Abstract
The negative predictive value of endoscopic ultrasonography fine-needle aspiration is relatively low. To achieve the improvement of the diagnostic yield, the following were proposed: a higher number of passes, the presence of the rapid on-site cytopathologist evaluation, the fanning technique, or the repetition of the fine needle biopsy. Harmonic contrast-enhanced endosonography may better identify the targeted area in the lesions by avoiding the inside necrosis and the vessels of fibrosis, so it can guide the fine-needle aspiration. Both techniques are complementary, not competitive, and they can be done in the same session. The combined technique is simple, safe, and requires only a few minutes with minimal extra costs compared to standard fine-needle aspiration. It minimally increases the diagnostic rate, and it permits the decrease of the number of passes. However, we will know its real clinical impact only in the future and whether it will be incorporated into the lesion assessment process.
Keywords: Contrast agents, contrast-enhanced endosonography, endoscopic ultrasound-guided fine-needle aspiration, endoscopic ultrasound-fine needle aspiration, endosonography, harmonic contrast-enhanced endosonography, lymph nodes, neuroendocrine tumors, pancreas
INTRODUCTION
Endoscopic ultrasound with fine-needle aspiration (EUS-FNA) has been a major step forward in tissue sampling in gastroenterology. One of the issues of EUS-FNA is its low negative predictive value (46%–80%), which is a critical parameter when considering the evaluation for malignancy.[1]
Different strategies have been proposed to achieve improvement in the diagnostic yield of EUS-FNA. The presence of fibrosis and necrosis inside the pancreatic tumors usually requires multiple fine-needle aspiration (FNA) passes (on average 3–6 needle passes) to obtain adequate samples.[2] The number of passes required is significantly greater in pancreatic masses coexisting with chronic pancreatitis due to the sensitivity of EUS-FNA being lower in these cases (54%–74%).[3,4]
The presence of a rapid on-site cytopathologist evaluation (ROSE) increases the diagnostic sensitivity of EUS-FNA (for pancreatic masses from 88% to 95%, compared with <80% in the absence of a cytopathologist).[5,6]
Compared to standard FNA, the fanning technique has demonstrated a better diagnostic accuracy (96.4% vs.76.9%, with 85.7% vs. 57.7% diagnostic rate on the first pass), and a lower number of passes are required to achieve diagnosis.[7] The use of ProCore needles proved no advantage in improving the diagnostic rate.[8]
Repeated EUS-FNA in inconclusive cases can increase the percentage of correct diagnosis in 61%–84% of patients.[9,10,11,12]
THE REASONS FOR USING CONTRAST-ENHANCED HARMONIC ENDOSCOPIC ULTRASONOGRAPHY FINE-NEEDLE ASPIRATION
Contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS) and elastography have been proposed as adjunctive methods for the better targeting of lesions.[13,14] CH-EUS is a reproducible tool, easy to learn, and with a short learning curve.[15] It improves the diagnostic yield because 80%–100% of false-negative cases in EUS-FNA are correctly classified by CH-EUS.[15,16,17] These results suggest that CH-EUS could help to decide between surgery and follow-up when the results of EUS-FNA are inconclusive.[15] However, we cannot rely only on the vascular aspect of mass to ascertain its nature, so FNA still represents the standard for the discrimination of mass. CH-EUS and EUS-FNA are complementary, not competitive, and are better performed together during the same investigation.[18]
The benefit of adding CH to EUS-FNA consists of a better identification of the targeted area in pancreatic lesions.[2] CH-EUS-FNA could decrease the false negative results due to less blood, necrosis, or fibrosis in retrieved samples. Necrosis, vessels, and cystic nonenhanced areas are more easily avoided and the lesion is better delineated with CH-EUS-FNA, so the best area for sampling can be more precisely targeted and with more visibility.[2,15,19]
CONTRAST HARMONIC-ENDOSCOPIC ULTRASONOGRAPHY EQUIPMENT AND TECHNIQUE
It is important to select the appropriate ultrasound machine and echoendoscopes with specific contrast harmonic imaging modes. The signal received by the transducer represents the nonlinear response of the microbubbles and ignores the fundamental signals from the background tissue. Other requirements for obtaining a good image are to set the ultrasound machine appropriately, to use correctly the contrast substance and to position the needle in time for performing the FNA under the contrast phase.
