Abstract
Over the last decade, the clinical applications of contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) have increased steadily. The development of second-generation ultrasound contrast agents has allowed superior visualization of the microvasculature and tissue perfusion of the target lesion. This methodology has proven useful in the differential diagnosis of solid pancreatic masses and lymph nodes. In addition, the applicability of CH-EUS has expanded to nonpancreas structures such as biliary, focal liver lesions, and vascular disease. This article focuses primarily on the novel applications of CH-EUS in biliary tract and visceral vascular diseases.
Keywords: Contrast agents, endoscopic ultrasound, biliary tract, vascular disease
INTRODUCTION
Contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) is an emerging technique used in many gastrointestinal disorders. CH-EUS has not only improved diagnostic performance in numerous pathological conditions but has also changed the status of EUS in some gastrointestinal disorders. Innovations continue to expand the capabilities of CH-EUS and push the boundaries of its indications.[1] This review focuses on new potential indications of CH-EUS.
NOVEL APPLICATIONS OF CONTRAST-ENHANCED HARMONIC ENDOSCOPIC ULTRASOUND
Gallbladder
The enhancement process of the gallbladder on CH-EUS is classified as early phase (10–30 s after contrast injection) and late phase (31–180 s after contrast injection), as the blood supply of the gallbladder is entirely arterial. The late phase persists for a short time compared to that of liver lesion.[2] While conventional EUS is one of the most common imaging investigations for diagnosing gallbladder diseases, it is often insufficient for determining the nature of complicated gallbladder diseases. This limitation of conventional EUS is mainly due to its poor ability to depict microvasculature in gallbladder diseases. Thus, CH-EUS can be applied to overcome the limitations of conventional EUS, as it can depict the hemodynamics in micro-and macro-circulations.
The use of CH-EUS in differentiating gallbladder polyps and gallbladder carcinoma has been studied previously.[3,4] A differential diagnosis between adenomas and cholesterol polyps is important because adenomas have the potential to develop into cancer over time. According to the study by Park et al., the sensitivity and specificity of CH-EUS for differentiating adenoma from cholesterol polyps based on enhancement pattern were 75% and 67%, respectively [Figure 1a].[4] Choi et al. investigated the value of CH-EUS for determining the malignant potential of gallbladder polyps >10 mm.[3] The identification of irregular tumor vessels and perfusion defects aided to differentiate malignant gallbladder polyps from benign ones with a sensitivity and specificity of 94% and 93%, respectively [Figure 1b].[3] Motionless sludge ball in the gallbladder occasionally mimics gallbladder polyp or cancer on EUS.[5] CH-EUS can very easily depict biliary sludge as avascular lesions in the gallbladder [Figure 1c] and the biliary tract, whereas it depicts apparent vessels in a gallbladder polyp and carcinoma.[3] Imazu et al. evaluated the utility of CH-EUS in differentiating gallbladder wall thickening and found that an inhomogeneous enhancement pattern is strongly predictive of a malignancy.[6]
Figure 1.
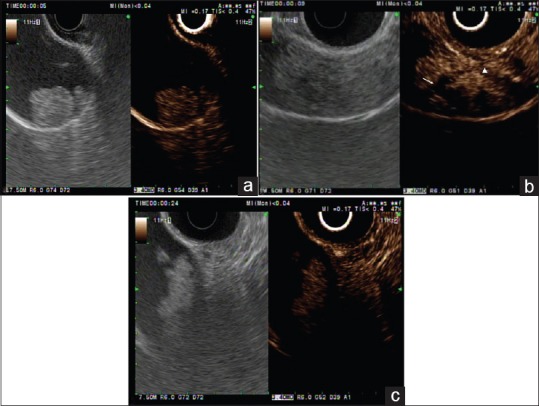
Conventional endoscopic ultrasound (left panel) and contrast-enhanced harmonic endoscopic ultrasound (right panel) images of gallbladder polypoid lesions. (a) Contrast-enhanced harmonic image (right panel) shows a homogeneous enhancement with regular spotty vessel (in this case, a gallbladder adenoma). (b) A patient with a gallbladder cancer. Contrast-enhanced harmonic endoscopic ultrasound (left panel) indicates that this area has irregular vessels (arrowhead) and perfusion defects (white arrow). (c) B-mode endoscopic ultrasound (left panel) shows a motionless isoechoic mass in the gallbladder. Contrast-enhanced harmonic endoscopic ultrasound (right panel) indicates that this area contains an unenhanced lesion (in this case, a gallbladder sludge ball)
