Abstract
Endoscopic ultrasound (EUS) has become as one the best diagnostic and therapeutic methods for the management of several intraintestinal and extraintestinal diseases, among them to highlight pancreaticobiliary indications, mediastinal evaluation, and the analysis of gastrointestinal lesions. Over the years, there has been an enormous evolution in the systems available to perform EUS. Newer processors and echoendoscopes are available nowadays, with the ability to perform new imaging analysis, such as elastography and contrast enhancement. In the present article, we will review which systems are available nowadays, focusing also in the technical advances associated.
Keywords: Contrast enhancement, echoendoscopes, echoprocessors, endoscopic ultrasound
INTRODUCTION
Endoscopic ultrasound (EUS) is considered mixed a diagnostic and therapeutic technique in the field of gastrointestinal and extraintestinal diseases, combining both endoscopy and ultrasound by the use of special transducers coupled at the tip of the endoscope.[1] This method enables the use of high-frequency scanning and different associated technologies.
Over the years, EUS is continuously expanding its clinical applications.[2] It is now considered as the method of choice for the evaluation of the pancreaticobiliary system such as the evaluation of pancreatic lesions,[3] gastrointestinal tract such as gastrointestinal stromal tumor lesions[4] and adjacent structures, and its corresponding diseases. Nowadays, EUS is considered as an excellent diagnostic tool, including the possibility of performing tissue acquisition and thus obtaining cytopathological or histopathological diagnosis of mediastinal, abdominal, retroperitoneal and pelvic lesions.[5] EUS has evolved to became an excellent therapeutic tool, allowing the performance of celiac plexus neurolysis,[6] drainage of fluid collections, or bile and pancreatic ducts.[7]
Recent innovations in EUS have attempted to even increase the diagnostic capabilities of this technique. Among them Doppler, real-time elastography,[8] and contrast enhancement,[9] the American Society for Gastrointestinal Endoscopy has published an article on the different systems available for the performance of this technique[1] as well as on some technical issues on contrast enhancement.[10]
In the present manuscript, we will review which area the current available echoendoscopes and specific processors for the performance of EUS, focusing on its capabilities to perform contrast-enhanced imaging.
INSTRUMENTATION
EUS equipment consists on ultrasound processor, connected to specific echoendoscopes, either with radial or linear transducer design coupled at the tip of an endoscope. Scopes are also connected to a standard video processor. The complete system allows a simultaneous endoscopic and ultrasound imaging. Nowadays, several systems are available from different companies.
Echoprocessors
Ultrasound processor can be considered, together with the development of the echoendoscopes as the key point in the development of EUS over the past years. The ultrasound processors interpret the electrical signals provided by the echoendoscopes, producing ultrasound image on the monitor. Basic image obtained is that considered to be B-mode, a two-dimensional image of the reflected sound waves. These new processors have increased resolution and incorporate additional imaging features including Doppler/power flow, tissue elastography, and an ability to visualize newer contrast agents. Focusing on the use of contrast agents, it is the development of contrast-enhanced EUS, which has evolved from simple power Doppler evaluation with contrast injection to a wideband dedicated transducer, which is now widely known as contrast-enhanced harmonic EUS.[10] This harmonic technology allows detection of microbubbles within slow-flow microvessels without Doppler-related artifacts.
Three major manufacturers, Fujifilm Endoscopy (Fujifilm Europe GmbH, Germany), Olympus (Olympus Europa SE and Co., KG), and Hitachi (Hitachi Medical Systems Europe, Zug, Switzerland), produce ultrasound processor, compatible with different types of echoendoscopes.[1]
Hitachi-Aloka processors
Processors are of various models, compatible either with Pentax or Olympus echoendoscopes.