There are two principles of harmonic imaging in endosonography: the dynamic contrast harmonic imaging presents on the Hitachi platform and the extended pure harmonic (ExpH) technique on Aloka platforms; both use a low mechanical index (0.1–0.4) to avoid bubble destruction. The CH-EUS-FNA has been reported only on the second platform until now.
There are two main contrast agents, which are present on the market. SonoVue (Bracco Imaging, Milan, Italy) is made of phospholipid-stabilized microbubbles of sulfur hexafluoride - a poorly soluble gas encountered by a lipid shell. The second - Sonazoid (Daiichi-Sankyo) - is made of perfluorobutane in a lipid shell and produces different signaling intensities and durations.
For SonoVue use, the arterial phase starts 25–30 s after contrast agent injection. After 30 s, the venous phase begins, and after 45 s from the contrast medium injection; the washout phase is seen as slow or fast. The contrast uptake in the lesion is defined as relative to the surrounding parenchyma; it is classified as hypoenhanced when the mass displayed shows less uptake of contrast medium compared to the surrounding parenchyma; in contrast, it is classified as iso- or hyperenhanced when the mass uptake is equal or superior to the surrounding parenchyma. The “fast washout” is defined in situ ations when contrast is almost no longer visible inside the target lesion during the venous phase, and “slow washout” when a significant amount of contrast is still visible inside the target lesion during the venous phase.
The dose of SonoVue (4.8 mL or less) required for an optimal view of the contrast depends on the sensitivity of the equipment used, on the type of transducer, and on the organ under investigation. When using higher frequency transducers, a dose of 4.8 mL performs better, as these frequencies are higher than those at which current contrast agents resonate most strongly.[19] The dynamic range has to be set in terms to image small differences in local contrast concentration.
The ExpH detection mode uses a different low mechanical index: 0.12–0.14 for the Alpha 7 ultrasound device,[20] 0.28 for the Alpha 10 ultrasound machine with 2.4 mL SonoVue administered[21,22] or 0.4 for the Alpha 10 ultrasound machine with 4.8 mL SonoVue administered,[2] and 0.20–0.22 for the Aloka F75 ultrasound machine (personal data). A good setting of the machine, especially the mechanical index and the gain obtained, together with the appropriate dosage of the contrast agents, may prevent the artifacts of the CH-EUS image[19] and facilitate the correct view of the EUS-FNA needle.
For the Sonazoid use, the mechanical index is 0.3, the arterial phase is longer-90s-, so it allows the entire lesion to be checked carefully before starting the puncture.
The SonoVue contrast agent is rapidly injected intravenously, followed by a 5–20 mL flush of saline.[2,20] Due to the SonoVue short arterial phase (25–30s) needed for the qualitative evaluation of the lesion, the puncture is usually possible during the late venous phase (45–60 s after injection). The needle is passed in the working channel and positioned in front of the targeted lesion before contrast medium injection.
The targeted area during FNA is different depending on the CH-EUS features. In the case of the hypoenhanced lesion, the needle is advanced into the lesion, targeting the least enhanced part of the lesion but avoiding the nonenhanced part of the lesion suggestive of necrosis.[20] Furthermore, the inside vessels are avoided. In case of iso- or hyper-enhanced lesions, the nonenhanced area should be avoided as they could represent an area of hemorrhage or necrosis (e.g., neuroendocrine tumors and submucosal neoplasms) [Figures 1 and 2].[2,23] In the case of a predominant cystic lesion, the septae or the mural nodules appearing hyerenhanced during arterial phase should be targeted by EUS-FNA [Figure 3]. In all situations, the difference of echogenicity between the needle and the surrounding tissue is high.
Figure 1.