Bile duct lesions
Bile duct wall thickening is a common feature in both benign and malignant bile duct lesions such as sclerosing cholangitis and cholangiocarcinoma. Studies suggested that homogeneous contrast enhancement in the biliary tract corresponds to nonneoplastic changes of the bile duct as in cholangitis.[7] In pancreatobiliary malignancies, accurate preoperative T-staging is important to guide the optimal treatment strategy. Although conventional EUS has been used widely in the diagnosis and T-staging of pancreatobiliary malignancies, the accuracy of EUS for vascular invasion appears to be lower than has previously been reported.[8] Tumor margin and the interface between tumor and vessel have been clearly demonstrated with contrast enhancement.[9] Imazu et al. compared the accuracy of EUS and CH-EUS for preoperative T-staging in 26 patients who underwent surgery.[9] CH-EUS was significantly better than EUS for T-staging, with an overall accuracy of 92% and 69%, respectively.[9] The depth of invasion and vascular involvement could be demonstrated more clearly with CH-EUS compared to EUS.[9]
Focal liver lesions
Ultrasound is suitable for liver imaging, and EUS provides very good imaging of the left lobe of the liver and a significant portion of the right lobe of the liver. Due to the dual blood supply system of the liver, CH-EUS is divided into arterial phase (<30 s from contrast injection), portal phase(30–120 s), and late phase (>120 s). On contrast enhancement imaging, arterial hyperenhancement and late-phase contrast washout indicate hepatocellular carcinoma, and peripheral rim-like hyperenhancement and the subsequent washout often indicate metastatic liver cancer.[10] The typical enhancement patterns of hemangioma are peripheral nodular hyperenhancement and sustained enhancement in the late phase.[10] Many studies have shown that compared with computed tomography (CT), CH-EUS is able to achieve the same or even superior characterization of focal liver lesions.[11]
Visceral vascular diseases
EUS can assess the large-caliber vasculatures adjacent to the gastrointestinal tract. Color Doppler EUS helps detect abnormal blood flow, along with flow direction and velocity within the vessel. The injection of ultrasound contrast agents (UCAs) can enhance the depiction of vascular flow and help to describe the vascular structure clearly.[12] Paik et al. demonstrated the value of CH-EUS in assessing visceral vascular disease. CH-EUS provides real-time angiography-like depiction of the vasculature and allows the accurate diagnosis of visceral vascular disease. A total of 12 patients with suspected visceral vascular disease on CT scan were included in the study.[12] EUS accurately identified all the visceral vascular lesions of 11 patients, and one patient with suspicious superior mesenteric artery (SMA) dissection on CT was proven to be normal by EUS.[12] CH-EUS showed intimal flap and flow within the true lumen of celiac axis, suggesting that what was presumed to be a periarterial soft tissue cuffing was actually organization of the false lumen [Figure 2].[12] While the focal stenosis of a visceral artery is not described on color Doppler EUS, it can be seen on CH-EUS [Figure 3].[12] Moreover, the stenotic area could be measured by using Doppler EUS. Two patients in the study underwent surgical thrombectomy and angioplasty because of total occlusion of SMA on CH-EUS.[12] The simultaneous assessment of morphologic and hemodynamic information using color Doppler EUS and CH-EUS would be useful in determining optimal management strategy in mesenteric artery dissection. CH-EUS has also been used in the hemodynamic assessment of esophageal varices.[13]
Figure 2.
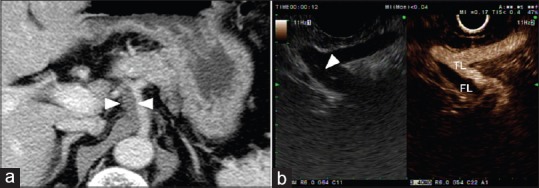
Computed tomography and endoscopic ultrasound findings in patient with celiac artery dissection. (a) Contrast-enhanced computed tomography scan shows periarterial soft tissue cuffing (arrowhead) around the celiac artery. (b) Contrast-enhanced harmonic endoscopic ultrasound shows the intimal flap (white arrowhead) inside the artery and true lumen and false lumen
Figure 3.
