Abstract
Incidental pancreatic cysts (PCs) are frequently encountered in the general population often in asymptomatic patients who undergo imaging tests to investigate unrelated conditions. The detection of a PC poses a significant clinical dilemma, as the differential diagnosis is quite broad ranging from benign to malignant conditions. Endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) has been reported to be an accurate tool in the differential diagnosis; however, its sensitivity is suboptimal and false negative results do occur. Contrast harmonic EUS (CH-EUS) was demonstrated to be a useful tool to investigate pancreatic solid lesions to differentiate between benign and malignant ones. In the setting of PCs, CH-EUS could help identify areas of malignant growth inside the cystic cavities. Several studies have reported promising results showing malignant areas in PCs as hyperenhanced lesions. Confirmation of malignancy can then be obtained by FNA, which should be precisely targeted according to the findings of the contrast harmonic study.
Keywords: Contrast agents, contrast harmonic endoscopic ultrasound, endoscopic ultrasound, fine-needle aspiration, pancreatic cysts
INTRODUCTION
The incidental detection of asymptomatic pancreatic cysts (PCs) in the general population has increased as a consequence of the wide use of computed tomography (CT) and magnetic resonance imaging (MRI), ranging from 0.5% to 45%.[1,2,3,4,5]
PCs comprise a large variety of different lesions, some of which are benign, i.e., pseudocysts and dysontogenetic cysts, whereas others are malignant or harbor malignant potential.
The latter cysts, from now on called pancreatic cystic lesions (PCLs), are mainly composed (90%) by intraductal papillary mucinous neoplasms (IPMN), mucinous cystadenoma (MCA), serous cystadenoma (SCA), and pseudopapillary neoplasms.
Recent evidence has shown that PCLs represent roughly half of PCs, the other half being represented by pseudocysts.[1,6]
PANCREATIC CYSTS DIFFERENTIAL DIAGNOSIS AND RISK STRATIFICATION
It is of paramount importance to obtain an accurate differential diagnosis of PCs to stratify the risks of malignant progression and decide the better clinical pathway for the patients that can comprise surgery or follow-up. Unfortunately, at the present time, the strategy to stratify the risk of malignant progression is poor and up to 75% of patients with PCLs undergo major surgery to remove cysts that demonstrate to have low malignant potential at histological examination of the surgical specimen.[7]
Recently, high-quality Italian guidelines focused on PCLs management in asymptomatic patients indicated MRI with magnetic resonance cholangiopancreatography as the first diagnostic step; if this technique turns out to be nondiagnostic or “suspicious” morphological aspects are detected, endoscopic ultrasound (EUS) with or without fine-needle aspiration (EUS-FNA) is suggested.[8]
THE ROLE OF TRADITIONAL B-MODE ENDOSCOPIC ULTRASOUND/ENDOSCOPIC ULTRASOUND-FINE-NEEDLE ASPIRATION
EUS can differentiate among PCLs and point out the so-called worrisome features, which are useful for the diagnosis of malignant or premalignant lesions (carcinoma/high-grade dysplasia) and can help in the definition of a diagnostic and therapeutic pathway.
According to the international guidelines published in 2012, PCLs’ worrisome features are represented by: size>3 cm, thickened enhanced cyst wall, main pancreatic duct (MPD) size of 5–9 mm, and abrupt change in the MPD caliber with distal pancreatic atrophy.[9]
Unfortunately, EUS is an operator-dependent technique and the differential diagnosis between PCLs still requires in most cases tissue confirmation with EUS-FNA. However, it should be emphasized that cytopathology analysis of aspirated specimens is diagnostic in no more than half of the cases[10,11] and that the detection of markers (e.g., carcinoembryonic antigen) in the cystic fluid can have a contributory role in the diagnostic process although it lacks optimal accuracy.[12] New intracystic molecular markers are currently under investigation, but their use is still limited due to costs and low sensitivity (not adding more than 10%–15% vs. cytology) and uneven availability among different laboratories.[13]
EUS-FNA is a safe procedure although a recent Italian study reported 6% complication rate in a selected population of patients with PCs.[14,15]
THE ROLE OF ULTRASOUND CONTRAST AGENTS IN PANCREATIC CYSTS
To overcome the limitations of EUS-FNA and improve the characterization of PCs, new techniques were developed.
