 5 HCMV-seropositive donors show that the addition of CD4+ T cells stopped the dissemination of HCMV into the fibroblast layer. The results for HCMV-seronegative donor CMV425 (Fig. 6G) clearly demonstrate that this is the action of CMV-specific CD4+ T cells, as the proportion of fibroblasts infected with the GFP-tagged virus was observed to be at levels similar to those for the infected control. The evidence from this functional assay, together with the polyfunctional responses described in Fig. 5, clearly shows that resting HCMV-specific CD4+ T cells isolated directly ex vivo have direct antiviral activity producing inflammatory cytokines and cytotoxic responses which enable these cells to prevent viral dissemination.
FIG 6.
HCMV-specific CD4+ T cells are able to prevent the dissemination of virus in vitro. moDCs from each donor were prepared and then mock or lytically infected with HCMV strain TB40/E-UL32-GFP at an MOI of 0.007 for 7 days. CD4+ T cells were coincubated with the infected moDCs with different E/T ratios for a further 7 days. Indicator fibroblasts were then added to the CD4+ T cells and infected moDCs after they had been coincubated for up to 28 days, and then the percentage of fibroblasts expressing GFP-tagged virus was measured by flow cytometry. (A) Representative dot plots showing GFP expression from one well of triplicate wells (top) and corresponding fluorescence microscope images (bottom) are shown. (B to G) The bar charts for 5 HCMV-seropositive donors (B to F) and 1 HCMV-seronegative donor (G) summarize the percentage of TB40/E-UL32-GFP-expressing fibroblasts corrected for the background response and as a percentage of the infected-only control. ctrl, control; UI uninfected; INF, infected.
DISCUSSION
Studies have implicated CMV to be associated with an increased risk of all-cause mortality (37) and to cause detrimental changes to the immune response (27–29) in older people. The paradox with the association of CMV seropositivity with the loss of immune function in older people is that overt CMV disease as a result of reactivation or new infections is not observed; however, there is an increase in detectable virus in urine in older people (44). This strongly suggests that the immune response to HCMV itself retains sufficient functionality within the older immunocompetent population but that immunomodulation as a consequence of lifelong carriage of HCMV may alter the immune response (57). Secretion of the immunomodulatory cytokine IL-10 (58) by CMV-specific CD4+ T cells is a candidate for mediating the immunomodulation of the CMV-specific T cell response during ageing. We have previously identified populations of CD4+ T cells specific for the HCMV proteins UL138 and LUNA, which secrete IL-10 (47). Others have observed secretion of IL-10 by CMV-specific CD4+ T cells in response to stimulation by pp65 and IE1, and they demonstrated that frequent exposure to CMV antigens drove the generation of an iTreg CD4+ T cell population specific to HCMV (49). We hypothesized that older CMV-seropositive donors may have increased numbers of CD4+ T cells secreting IL-10 following CMV antigen stimulation due to longer periods of exposure to viral antigens and that this subset of CMV-specific CD4+ T cells may inhibit efficient recognition of the virus by IFN-γ-secreting CMV-specific CD4+ T cells.
We did not see any influence of donor age on the magnitude of the total CMV-specific CD4+ T cell IFN-γ or IL-10 response to gB, pp65, pp71, and IE2 stimulation. There was a significant decline in the IE1 IL-10-specific CD4+ T cell response in older donors, but for each donor, the relationship of the magnitude of the total IFN-γ and IL-10 response to all 6 proteins was not affected by donor age. We have therefore demonstrated that a proportion of CD4+ T cells specific to the 6 different HCMV proteins examined here produced IL-10 but that this response was limited overall compared to the IFN-γ response observed. However, we did observe that some donors (approximately 1 in 6) within the cohort had an IL-10 response to 3 or more of the CMV protein responses examined that was equal to the IFN-γ response or that occurred at a higher frequency (number of sfu per million cells) than the IFN-γ response. Overall, in this study there was no alteration in the balance of IL-10 and IFN-γ secretion with increasing donor age and putative increased lengths of viral carriage and exposure to viral antigens. It would be interesting to examine whether donors in a suitably sized cohort with an IL-10 bias in the CD4+ T cell response to CMV antigens differ in other aspects of their CMV immune response, such as CMV IgG titers or viral carriage.