Processors compatible with Pentax echoendoscopes
The Hitachi-Aloka HI VISION series, which includes Avius, Preirus, and Ascendus [Figure 1], classified in this order according to their capabilities, being the latter one the most advance and powerful system. All three of them include the option for contrast-enhanced examination with the administration of microbubble-based contrast agents since they had a contrast specific mode on the processor intended to aid recognition of microvasculature and staging. The system also includes the option to perform real-time elastographic evaluation (including all options for quantitative evaluation, such as strain ratio and strain histogram, as well as the option for frame selection and frame average). As additional option, system included HI-COM, HI-REZ, and dTHI. A smaller processor has recently launched, the HI VISION Noblus [Figure 2], a tabletop processor including an integrated monitor and console, similar to and not much larger than a laptop computer, also with all technical options available.
Figure 1.

Detail image of the echoprocessor Hitachi-Aloka HI VISION Ascendus (courtesy of Hitachi-Medical Systems and Pentax Medical) to be used with Pentax-Medical echoendoscopes
Figure 2.
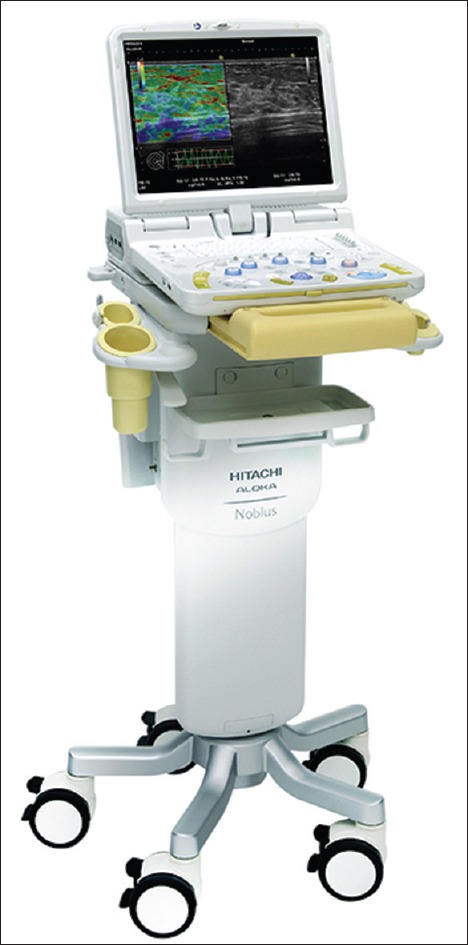
Detail image of the echoprocessor Hitachi-Aloka HI VISION Noblus (courtesy of Hitachi-Medical Systems and Pentax Medical) to be used with Pentax-Medical echoendoscopes (courtesy of)
A new system has recently launched the market, compatible with all Pentax echoendoscopes, Hitachi-Aloka Arietta V70 [Figure 3]. A multilayered and single crystal technology allowing more efficient transmission and reception of the ultrasound pulse with minimal energy loss, increasing both the sensitivity and clarity of the images. The processor associates the compound pulsed wave generator (CPWG+). Finally, system is equipped with advanced technologies, such as real-time virtual sonography and real-time tissue elastography. Apart from including latest versions of contrast enhancement advance technologies, it associates a high-resolution B-mode imaging coupled with eFLOW, the advanced flow mapping mode that offers increased sensitivity to flow in minute vessels.
Figure 3.
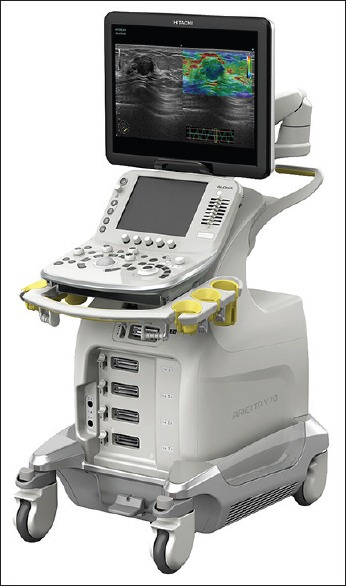
Detailed image of the echoprocessor Hitachi-Aloka Arietta V70 (courtesy of (courtesy of Hitachi-Medical Systems and Pentax Medical) to be used with Pentax-Medical echoendoscopes
Processors compatible with Olympus echoendoscopes
The latest version is the system ProSound F75 [Figure 4], manufactured by Hitachi-Aloka. It is a new digital platform designed for use with all Olympus echoendoscopes. This new system included the broadband harmonics, a spatial compound imaging, the use of trapezoidal scan, and the possibility to perform contrast echo with the newest contrast agents.