Concerning PCLs, mural nodules are considered the most important predictive factor of malignancy, particularly in cases showing features of hyperenhancement at CT and/or lesions >7 mm in size.[9,16,17,18]
Unfortunately, CT and MRI are specific in nodules detection but have a low sensitivity.[19,20] B-mode EUS is sensitive, but less specific due to the difficulty in distinguishing nodules from mucous clots and debris.[19]
Consequently, research is oriented in finding new techniques with high intracystic nodules detection and able to distinguish malignant/premalignant nodules from benign nodules such as mucus clots mimicking solid components or pseudocysts with internal debris.
Recently, contrast harmonic EUS (CH-EUS) has been reported as a useful adjunct in the differential diagnosis of pancreatic solid tumors.[21,22,23,24] The interest and experience in CH-EUS for the study of PCs are growing due to capability of the technique in visualizing the microvascularization of cystic walls, septae, and most of all, nodules.
The technique for performing CH-EUS has been detailed in other articles of this special issue of Endoscopic Ultrasound. CH-EUS, which takes advantage of the intravenous infusion of a contrast medium to visualize the blood flow even in very fine vessels, should be adequate to investigate the small solid components that are sometimes detected inside PCs. In theory, the small neoplastic solid components should exhibit some signs of vascularization [Figure 1] as opposed to debris and mucus that are expected to be completely avascular [Figure 2].
Figure 1.
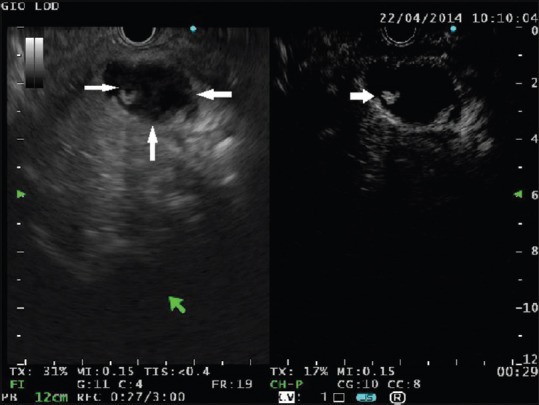
A cystic lesion is seen in the pancreatic head containing solid components with mixed echogenicity (left panel, thin arrows). At contrast harmonic-endoscopic ultrasound, a small area of hyperenhancement is clearly visible (right panel, arrow). This finding is highly suggestive of malignancy
Figure 2.
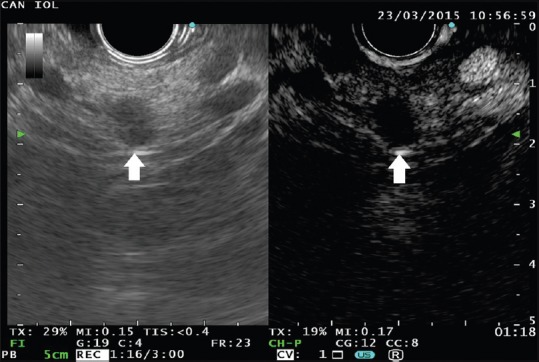
A small cyst is detected in the pancreatic body. At B-mode endoscopic ultrasound, the lesion appears full of echogenic material (left panel, arrow). At contrast harmonic-endoscopic ultrasound, the cyst is clearly unenhanced thus ruling out malignancy (right panel, arrow)
Initially, no CH-EUS dedicated platforms were available; therefore, the first experiences were based on the study of the contrast medium distribution with color Doppler or power Doppler. The latter technique is named contrast-enhanced-EUS (CE-EUS).