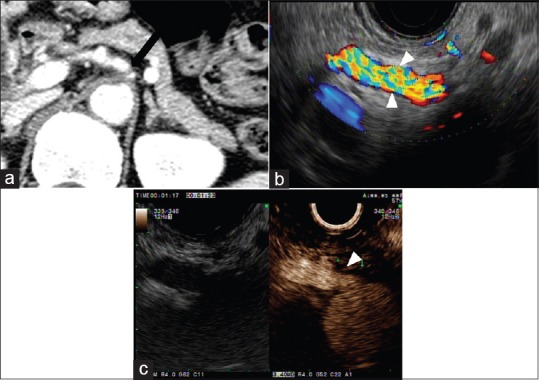
Computed tomography and endoscopic ultrasound findings in patient with initially suspected celiac artery stenosis. (a) Contrast-enhanced computed tomography shows significant stenosis at the root of celiac artery (arrow). (b) Doppler endoscopic ultrasound shows normal blood flow in celiac artery (arrowhead). (c) Contrast-enhanced harmonic endoscopic ultrasound shows focal stenosis at the origin of celiac artery (arrowhead). The distal flow signal of celiac artery is preserved
Treatment response evaluation
Recent research has focused on the potential of EUS-guided local ablation for treating pancreatobiliary tumor. In these local therapies, the tumor is not eradicated, but is devascularized or coagulated. Therefore, it is important to evaluate the local treatment response and detect residual viable tumor. Conventional EUS does not provide any reliable information regarding the efficacy of ablative treatment. When EUS is used as the imaging modality for guiding intervention, the addition of UCAs can provide useful information.[14,15] The pretreatment assessment of tumor vascularity to compare pre- and post-ablation patterns at the end of ablation treatment is mandatory. The typical imaging that indicates complete ablation is the disappearance of any previously visualized intra-lesional enhancement on CH-EUS.[14] The size of the nonperfused volume of the necrosis achieved can be compared with the size of the pretreatment volume of the tumor. In addition, CH-EUS can be used as a complement to CT or magnetic resonance imaging for conducting pretreatment assessments of vascularity and treatment responses.[14]
CONCLUSIONS
CH-EUS provides improved characterizations of various gallbladder and bile duct lesions. Its applicability has expanded to focal liver lesions and visceral vascular disease. Novel applications include assessing treatment response after local therapies. The advent of CH-EUS has greatly enhanced the usefulness of EUS and even changed the status of EUS in clinical practice. The role of CH-EUS is continuously evolving and it is expected that its use will be expanded further in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Choi JH, Seo DW. The expanding role of contrast-enhanced endoscopic ultrasound in pancreatobiliary disease. Gut Liver. 2015;9:707–13. doi: 10.5009/gnl15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie XH, Xu HX, Xie XY, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239–48. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Seo DW, Choi JH, et al. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos) Gastrointest Endosc. 2013;78:484–93. doi: 10.1016/j.gie.2013.03.1328. [DOI] [PubMed] [Google Scholar]
- 4.Park CH, Chung MJ, Oh TG, et al. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–21. doi: 10.1007/s00464-012-2620-x. [DOI] [PubMed] [Google Scholar]
- 5.Choi WB, Lee SK, Kim MH, et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000;52:372–9. doi: 10.1067/mge.2000.108041. [DOI] [PubMed] [Google Scholar]
- 6.Imazu H, Mori N, Kanazawa K, et al. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2014;59:1909–16. doi: 10.1007/s10620-014-3115-5. [DOI] [PubMed] [Google Scholar]
- 7.Hyodo T, Hyodo N, Yamanaka T, et al. Contrast-enhanced intraductal ultrasonography for thickened bile duct wall. J Gastroenterol. 2001;36:557–9. doi: 10.1007/s005350170059. [DOI] [PubMed] [Google Scholar]
- 8.Meining A, Dittler HJ, Wolf A, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002;50:599–603. doi: 10.1136/gut.50.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imazu H, Uchiyama Y, Matsunaga K, et al. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732–8. doi: 10.3109/00365521003690269. [DOI] [PubMed] [Google Scholar]
- 10.Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15–24. doi: 10.4329/wjr.v1.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu GJ, Xu HX, Lu MD, et al. Enhancement pattern of hepatocellular carcinoma: Comparison of real-time contrast-enhanced ultrasound and contrast-enhanced computed tomography. Clin Imaging. 2006;30:315–21. doi: 10.1016/j.clinimag.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Paik WH, Choi JH, Seo DW, et al. Clinical usefulness with the combination of color Doppler and contrast-enhanced harmonic EUS for the assessment of visceral vascular diseases. J Clin Gastroenterol. 2014;48:845–50. doi: 10.1097/MCG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Yamazaki K, Toyota J, et al. Evaluation of hemodynamics in esophageal varices. Value of endoscopic color Doppler ultrasonography with a galactose-based contrast agent. Hepatol Res. 2003;25:55–61. doi: 10.1016/s1386-6346(02)00168-7. [DOI] [PubMed] [Google Scholar]
- 14.Giday SA, Magno P, Gabrielson KL, et al. The utility of contrast-enhanced endoscopic ultrasound in monitoring ethanol-induced pancreatic tissue ablation: A pilot study in a porcine model. Endoscopy. 2007;39:525–9. doi: 10.1055/s-2007-966391. [DOI] [PubMed] [Google Scholar]
- 15.Park DH, Choi JH, Oh D, et al. Endoscopic ultrasonography-guided ethanol ablation for small pancreatic neuroendocrine tumors: Results of a pilot study. Clin Endosc. 2015;48:158–64. doi: 10.5946/ce.2015.48.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]