Figure 4.
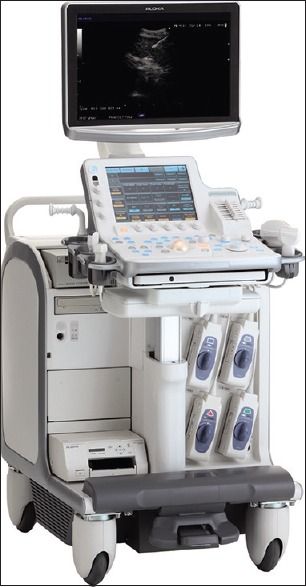
Image corresponding to the echoprocessor Hitachi-Aloka system ProSound F75 (courtesy of OLYMPUS EUROPA SE and CO., KG)
The ProSound Alpha 7 is the older version of the Hitachi-Aloka processors compatibles with Olympus echoendoscopes. This system includes the broadband harmonics technology and the directional eFLOW which enhance spatial resolution for greater detail of blood flow information. Contrast harmonic echo is compatible with all high-, medium-, and low-sound pressure contrast agents.
Olympus processor
It includes one model called the EU-ME2 [Figure 5] designed to be used with Olympus echoendoscopes and for integration with conventional endoscopy on a single workstation. This compact processor allows a broad range of EUS frequencies (5–20 MHz), elastography (optional), and miniprobe three-dimensional rendering. The processor has other features including pulse wave Doppler and high-resolution flow mode with the contrast harmonics. This processor does not come equipped with a monitor but can be set up with existing monitors (depending on resolution and input jacks).
Figure 5.

Image of the compact echoprocessor EU-ME2 (courtesy of OLYMPUS EUROPA SE and CO., KG), to be used with the Olympus echoendoscopes
Fujifilm processor
It includes one model namely the SU-8000 US processor which is the smallest processor. It uses Zone Sonography Technology that delivers wide ultrasound beams and acquires large amount of echoendoscopic data in zones. The data are processed by estimating and selecting the optimal ultrasound speed that produces the highest lateral resolution to construct the EUS image. The SU-8000 also has a range of 5–12 MHz. Although this processor does not offer elastography, it does have color Doppler capabilities.
The latest processor for Fujifilm echoendoscopes is the SU-1 [Figure 6], which provides with frequencies ranging from 5 to 12 MHz, the option of contrast harmonic imaging, optimize function and sound speed correction, and elastography.
Figure 6.

SU-1 echoproccesor (courtesy of Fujifilm España, Fujifilm Europe GmbH) to be connected to Fujifilm echoendoscopes
Echoendoscopes
As previously stated, echoendoscopes consist on an ultrasound transducer attached to the tip of an endoscope. Initial echoendoscopes used a mechanical scanner, however, latest versions and advances in ultrasound imaging, including EUS bases, moved to the electronic transducers. The transmitter energizes the transducer elements by timed, high-amplitude voltages. This transducer contains piezoelectric crystals that change shape responding to the applied voltage. Crystals convert electrical energy to mechanical energy, the so-called sound waves. Sound waves will be transmitted to the specific tissue under evaluation, and reflected sound waves are captured by the transducer and converted into electrical signals by the reverse piezoelectric effect. The variations in image glare are a consequence of different amplitudes of sound wave signals reflected from tissues or organs under evaluation. The number of this crystal contained in the transducers is variable, with the ability to alter the focal distance and use tissue harmonic enhancement, improving the resolution of the image. By increasing the number of piezoelectric elements, the lateral resolution of the image also increases. Echoendoscopes have variable frequencies, being the most common between 5 and 12 MHz. Higher frequencies limit the penetration of the ultrasound beam but improve resolution.