In 2008, Dietrich et al. showed that CE-EUS (using Levovist, a first generation ultrasound contrast agent) was useful in differentiating small solid pancreatic tumors that resulted hypovascular in comparison with the surrounding pancreatic parenchyma. The study included for the related risk of detection of ductal adenocarcinoma, also 10 (<40 mm) SCAs that resulted in all hyperenhanced. They collected data also about other PCs, reporting that hyperenhanced mural nodules were identified in 6/6 IPMNs and in 79% (15/19) MCAs. They also reported that only 8/90 pseudocysts presented contrast enhancing vessels. According to the authors, CE-EUS was not useful in differentiating between benign and malignant PCLs.[25]
In 2009, Ohno et al. used CE-EUS to identify malignant predictors in IPMN. The study was conducted on 87 patients affected by IPMN with mural nodules. The authors classified mural nodules into four types according to the morphology and studied the vascular characteristics of mural nodules with color Doppler after the injection of Levovist. CE-EUS results were compared to CT scan. The study showed that hyperenhanced nodules were significantly correlated to malignancy and that CE-EUS detected more and smaller mural nodules than CT.[26]
In 2013, Yamashita et al. used CH-EUS (with Sonazoid) to investigate 17 patients with IPMN with mural nodules. CH-EUS allowed detecting the microvasculature within nodules in 13 patients (76%) unlike CT scan, which showed vascularized mural nodules only in seven patients (41%). Interestingly, in that study, 75% of mural nodules identified by CH-EUS turned out to be intraductal papillary mucinous carcinoma at histopathology.[21] One case of a cystic septum that was erroneously interpreted as a hyperenhanced solid component accounted for a false-positive result of CH-EUS.
In 2014, Hocke et al. performed CH-EUS with Sonovue in 125 patients with undetermined PCs. They evaluated the contrast medium behavior in cystic walls, septae, and nodules. Concerning walls enhancement, the study showed that CH-EUS could identify benign lesions as pseudocysts and dysontogenetic cysts because cystic walls show no vascularization. On the other hand, CH-EUS could not differentiate between serous and mucinous PCLs because the enhancement of walls and septae was similar between the two entities. The authors maintain that EUS-FNA is still required for the differential diagnosis of PCLs and that the usefulness of CH-EUS consist in targeting FNA on those lesions, assumed to be cystic pancreatic tumors, with visible walls, septae, or nodules vascularization.[27]
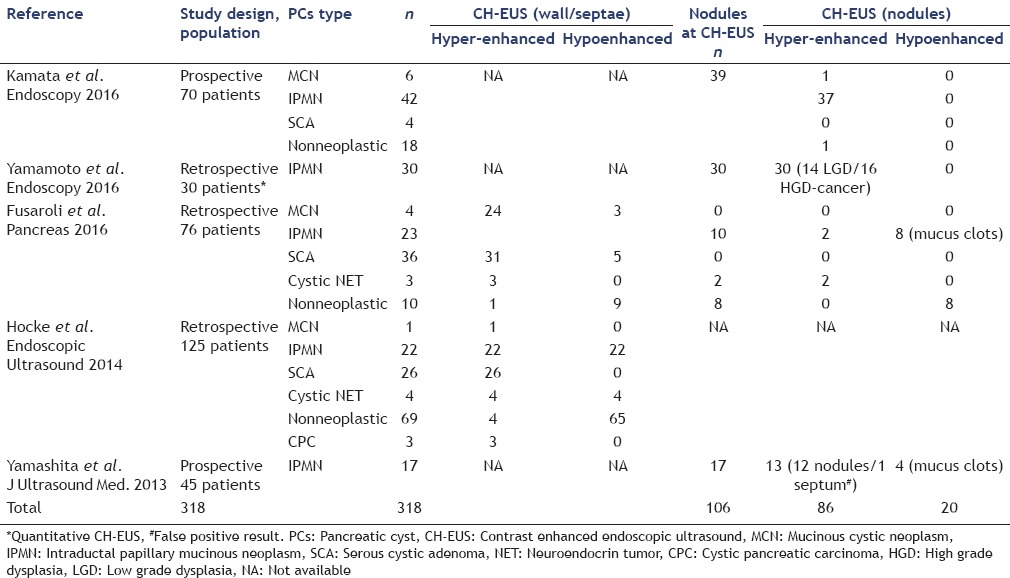
As a demonstration of the increasing interest on CH-EUS, three different articles have already been published in the current year [Table 1].