The observations from this cohort regarding the impact of donor age on CMV-specific CD4+ T cell responses are in contrast to those from some other studies which have shown an accumulation of IFN-γ-secreting CD4+ T cells in older donors (19, 21, 22). These studies used the responses obtained by the use of viral lysate to stimulate HCMV-specific CD4+ T cells rather than the responses to particular HCMV proteins and intracellular flow cytometry to measure the IFN-γ response. The current study used FluoroSpot assays to measure IFN-γ production by CD4+ T cells; FluoroSpot is an enzyme-linked immunosorbent spot (ELISPOT) assay which enables measurement of multiple cytokines simultaneously (59). Experience from our own studies and other groups suggests that the ELISPOT assay is more sensitive than other methods for detecting T cell responses to HCMV antigens (60), so the contrasting observations obtained in this study compared to those obtained in previously published work are not explained by the use of different techniques to measure IFN-γ responses. The studies demonstrating an increased frequency of CMV-specific CD4+ T cells in older donors (19, 21, 22) did not investigate the absolute size of the T cell compartment in peripheral blood. This is important, as the increased percentage measured in these studies may equate to the same numbers of IFN-γ-producing CD4+ T cells only if the size of the total CD4+ T cell compartment decreases, for instance. It is therefore difficult to compare the conclusions from studies where the methods of reporting the results differ between frequency and percentages, particularly when the information required for interpreting the presented percentage data is absent. It has previously been observed that donor cohorts from different geographical locations, e.g., older Sicilians with a 70% rate of CMV seropositivity (61), did not show an accumulation of CMV-specific CD8+ T cells in the older donor group compared to the young donor group. Understanding that HCMV infection does not have the same effect on all older donor cohorts is important when interpreting the results of studies which propose that medical intervention in CMV-seropositive older people is necessary to improve their immune response and promote a healthy ageing phenotype.
It is becoming increasingly clear in both infections with CMV (26, 31, 52) and infections with other viruses, e.g., influenza virus, West Nile virus, rotavirus, and Sendai virus (reviewed in reference 62), that CD4+ T cells can exhibit direct effector functions, including cytotoxicity and the secretion of proinflammatory effector molecules, that help to control or resolve viral infections. We saw that CD4+ T cells specific to all 6 HCMV proteins upregulated the expression of CD107a, a marker of degranulation used as a surrogate indicator of potential cytotoxic activity (30), and produced the proinflammatory chemokine MIP-1β. Our observations on the memory cell phenotype of CMV-specific CD4+ T cells do confirm the findings of previous studies performed using pp65 peptides and viral lysate stimulation, which have described CMV-specific CD4+ T cells as having an effector memory cell phenotype (19, 21, 30). The use of in vitro stimulation of CD4+ T cells with HCMV peptide pools or viral lysate allows the determination of T cell effector functions, but this is in the absence of immune evasion molecules expressed by CMV during its lytic life cycle. Examination of CMV-specific T cell responses in the absence of viral immunomodulation is not representative of the situation during CMV infection or reactivation in the host (57). CMV genome-encoded proteins target many aspects of the immune response, including the evasion of natural killer cell responses, the interferon response, and perturbation of immunomodulatory pathways (reviewed in reference 53). Pertinent to affecting host CD4+ T cell effector responses, the proteins encoded by CMV US3 (63) and US2 (64) interfere with MHC class II presentation at the cell surface. UL82, which encodes the phosphoprotein pp65, can mediate the destruction of HLA-DR molecules (65) and the loss of MHC class II expression by CMV-infected dendritic cells (66, 67). The combined effect of these immune evasion and modulatory proteins in an active infection with CMV or a CMV reactivation event in the host could lead to effector behaviors by CMV-specific CD4+ T cells very different from those observed in response to isolated viral proteins. This problem has been addressed to an extent in the murine model with MCMV infection. A recent study using novel epitope class II-restricted tetramers in vivo observed direct killing of infected cells (68). With respect to HCMV infections, Sinzger et al. have shown that IE1-specific CD4+ T cell clones are able to produce IFN-γ in response to stimulation by TB40/E-infected macrophages which have downregulated MHC class II expression (69).
We used an experimental model of lytic infection in vitro to measure the effector functions of CD4+ T cells isolated directly ex vivo in response to HCMV-infected monocyte-derived dendritic cells. The predominant effector function produced was IFN-γ expression followed by CD107a expression. Low levels of the cytolytic enzymes granzymes A and B and MIP-1β were also detected. The polyfunctional CD4+ T cell responses observed are important, as polyfunctional CD4+ T cells have been shown to be better effector cells (50) and reduced frequencies of polyfunctional CMV-specific CD4+ T cells are associated with the occurrence of congenital CMV infections (70). However, these assays still just measure effector mechanisms and do not give any indication whether the T cells could mediate direct antiviral activity. In order to measure the effectiveness of these CMV-specific CD4+ T cells, we performed a viral dissemination assay using the nonattenuated clinical strain TB40/E-UL32-GFP. We performed the assay with cells from 5 HCMV-seropositive donors and clearly observed that the CD4+ T cells prevented viral dissemination from the virus-infected dendritic cells. By performing the assay with cells from a CMV-seronegative donor, we confirmed that this was a CMV-specific CD4+ T cell effect, as there was no control of viral dissemination in the presence of nonspecific CD4+ T cells. This assay convincingly shows that CD4+ T cells can respond directly to CMV-infected cells probably using both the cytokine expression and cytotoxicity mechanisms observed in the polyfunctional flow cytometry experiments. The ability of CMV-specific CD4+ T cells to control viral dissemination is in spite of the large array of immune evasion mechanisms encoded by the virus genome and confirms the observations made in vivo in the murine model (68) and previously in infected macrophages in in vitro models of HCMV infection (69). Further interrogation of the CMV-specific CD4+ T cell response using this in vitro model will be able to determine whether the cytokines or cytotoxicity produced by CD4+ T cells is more important in resolving CMV infection or reactivation in the host.