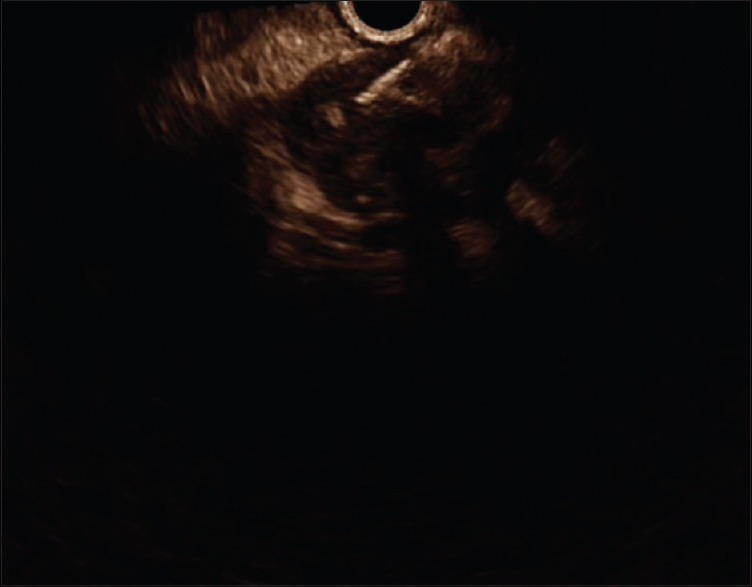
The contrast harmonic endoscopic ultrasonography-fine-needle aspiration of a pancreatic lesion (adenocarcinoma). The pancreatic lesion is hypoenhanced compared to the surrounding tissue, with some vessels inside and a central anechoic necrosis. The needle is inserted into the lesion during the late venous phase (Aloka alpha 7 platform)
Figure 2.
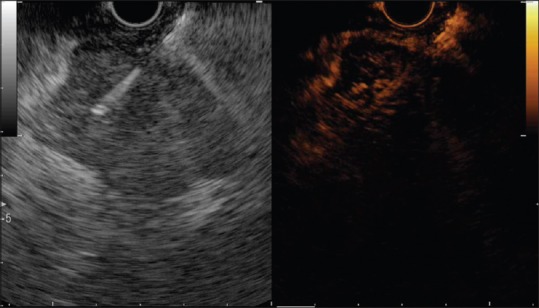
The contrast harmonic endoscopic ultrasonography-fine-needle aspiration of a pancreatic lesion (neuroendocrine tumor). The lesion is hyperenhanced in the arterial phase, with fast washout in the venous phase. There are some remains of contrast inside the lesion during the late venous phase. The needle is inserted into the hyperenhanced region, avoiding the hypoenhanced part situated behind the needle (Aloka F75 platform)
Figure 3.
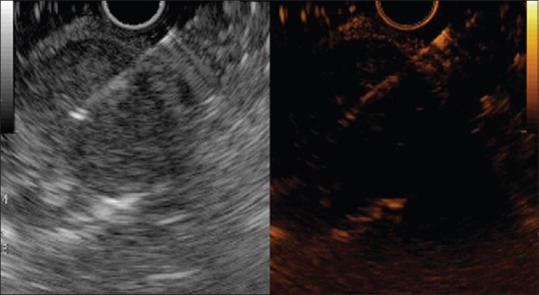
The contrast harmonic endoscopic ultrasonography-fine-needle aspiration of a submucosal gastric neoplasm (gastrointestinal stromal tumor). The lesion is hyperenhanced in the arterial phase, with fast washout. The needle is inserted into the lesion during the late venous phase, but very little contrast is still remaining inside (Aloka F75 platform)
When Sonazoid is used as a contrast agent, which persists more in the microvessels (several minutes), the insertion of the needle may be done after the entire assessment of the lesion under contrast enhancement. After infusion of 0.015 mL/kg of Sonazoid, the area with the widest contrast-enhanced area is identified and then the biopsy is directed toward that area while avoiding unenhanced areas.[24]
The normal EUS-FNA may be done (back-and-fro or fanning technique), with or without suction; different needles sizes can be used, and ROSE may be applied if possible.[24]
Indications
CONTRAST-ENHANCED HARMONIC ENDOSCOPIC ULTRASONOGRAPHY ORIENTED FINE-NEEDLE ASPIRATION OF PANCREATIC MASSES
Kitano reported that the use of Sonazoid gave an Area under the curve (AUC) of 92%; meanwhile, the AUC for EUS-FNA was 96%. The authors found that all ductal carcinomas with false-negative EUS-FNA findings had hypoenhancement, so the combination between the two methods has increased the sensitivity yield of the diagnostic to 100%. They concluded that it is likely that CH-EUS facilitates EUS-FNA of lesions by helping to identify the target tissue submitted to EUS-FNA[17]
In a multicenter study of solid pancreatic mass, the decision of FNA was guided by the hypoenhanced aspect in 26% of cases with a mixed pattern of adenocarcinoma, which turned into a 95% accuracy of EUS-FNA[15]
In cases of cystic pancreatic lesions, the hyperenhanced solid component directed the EUS-FNA in the potential neoplastic area in two intraductal papillary mucinous neoplasma and two neuroendocrine tumors, avoiding the puncture of debris and mucin plugs.[21]
CONTRAST-ENHANCED HARMONIC ENDOSCOPIC ULTRASONOGRAPHY-GUIDED FINE-NEEDLE ASPIRATION
This was reported for two cases of acinar cell carcinoma and pancreatic metastasis from lung cancer[9] and for one case of portal thrombosis.[25] The needle is clearly seen during the procedure, and the necrotic area or vessels are easy to avoid.