Table 1.
Literature pertaining the role of CH-EUS in pancreatic cysts
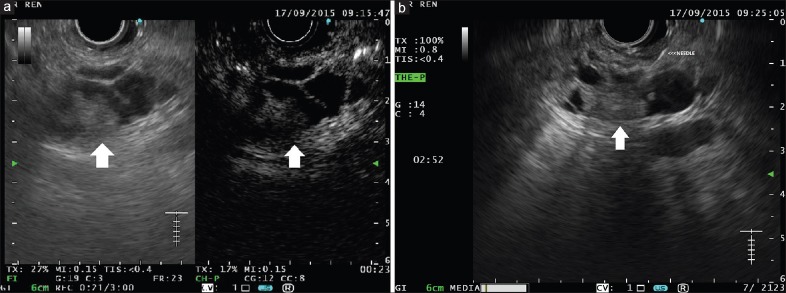
Fusaroli et al. evaluated the role of CH-EUS in the differential diagnosis of PCs and in the detection of malignancy. The evaluation of 76 patients showed that CH-EUS could distinguish between pseudocysts and other cysts but cannot differentiate between serous and mucinous PCLs because the wall and septae enhancement is not different between these lesions. On the other hand, EUS is a very useful tool in visualizing nodules inside cysts and the nodule enhancement after injection of Sonovue can identify malignant nodules that are clearly hyperenhancing [Figure 3]. In conclusion, CH-EUS can be used as a tool in targeting FNA and avoiding puncture of mucus plugs or debris.[28]
Figure 3.
(a) A complex multiloculated cystic lesion is visible in the pancreatic head, containing a hypoechoic solid component (left panel, arrow). At contrast harmonic-endoscopic ultrasound, the solid component appears hyperenhanced (right panel, arrow), as well as septae and cystic wall. (b) As the contrast harmonic-endoscopic ultrasound finding is highly suggestive of malignancy, selective endoscopic ultrasound-fine-needle aspiration is performed
Kamata et al. showed that standard B-mode EUS had high sensitivity but low specificity (40%) in the study of mural nodules. The addition of CH-EUS increases the specificity up to 75%, whereas maintaining high sensitivity (95%). The Authors have also assessed that the presence of an enhancing nodule ≥4 mm represented the optimal threshold to discriminate between benign and malignant PCLs.[29]
Yamamoto et al. evaluated by a time-intensity curve analysis, the diagnostic accuracy of CH-EUS (with Sonazoid) in differentiating the grade of dysplasia inside nodules (low-grade vs. high-grade dysplasia/carcinoma). In thirty patients, they observed that among the other parameters taken into account, nodule versus pancreatic parenchyma contrast ratio was the most accurate parameter to quantify the blood flow in intracystic nodules. They also correlated the blood flow with contrast enhancement and demonstrated, through histopathology, a correlation between nodule vascularity and dysplasia.[30]
CONCLUSION
The differential diagnosis of PCs is quite broad encompassing benign, borderline, and malignant entities. Current imaging methods, including EUS and EUS-FNA, show suboptimal accuracy in differentiating PCLs and detecting malignancy. CH-EUS shows no significant differences between the enhancing patterns of cystic walls and septae of mucinous and nonmucinous PCLs. However, CH-EUS appears to be a sensitive and specific adjunct in the differential diagnosis of intracystic solid components to detect malignancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Freeny PC, Saunders MD. Moving beyond morphology: New insights into the characterization and management of cystic pancreatic lesions. Radiology. 2014;272:345–63. doi: 10.1148/radiol.14131126. [DOI] [PubMed] [Google Scholar]
- 2.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: Observe or operate. Ann Surg. 2004;239:651–7. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–53. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–84. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 6.Mohamadnejad M, Eloubeidi MA. Cystsic lesions of the pancreas. Arch Iran Med. 2013;16:233–9. [PubMed] [Google Scholar]
- 7.Polkowski M. Endoscopic ultrasonography for pancreatic cystic lesions: Let's enhance it. Endoscopy. 2016;48:4–6. doi: 10.1055/s-0035-1569561. [DOI] [PubMed] [Google Scholar]