In summary, we have shown that the CD4+ T cell response to lytic HCMV antigens and infection is not obviously attenuated in older donors. CD4+ T cells specific to HCMV have a cytotoxic capability and secrete MIP-1β and IFN-γ, which are known to be essential to control viral replication (13, 20, 70) and can control viral dissemination in vitro. Previous studies focusing solely on T cell responses to limited HCMV ORF-encoded proteins or inactive viral lysate in the immune response of older people (71) may have resulted in a perspective on understanding the etiology of the CMV infection and diseases in healthy older people that was too narrow. An understanding of why older donors may reactivate virus more frequently than younger donors (44) will require further study of CD4+ T cell responses in the context of viral infection models. By interrogating the immune response to the entire HCMV proteome expressed during both lytic and latent infection using direct antiviral assays instead of relying on responses to isolated peptide stimulation will help to identify whether the HCMV-specific T cell response is impaired in older or immunocompromised patients. This will also enable the development of effective immunotherapeutic treatments for HCMV infection and widen our knowledge of the functional capacity of CD4+ T cells in response to virus infection.
MATERIALS AND METHODS
Ethics and donor cohort information.
Healthy HCMV-seropositive and HCMV-seronegative donors were recruited locally with ethical approval from the Addenbrookes National Health Service Hospital Trust Institutional Review Board (Cambridge Research Ethics Committee); informed written consent was obtained from all volunteers in accordance with the Declaration of Helsinki (LREC 97/092). Twenty-two HCMV-seropositive donors (5 females, 17 males) aged 23 to 77 years were recruited. A second healthy donor cohort was recruited from the Cambridge National Institute of Health Research (NIHR) BioResource. Ethical approval was obtained from the University of Cambridge Human Biology Research Ethics Committee. Informed written consent was obtained from all donors in accordance with the Declaration of Helsinki (HBREC.2014.07). A cohort of 84 HCMV-seropositive donors (48 females, 36 males) aged 23 to 74 years and 24 seronegative donors (14 females, 10 males) aged 29 to 78 years was included in this study. The CMV serostatus of all donors was confirmed by serological assessment of CMV IgG levels using a Captia CMV IgG enzyme immunoassay (Trinity Biotech, Ireland) following the manufacturer's instructions.
PBMC isolation.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples using Lymphoprep (Axis-Shield; Alere Ltd., Stockport, UK) density gradient centrifugation. PBMCs were either used fresh or frozen in a 10% dimethyl sulfoxide (Sigma-Aldrich, Poole, UK) and 90% fetal bovine serum (FBS; Gibco-Thermo Fisher Scientific, Paisley, UK) solution at a high cell concentration. Frozen PBMCs were resuscitated before use in prewarmed serum-free medium in the presence of 10 U/ml DNase I (Roche Diagnostics Ltd., Burgess Hill, UK) or Benzonase nuclease (Millipore, Watford, UK), followed by a 1-h incubation in warmed serum-free medium with DNase I or Benzonase nuclease at 37°C, and the PBMCs were then further rested in X-Vivo 15 medium (Lonza, Slough, UK) at 37°C before use in subsequent assays.
HCMV ORF peptide mixes.
Ten HCMV ORFs (UL28, UL48, UL55 [gB], UL82 [pp71], UL99, UL122 [IE2], UL123 [IE1], US3, US29, and US32) were selected, and libraries of consecutive 15-mer peptides overlapping by 10 amino acids were synthesized by ProImmune PEPScreen (Oxford, UK) from sequences detailed in the study of Sylwester et al. (17). A UL83 (pp65) ORF 15-mer peptide library was synthesized by JPT Peptide Technologies GmbH (Berlin, Germany). The individual lyophilized peptides from each ORF library were reconstituted and used as previously described (54).
Virus.
HCMV strain TB40/E-UL32-GFP (a gift of Christian Sinzger, Universitätsklinikum Ulm, Institut für Virologie, Germany) was used in this study. The infectious titer of the endothelium-tropic passaged strain was determined using ARPE-19 cells. The number of PFU per milliliter was used to calculate the multiplicity of infection used to infect monocyte-derived dendritic cells. UV treatment of TB40/E-UL32-GFP for 60 min was performed to inactivate the virus stock.