This technique was reported in three studies so far.
Afirst study used two arms, a randomized arm and consecutively an EUS-FNA arm with twenty patients for each group. The diagnostic rate was similar - 90% with contrast versus 85% without contrast, but the difference was important only for the first pass; this was performed by nonexperienced endosonographers and gave better results for the contrast groups - 60% versus 25%. The contrast technique limited the number of passes because 12 of the contrast group required only one pass for the diagnosis.[24]
A second retrospective study compared the CH-EUS-FNA group of 58 patients with a conventional EUS-FNA group of 105 patients. There were on average 3.7 passes per patient with a 22-G needle for the first group and 3.6 passes per patient for the second group. Cytology and cell blocks were prepared. SonoVue 4.8 mL was used as contrast substance, and tissue cytology was used for assessing the diagnosis. The accuracy was 87.9% in the CH-EUS-FNA group compared to 80% for the conventional EUS-FNA group of patients.[2]
A third prospective trial, conducted by our group, obtained 86% accuracy for two passes of CH-EUS-FNA compared to 78% for the two passes of EUS-FNA. When the two results were combined, the accuracy increased to 94% with a likelihood negative ratio of only 0.04.[20]
FACTS
Feasibility
The method is simple and the duration of the procedure is prolonged only a few minutes.
Reliability
All three studies on CH-EUS-FNA proved some superiority of the diagnosis rate by the simultaneous use of CH-EUS and EUS-FNA although the differences were nonsignificant. There are some limitations in these studies. First, none of them were multicenter trials and none of them reached the limit number of about 200 patients to produce a powerful statistical analysis. Second, the randomized study proved that the number of passes can be decreased when contrast CH-EUS is used simultaneously, but more studies are required, and it is too early to predict the future of this method.
Cost
The incremental cost per diagnostic sample was lower than repeating the EUS-FNA.[2] This is possible with up-to-date equipment which exists in many units, with the price of the vial of SonoVue which is not too high, and with the possibility that half of the vial is enough for one or two passes of FNA. Although CH-EUS-FNA is slightly more expensive and lasts longer, it can be considered cost-effective for EUS-FNA in pancreatic solid tumors.[2]
Safety
The risk profile is very low: anaphylactoid reactions (0.0002%) and in patients with severe coronary artery disease and pulmonary hypertension.[19] The complication rate for EUS-FNA is reported as 2.1% (acute pancreatitis, bleeding, and pain)[26,27] and no complications were reported in the above-mentioned studies with regard to the nonthermal effects induced by the contrast agents.
OPENED QUERIES
There are some questions which remain to be answered.
The best ultrasound machine and the best contrast agent. The use of Sonazoid gives the endosonographer more time for inserting the needle after the assessment of the entire lesion under CH-EUS. On the other hand, when SonoVue is used, it is preferable to have the needle inserted in the working channel before the injection of the contrast, and the lesion is targeted during the late venous phase. Then, there is a little contrast in the microvessels and even in the parenchyma (rapid wash-out patients), so the visibility of structures to be avoided during puncture such as the microvessels and necrosis area can be decreased. Better sensitivity of the ultrasound machines extends the duration of useful contrast enhancement[19]
The best area to be targeted depends also on the type of contrast agent used. All the authors consider that it is important to avoid the unenhanced areas suggestive of necrosis.[20,24] Furthermore, the inflammatory part of the malignant mass or the scirrhous part of the inflammatory mass has to be avoided; by doing so, the rate of insufficient material and the need to repeat EUS-FNA are reduced.[2] With Sonazoid, the widest contrast-enhanced area is targeted, whereas with SonoVue, the most hypoenhanced area must be targeted although the risk of puncturing areas of dense fibrosis still exists
The number of passes required to obtain a tissue diagnosis depends again on the type of assessment done: cytology, cell blocks, or core – histology
The risk for bleeding during puncture under contrast might be increased due to the presence of contrast which may impede the immediate blood coagulation.