- 8.Italian Association of Hospital Gastroenterologists and Endoscopists, Italian Association for the Study of the Pancreas. Buscarini E, et al. Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis. 2014;46:479–93. doi: 10.1016/j.dld.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Palazzo L, O’Toole D. Endoscopic ultrasound in cystic pancreatic lesions: Operator training needs to be improved, EUS-guided sampling should be standardized, and decision-making should be multidisciplinary and evidence-based. Endoscopy. 2011;43:557–9. doi: 10.1055/s-0030-1256614. [DOI] [PubMed] [Google Scholar]
- 11.de Jong K, Poley JW, van Hooft JE, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy. 2011;43:585–90. doi: 10.1055/s-0030-1256440. [DOI] [PubMed] [Google Scholar]
- 12.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 13.Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 14.ASGE Standards of Practice Committee. Early DS, Acosta RD, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839–43. doi: 10.1016/j.gie.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Tarantino I, Fabbri C, Di Mitri R, et al. Complications of endoscopic ultrasound fine needle aspiration on pancreatic cystic lesions: Final results from a large prospective multicenter study. Dig Liver Dis. 2014;46:41–4. doi: 10.1016/j.dld.2013.08.134. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883–8. doi: 10.1097/MPA.0b013e31827a7b84. [DOI] [PubMed] [Google Scholar]
- 17.Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–48.e22. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Ann Surg. 2014;259:72–81. doi: 10.1097/SLA.0b013e31829385f7. [DOI] [PubMed] [Google Scholar]
- 19.Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192–8. doi: 10.1016/j.cgh.2011.09.029. 198.e1-2. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Ueda K, Itonaga M, et al. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J Ultrasound Med. 2013;32:61–8. doi: 10.7863/jum.2013.32.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Fusaroli P, Spada A, Mancino MG, et al. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–34.e1-2. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–10. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 23.Gincul R, Palazzo M, Pujol B, et al. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: A prospective multicenter trial. Endoscopy. 2014;46:373–9. doi: 10.1055/s-0034-1364969. [DOI] [PubMed] [Google Scholar]
- 24.Fusaroli P, D’Ercole MC, De Giorgio R, et al. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video) Pancreas. 2014;43:584–7. doi: 10.1097/MPA.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–34. doi: 10.1097/SLA.0b013e3181a189a8. [DOI] [PubMed] [Google Scholar]
- 27.Hocke M, Cui XW, Domagk D, et al. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123–30. doi: 10.4103/2303-9027.131040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusaroli P, Serrani M, De Giorgio R, et al. Contrast harmonic-endoscopic ultrasound is useful to identify neoplastic features of pancreatic cysts (With Videos) Pancreas. 2016;45:265–8. doi: 10.1097/MPA.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 29.Kamata K, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35–41. doi: 10.1055/s-0034-1393564. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Kato H, Tomoda T, et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy. 2016;48:26–34. doi: 10.1055/s-0034-1393563. [DOI] [PubMed] [Google Scholar]