Dual FluoroSpot assays.
PBMCs were depleted of CD8+ T cells by magnetically activated cell sorting (MACS) using anti-CD8+ direct beads (Miltenyi Biotech), according to the manufacturer's instructions, and separated on either LS columns with a VarioMACS stand (Miltenyi Biotech) or by use of an autoMACS Pro separator (Miltenyi Biotech). The efficiency of depletion was determined by staining cells with a CD3-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE), and CD8-peridinin chlorophyll protein (PerCP)-Cy5.5 antibody mix (all from BioLegend) and analyzed by flow cytometry. Depletions performed in this manner resulted in 0.2 to 4.3% residual CD8+ T cells (n = 40). Triplicate wells of 2 × 105 CD8+ T cell-depleted PBMCs suspended in X-Vivo 15 medium supplemented with 5% human type AB serum (Sigma-Aldrich) in precoated FluoroSpot plates (human IFN-γ and IL-10 FluoroSpot plates [Mabtech AB, Nacka Strand, Sweden]) were incubated with a mix of the ORF-encoded peptides (final peptide concentration, 2 μg/ml/peptide) and an unstimulated and positive-control mix containing anti-CD3 antibody (Mabtech AB) as well as Staphylococcus enterotoxin B (SEB), phytohemagglutinin (PHA), pokeweed mitogen (PWM), and lipopolysaccharide (LPS) (all from Sigma-Aldrich) at 37°C in a humidified CO2 atmosphere for 48 h. The cells and medium were decanted from the plate, and the assay was developed following the manufacturer's instructions. The results on the developed plates were read using an AID iSpot reader (Autoimmun Diagnostika [AID] GmbH, Strassberg, Germany) and counted using EliSpot (version 7) software (Autoimmun Diagnostika). The cutoff for a positive response for IFN-γ and IL-10 was determined by comparing the distribution of the responses from HCMV-seropositive and HCMV-seronegative donors to all HCMV ORFs and the positive-control response after correction for the background response. This analysis determined that the positive response for IFN-γ was greater than 100 spot forming units (sfu)/million cells (Fig. 1B) and that the positive response for IL-10 was greater than 50 sfu/million cells (Fig. 2A).
Measurement of degranulation and cytokine secretion and phenotyping of antigen-specific CD4+ T cells.
PBMCs (2.5 × 106) suspended in X-Vivo 15 medium with 5% human type AB serum were stimulated with ORF-encoded peptide mixes in the presence of CD107a-Alexa Fluor 647 (BioLegend), were unstimulated, or were simulated with a positive-control mix (SEB, αCD3, PHA, PWM, and LPS) for 1 h, and then 5 μg/ml brefeldin A and 2 μM monensin (both from BioLegend) were added and the cells were incubated overnight at 37°C in a humidified CO2 atmosphere. The cells were then washed, stained with a combination of surface antibodies, including CD3-Brilliant Violet 650, CD45RA-PE-Cy7, CD27-allophycocyanin (APC)-eFluor 780 (eBioscience), or CD14- and CD19-FITC-dump channel (eBioscience) and LIVE/DEAD fixable yellow dead cell stain (Invitrogen), at 4°C. The cells were fixed, permeabilized using a Fix&Perm cell fixation and permeabilization kit (Nordic-MuBio, Susteren, Holland), and stained intracellularly with CD69-Pacific Blue, 4-1BB–PE–Cy5, CD8-Alexa Fluor 700, CD4-PE-Dazzle (BioLegend), CD40L-PerCP-eFluor 710 (eBioscience), and MIP-1β–PE (BD Biosciences) at 4°C in the dark. Samples were washed and fixed in a paraformaldehyde (PFA) solution (final PFA concentration, 1%) and acquired on a BD LSR Fortessa cytometer using FACSDiva software. The data were analyzed using FlowJo software, and antigen-specific CD4+ T cell populations were identified to be CD40L+ and CD69high by their level of expression above the background level of expression observed in the unstimulated control following the elimination of doublets and the removal of monocytes, B cells, and dead cells from the analyzed population (an example of CD40L+ and CD69high expression is shown in Fig. 3A). CD69 expression was lower due to incubation overnight as opposed to incubation for 6 h, which is why a criterion of CD69high expression was used to identify the activated cell populations. The level of CD107a staining was set on the basis of the level of expression measured in activated CD8+ T cells compared to that measured in unstimulated cells for each donor. The positive-control sample was used to verify the expression of the activation markers CD107a and MIP-1β for each donor.
Measurement of polyfunctional T cell responses to HCMV-infected dendritic cells.