CONCLUSION
CH-EUS-FNA is easy to perform and safe, with minimum added extra costs. The results are promising, but we will know only in the future its clinical impact and whether the lesion assessment is to be changed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.eusjournal.com
REFERENCES
- 1.Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(Suppl 1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 2.Hou X, Jin Z, Xu C, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: A retrospective study. PLoS One. 2015;10:e0121236. doi: 10.1371/journal.pone.0121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritscher-Ravens A, Brand L, Knöfel WT, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–75. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 4.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 9.Ueda K, Yamashita Y, Itonaga M. Real-time contrast-enhanced endoscopic ultrasonography-guided fine-needle aspiration (with video) Dig Endosc. 2013;25:631. doi: 10.1111/den.12165. [DOI] [PubMed] [Google Scholar]
- 10.Eloubeidi MA, Varadarajulu S, Desai S, et al. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567–70. doi: 10.1111/j.1440-1746.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 11.DeWitt J, McGreevy K, Sherman S, et al. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610–9. doi: 10.1016/j.gie.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Nicaud M, Hou W, Collins D, et al. The utility of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. Gastroenterol Res Pract 2010. 2010:268290. doi: 10.1155/2010/268290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu A, Saftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound. 2014;3:109–17. doi: 10.4103/2303-9027.123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitano M, Sakamoto H, Kudo M. Contrast-enhanced endoscopic ultrasound. Dig Endosc. 2014;26(Suppl 1):79–85. doi: 10.1111/den.12179. [DOI] [PubMed] [Google Scholar]
- 15.Gincul R, Palazzo M, Pujol B, et al. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: A prospective multicenter trial. Endoscopy. 2014;46:373–9. doi: 10.1055/s-0034-1364969. [DOI] [PubMed] [Google Scholar]
- 16.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy. 2010;42:564–70. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 17.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–10. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 18.Fusaroli P, Eloubeidi MA. Diagnosis of pancreatic cancer by contrast-harmonic endoscopic ultrasound (EUS): Complementary and not competitive with EUS-guided fine-needle aspiration. Endoscopy. 2014;46:380–1. doi: 10.1055/s-0034-1365425. [DOI] [PubMed] [Google Scholar]
- 19.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 20.Seicean A, Badea R, Moldovan-Pop A, et al. Harmonic contrast-enhanced endoscopic ultrasonography for the guidance of fine-needle aspiration in solid pancreatic masses. Ultraschall Med. 2015 doi: 10.1055/s-0035-1553496. [In press] [DOI] [PubMed] [Google Scholar]
- 21.Fusaroli P, Serrani M, De Giorgio R, et al. Contrast harmonic-endoscopic ultrasound is useful to identify neoplastic features of pancreatic cysts (With videos) Pancreas. 2016;45:265–8. doi: 10.1097/MPA.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 22.Fusaroli P, Kypraios D, Mancino MG, et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063–9. doi: 10.1111/j.1440-1746.2012.07115.x. [DOI] [PubMed] [Google Scholar]
- 23.Faccioli N, Crippa S, Bassi C, et al. Contrast-enhanced ultrasonography of the pancreas. Pancreatology. 2009;9:560–6. doi: 10.1159/000225960. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Takagi T, Hikichi T, et al. Conventional versus contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538–41. doi: 10.1016/j.pan.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Moreno M, Gimeno-García AZ, Corriente MM, et al. EUS-FNA of a portal vein thrombosis in a patient with a hidden hepatocellular carcinoma: Confirmation technique after contrast-enhanced ultrasound. Endoscopy. 2014;46:E590–1. doi: 10.1055/s-0034-1390734. [DOI] [PubMed] [Google Scholar]
- 26.Jenssen C, Alvarez-Sánchez MV, Napoléon B, et al. Diagnostic endoscopic ultrasonography: Assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659–76. doi: 10.3748/wjg.v18.i34.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy Technical Guideline. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