Monocytes were isolated from donor PBMCs by MACS using anti-CD14+ direct beads (Miltenyi Biotech) according to the manufacturer's instructions and separated on LS columns with a VarioMACS system. Purified monocytes were allowed to adhere to a 48-well tissue culture plate at a density of 0.3 × 106 cells per well and then incubated in X-Vivo 15 medium supplemented with 2.5 mM l-glutamine (Sigma-Aldrich) and with 1,000 IU/ml IL-4 and 1,000 IU/ml granulocyte-macrophage colony-stimulating factor (Miltenyi Biotec) for 6 days at 37°C in a humidified CO2 atmosphere. The differentiated monocytes were matured by exchanging the medium for X-Vivo 15 medium supplemented with 2.5 mM l-glutamine and 50 ng/ml LPS for 24 h. The dendritic cells (moDCs) were then infected with HCMV strain TB40/E-UL32-GFP at an MOI of 0.1 or the equivalent amount of UV-inactivated virus for 3 h in l-glutamine-supplemented X-Vivo 15 medium. The medium was then replaced, and the infected cells were incubated in fresh supplemented X-Vivo 15 medium for 7 days at 37°C in a humidified CO2 atmosphere. Infection was confirmed by the observation of GFP expression in dendritic cells by fluorescence microscopy but the lack of GFP expression in mock-infected and UV-inactivated virus-treated cells and by quantitative reverse transcription-PCR. CD4+ T cells were purified from defrosted autologous PBMCs by MACS using anti-CD4+ beads following the manufacturer's instructions using LS columns and a VarioMACS system. CD4+ T cells (0.5 × 106) suspended in X-Vivo 15 medium supplemented with l-glutamine were added to each well of uninfected, infected, UV-inactivated virus-infected, and positive-control mix-pulsed and pp65 and gB protein-pulsed moDCs in the presence of CD107a-Alexa Fluor 647 (BioLegend) for 1 h. Then, 5 μg/ml brefeldin A and 2 μM monensin (both from BioLegend) were added and the cells were incubated overnight at 37°C in a humidified CO2 atmosphere. The cells were harvested, washed, and stained with a combination of surface antibodies, CD45RA-PE-Cy7, CD27-APC-eFluor 780 (eBioscience), CD3-Brilliant Violet 650, CD57-PE-Dazzle, CD28-Alexa Fluor 700, CD14- and CD19-Brilliant Violet 510 (BioLegend), or LIVE/DEAD Fixable Aqua Dead cell stain (Invitrogen)-dump channel at 4°C. Cells were fixed and permeabilized using a Fix&Perm cell fixation and permeabilization kit and stained intracellularly with CD69-Pacific Blue, 4-1BB–PE–Cy5, CD8-Brilliant Violet 570, CD4-Brilliant Violet 605, granzyme A-FITC (BioLegend), granzyme B-FITC (Miltenyi Biotec), CD40L-PerCP-eFluor 710 (eBioscience), IFN-γ–Brilliant Violet 786, and MIP-1β–PE (BD Biosciences) at 4°C in the dark. Samples were washed and fixed in a final 1% paraformaldehyde solution and acquired on a BD LSR Fortessa cytometer using FACSDiva software. Data were analyzed using FlowJo software, and CD4+ T cells were identified following elimination of doublets and removal of monocytes, B cells, and dead cells from the analyzed population. Antigen-specific CD4+ T cell populations were identified by the expression of CD40L and 4-1BB above the background level of expression observed in the unstimulated sample. The percentage of antigen-specific CD4+ T cells producing combinations of the functional markers CD107a, granzymes A and B, IFN-γ, and MIP-1β above background levels was identified (gating of populations was determined by the use of fluorescence minus one samples and the unstimulated control).
Measurement of viral dissemination control by CD4+ T cells.
Monocytes were isolated, differentiated, and matured to moDCs in 96-well plates at a density of 0.1 × 106 cells per well, as described in the previous paragraph. The moDCs were then infected with HCMV strain TB40/E-UL32-GFP at an MOI of 0.007 or the equivalent amount of UV-inactivated virus for 3 h in l-glutamine-supplemented X-Vivo 15 medium. The medium was then replaced and the infected cells were incubated in fresh supplemented X-Vivo 15 medium for 7 days at 37°C in a humidified CO2 atmosphere. Infection was confirmed by observation of GFP expression in moDCs by fluorescence microscopy compared to the lack of observation of GFP expression in mock-infected and UV-inactivated virus-treated cells. Autologous CD4+ T cells were purified from frozen PBMCs as described above and then added to the wells with infected moDCs at different E/T ratios (1.2:1, 0.6:1, and 0.3:1) in supplemented X-Vivo 15 medium. The CD4+ T cells were coincubated with infected moDCs, and after 7 days, indicator dermal fibroblasts were added to the moDC monolayer following removal of the well supernatant and nonadherent cells. The fibroblast coculture was maintained in Dulbecco modified Eagle medium (Gibco) supplemented with 20% FBS (Gibco) for up to 21 days at 37°C in a humidified CO2 atmosphere. The spread of TB40/E-UL32-GFP to fibroblasts after 21 days was measured by flow cytometry acquisition on a BD Accuri C6 flow cytometer following fibroblast harvest with trypsin and fixation with 2% PFA.
Statistics.
Statistical analysis was performed using GraphPad Prism software (version 6.00 for Windows; GraphPad Software, San Diego, CA, USA). The correlation between age and the T cell response to CMV was assessed by use of the Spearman rank correlation for nonnormally distributed data. The results of analyses of CD107a, MIP-1β, and the populations with the different peptide-specific memory cell phenotypes were compared using a 1-way ANOVA Kruskal-Wallis test with Dunn's post hoc multiple-comparison test and the Wilcoxon signed-rank test. In the case of repeated analyses of the same donor cohort, results were considered significant only if P was ≤0.01.
ACKNOWLEDGMENTS
We gratefully acknowledge the participation of all NIHR Cambridge BioResource volunteers, and thank the NIHR Cambridge BioResource Centre and staff for their contribution. We thank the National Institute for Health Research and NHS Blood and Transplant. Further information can be found at www.cambridgebioresource.org.uk.
This research was supported by the Cambridge NIHR BRC Cell Phenotyping Hub. This work was funded by U.K. Medical Research Council (MRC) grants G0701279 and MR/K021087/1 and an MRC Sackler Ph.D. award to G.X.S.
REFERENCES
- 1.Gandhi MK, Khanna R. 2004. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair J, Sissons P. 2006. Latency and reactivation of human cytomegalovirus. J Gen Virol 87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 3.Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson S, Mason G, Wills M. 2011. Human cytomegalovirus immunity and immune evasion. Virus Res 157:151–160. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Benedict CA, Crozat K, Degli-Esposti M, Dalod M. 2013. Host genetic models in cytomegalovirus immunology, p 259–285. In Reddehase MJ. (ed), Cytomegaloviruses: from molecular pathogenesis to intervention, vol II Caister Academic Press, Poole, United Kingdom. [Google Scholar]
- 6.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916–3922. doi: 10.1182/blood.V99.11.3916. [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. 2003. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 8.Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, Löwenberg B, Cornelissen JJ. 2008. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom 74:211–220. doi: 10.1002/cyto.b.20420. [DOI] [PubMed] [Google Scholar]
- 9.Sester M, Sester U, Gartner B, Heine G, Girndt M, Mueller-Lantzsch N, Meyerhans A, Kohler H. 2001. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 10.Gamadia LE, Remmerswaal EBM, Weel JF, Bemelman F, van Lier RAW, ten Berge IJM. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 11.Lilleri D, Fornara C, Revello MG, Gerna G. 2008. Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J Infect Dis 198:536–543. doi: 10.1086/590118. [DOI] [PubMed] [Google Scholar]
- 12.Rentenaar RJ, Gamadia LE, van der Hoek N, van Diepen FNJ, Boom R, Weel JFL, Wertheim-van Dillen PME, van Lier RAW, ten Berge IJM. 2000. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J Clin Invest 105:541–548. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, Adler S, Arvin A, Lewis DB. 2004. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol 172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 14.Wills MR, Mason GM, Sissons JGP. 2013. Adaptive cellular immunity to human cytomegalovirus, p 142–172. In Reddehase MJ. (ed), Cytomegaloviruses: from molecular pathogenesis to intervention, vol II Caister Academic Press, Poole, United Kingdom. [Google Scholar]
- 15.Elkington R, Shoukry NH, Walker S, Crough T, Fazou C, Kaur A, Walker CM, Khanna R. 2004. Cross-reactive recognition of human and primate cytomegalovirus sequences by human CD4 cytotoxic T lymphocytes specific for glycoprotein B and H. Eur J Immunol 34:3216–3226. doi: 10.1002/eji.200425203. [DOI] [PubMed] [Google Scholar]
- 16.Pachnio A, Zuo J, Ryan GB, Begum J, Moss PAH. 2015. The cellular localization of human cytomegalovirus glycoprotein expression greatly influences the frequency and functional phenotype of specific CD4+ T cell responses. J Immunol 195:3803–3815. doi: 10.4049/jimmunol.1500696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sester M, Sester U, Gartner B, Kubuschok B, Girndt M, Meyerhans A, Kohler H. 2002. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol 76:3748–3755. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. 2007. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen EMM, Remmerswaal EBM, Vossen MTM, Rowshani AT, Wertheim-van Dillen PME, van Lier RAW, ten Berge IJM. 2004. Emergence of a CD4+ CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol 173:1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 21.Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, Vukmanovic-Stejic M, Yong K, Battistini L, Kern F, Soares MV, Akbar AN. 2011. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+ CD45RA+ CD27 T cells: the potential involvement of interleukin-7 in this process. Immunology 132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 23.Raeiszadeh M, Pachnio A, Begum J, Craddock C, Moss P, Chen FE. 2015. Characterisation of CMV-specific CD4+ T-cell reconstitution following stem cell transplantation through the use of HLA class II-peptide tetramers identifies patients at high risk of recurrent CMV reactivation. Haematologica 100:e318–e322. doi: 10.3324/haematol.2015.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weekes MP, Wills MR, Sissons JGP, Carmichael AJ. 2004. Long-term stable expanded human CD4+ T cell clones specific for human cytomegalovirus are distributed in both CD45RAhigh and CD45ROhigh populations. J Immunol 173:5843–5851. doi: 10.4049/jimmunol.173.9.5843. [DOI] [PubMed] [Google Scholar]
- 25.Tovar-Salazar A, Patterson-Bartlett J, Jesser R, Weinberg A. 2010. Regulatory function of cytomegalovirus-specific CD4+ CD27− CD28− T cells. Virology 398:158–167. doi: 10.1016/j.virol.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ. 2002. Characterization of CD4+ CTLs ex vivo. J Immunol 168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 27.Dirks J, Tas H, Schmidt T, Kirsch S, Gärtner BC, Sester U, Sester M. 2013. PD-1 analysis on CD28− CD27− CD4 T cells allows stimulation-independent assessment of CMV viremic episodes in transplant recipients. Am J Transplant 13:3132–3141. doi: 10.1111/ajt.12480. [DOI] [PubMed] [Google Scholar]
- 28.Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. 2013. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine 31:685–690. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. 2010. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 30.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crompton L, Khan N, Khanna R, Nayak L, Moss PAH. 2008. CD4+ T cells specific for glycoprotein B from cytomegalovirus exhibit extreme conservation of T-cell receptor usage between different individuals. Blood 111:2053–2061. doi: 10.1182/blood-2007-04-079863. [DOI] [PubMed] [Google Scholar]
- 32.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F. 2012. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol 86:1001–1009. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Čičin-Šain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, Fischer MB, Koudelka CW, Axthelm MK, JNikolich-Žugich Picker LJ. 2011. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol 187:1722–1732. doi: 10.4049/jimmunol.1100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJL, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, Thomas PG, Davis MM. 2015. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 7:281ra43. doi: 10.1126/scitranslmed.aaa2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marandu TF, Finsterbusch K, Kröger A, Čičin-Šain L. 2014. Mouse CMV infection delays antibody class switch upon an unrelated virus challenge. Exp Gerontol 54:101–108. doi: 10.1016/j.exger.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. 2009. The cytolytic enzymes granzyme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol 85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weltevrede M, Eilers R, de Melker HE, van Baarle D. 2016. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: a systematic review. Exp Gerontol 77:87–95. doi: 10.1016/j.exger.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Denkinger MD, Leins H, Schirmbeck R, Florian M, Geiger H. 2015. HSC aging and senescent immune remodeling. Trends Immunol 36:815–824. doi: 10.1016/j.it.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kline KA, Bowdish DME. 2016. Infection in an aging population. Curr Opin Microbiol 29:63–67. doi: 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, Moss PAH, Medical Research Council Cognitive Function and Ageing Study . 2013. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell 12:381–387. doi: 10.1111/acel.12059. [DOI] [PubMed] [Google Scholar]
- 41.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. 2011. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. 2013. Decreased naive and increased memory CD4+ T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 8:e71498. doi: 10.1371/journal.pone.0071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. 2012. Higher immunoglobulin G antibody levels against cytomegalovirus are associated with incident ischemic heart disease in the population-based EPIC-Norfolk cohort. J Infect Dis 206:1897–1903. doi: 10.1093/infdis/jis620. [DOI] [PubMed] [Google Scholar]
- 44.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. 2007. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol 42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz AR, Malzer JN, Domingo C, Jurchott K, Grutzkau A, Babel N, Nienen M, Jelinek T, Niedrig M, Thiel A. 2015. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J Immunol 195:4699–4711. doi: 10.4049/jimmunol.1500598. [DOI] [PubMed] [Google Scholar]
- 46.Lelic A, Verschoor C, Ventresca M, Parsons R, Evelegh C, Bowdish D, Betts M, Loeb M, Bramson J. 2012. The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog 8:e1003076. doi: 10.1371/journal.ppat.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason G, Jackson S, Okecha G, Poole E, Sissons JGP, Sinclair J, Wills M. 2013. Human cytomegalovirus latency-associated proteins elicit immune-suppressive IL-10 producing CD4+ T cells. PLoS Pathog 9:e1003635. doi: 10.1371/journal.ppat.1003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayamada S, Takahashi H, Kanno Y, O'Shea J. 2012. Helper T cell diversity and plasticity. Curr Opin Immunol 24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwele S, Fischer A, Brestrich G, Wlodarski M, Wagner L, Schmueck M, Roemhild A, Thomas S, Hammer M, Babel N, Kurtz A, Maciejewski J, Reinke P, Volk HD. 2012. Cytomegalovirus-specific regulatory and effector T cells share TCR clonality—possible relation to repetitive CMV infections. Am J Transplant 12:669–681. doi: 10.1111/j.1600-6143.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 50.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. 2007. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chattopadhyay PK, Yu J, Roederer M. 2005. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med 11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 52.Pachnio A, Ciaurriz M, Begum J, Lal N, Zuo J, Beggs A, Moss P. 2016. Cytomegalovirus infection leads to development of high frequencies of cytotoxic virus-specific CD4+ T cells targeted to vascular endothelium. PLoS Pathog 12:e1005832. doi: 10.1371/journal.ppat.1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noriega V, Redmann V, Gardner T, Tortorella D. 2012. Diverse immune evasion strategies by human cytomegalovirus. Immunol Res 54:140–151. doi: 10.1007/s12026-012-8304-8. [DOI] [PubMed] [Google Scholar]
- 54.Jackson SE, Mason GM, Okecha G, Sissons JGP, Wills MR. 2014. Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T cells. J Virol 88:10894–10908. doi: 10.1128/JVI.01477-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. 1994. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair J, Reeves M. 2014. The intimate relationship between human cytomegalovirus and the dendritic cell lineage. Front Microbiol 5:389. doi: 10.3389/fmicb.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wills MR, Poole E, Lau B, Krishna B, Sinclair JH. 2015. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol Immunol 12:128–138. doi: 10.1038/cmi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore KW, de Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 59.Janetzki S, Rueger M, Dillenbeck T. 2014. Stepping up ELISpot: multi-level analysis in FluoroSpot assays. Cells 3:1102–1115. doi: 10.3390/cells3041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tischer S, Dieks D, Sukdolak C, Bunse C, Figueiredo C, Immenschuh S, Borchers S, Stripecke R, Maecker-Kolhoff B, Blasczyk R, Eiz-Vesper B. 2014. Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein-Barr virus-specific T cells as targets of interest in immunotherapeutic approaches. J Immunol Methods 408:101–113. doi: 10.1016/j.jim.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, Ammatuna P, Fletcher JM, Caruso C, Pawelec G. 2007. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol 42:995–1002. doi: 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Swain S, McKinstry K, Strutt T. 2012. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol 12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hegde NR, Tomazin RA, Wisner TW, Dunn C, Boname JM, Lewinsohn DM, Johnson DC. 2002. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J Virol 76:10929–10941. doi: 10.1128/JVI.76.21.10929-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomazin R, Boname J, Hegde NR, Lewinsohn DM, Altschuler Y, Jones TR, Cresswell P, Nelson JA, Riddell SR, Johnson DC. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med 5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 65.Odeberg J, Plachter B, Brandén L, Söderberg-Nauclér C. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 101:4870–4877. doi: 10.1182/blood-2002-05-1504. [DOI] [PubMed] [Google Scholar]
- 66.Beck K, Meyer-König U, Weidmann M, Nern C, Hufert FT. 2003. Human cytomegalovirus impairs dendritic cell function: a novel mechanism of human cytomegalovirus immune escape. Eur J Immunol 33:1528–1538. doi: 10.1002/eji.200323612. [DOI] [PubMed] [Google Scholar]
- 67.Cebulla C, Miller D, Zhang Y, Rahill B, Zimmerman P, Robinson J, Sedmak D. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J Immunol 169:167–176. doi: 10.4049/jimmunol.169.1.167. [DOI] [PubMed] [Google Scholar]
- 68.Verma S, Weiskopf D, Gupta A, McDonald B, Peters B, Sette A, Benedict CA. 2015. Cytomegalovirus-specific CD4 T cells are cytolytic and mediate vaccine protection. J Virol 90:650–658. doi: 10.1128/JVI.02123-15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinzger C, Eberhardt K, Cavignac Y, Weinstock C, Kessler T, Jahn G, Davignon J-L. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J Gen Virol 87:1853–1862. doi: 10.1099/vir.0.81595-0. [DOI] [PubMed] [Google Scholar]
- 70.Gibson L, Barysauskas CM, McManus M, Dooley S, Lilleri D, Fisher D, Srivastava T, Diamond DJ, Luzuriaga K. 2015. Reduced frequencies of polyfunctional CMV-specific T cell responses in infants with congenital CMV infection. J Clin Immunol 35:289–301. doi: 10.1007/s10875-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R. 2015. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]