There are two types of echoendoscopes, radial and linear.[1,11] Radial EUS is mainly used for luminal imaging and evaluation of the wall layers of the gastrointestinal tract although it is possible to evaluate almost same structures as with the linear EUS, such as the pancreaticobiliary systems and the mediastinum. Linear EUS has an important add value since it allows tissue sampling and therapeutic applications in real time, and it has also been considered to be a better tool also for pure diagnostic imaging purposes. All new advance image technologies, such as elastography and contrast-enhanced imaging, can be performed with both type of scopes.
Three major manufacturers, Fujifilm Endoscopy (Fujifilm Europe GmbH, Germany), Olympus (Olympus Europa SE and Co., KG), and Pentax (Pentax Europe GmbH, Hamburg, Germany), produce both radial and linear echoendoscopes.
Radial echoendoscopes
Radial transducers orient the individual piezoelectric elements around the distal tip in a 360° radial array, producing an image in a plane perpendicular to the long axis of the echoendoscope. As we have stated, these radial echoendoscopes are used only for diagnostic purposes since they do not allow tissue sampling and therapeutic interventions.
The echoendoscopes are very similar in shape, and all provide a 360 field of view. The endoscopic camera is end-viewing on the Pentax (EG-3630URK) and Fujifilm echoendoscopes (EG-530UR2 and EG-580UR), whereas Olympus echoendoscopes provide with an oblique view (GF-UE160-AL5). Most of the radial echoendoscopes image at an adjustable frequency of 5, 7.5, or 10 MHz. The radial echoendoscopes also come in slightly different shaft diameters. Fujifilm offers the slimmest (11.5 mm) and most flexible echoendoscope, whereas the Olympus echoendoscope has the widest diameter (13.8 mm) at the junction of the shaft and the ultrasound transducer.
Linear echoendoscopes
All currently available electronic linear instruments produce ultrasound images in a plane parallel to the long axis of the echoendoscope, usually in a sector between 100° and 180°. This ultrasound image orientation is a key point for tissue acquisition and therapeutic interventions as EUS devices are advanced from the distal tip of the echoendoscope in the same plane as the ultrasound image. This allows for simultaneous visualization of the target lesion and the EUS device during the complete procedure. In addition, all linear instruments incorporate an elevator at the distal end of the working channel allowing control of the angle of exit of EUS needles or other devices from the working channel.
Among different companies, echoendoscopes differ in the ultrasound tip design, flexibility, balloon insufflation control design, and bend points at the distal end of the echoscopes. All of the EUS transducers have a curved design and are located distal to the oblique-viewing endoscopic camera lens.
Olympus linear platform consists of three main scopes:
GF-UCT180 allowing higher resolution imaging compared with older models. It has a 3.7-mm working channel, a 14.6-mm diameter distal tip, and as well as a detachable cable allowing easier insertion of the echoendoscope into automated endoscopic processors [Figure 7]
GF-UC140P-AL5 with a working channel of 2.8 mm, and the therapeutic echoendoscope (GF-UCT140-AL5) with 3.7-mm working channel. Those are the older version of linear echoendoscopes
TGF-UC180J, a forward-viewing linear echoendoscopes, designed mainly for therapeutic applications. The working channel diameter is 3.7 mm, and the distal tip diameter is 14.6 mm. This forward-viewing echoendoscope has a short transducer and an end-viewing camera but no balloon or elevator. Ultrasound image is limited to a 90 range.
Figure 7.

Detailed image of the tip of the Olympus echoendoscope GF-UCT180 (courtesy of OLYMPUS EUROPA SE and CO., KG) suitable for Hitachi-Aloka ProSound F75, Alfa 7, and EU-ME2 echoprocessors
All current Olympus linear echoendoscopes are compatible with B-mode, color Doppler, pulse wave Doppler, H-Flow, and tissue harmonic echo (except the GF-UC140P-AL5 and GF-UCT140-AL5). Elastography is available for all linear echoendoscopes on the EU-ME2.
Pentax linear platform has two linear scopes:
EG-387OUTK with a 3.8-mm working channel. The diameter of the distal tip is 14.3 mm. This scope is suitable for all diagnostic and therapeutic procedures [Figure 8]
EG-327OUK is a recently designed echoendoscopes, considered mainly for diagnostic purposes including tissue acquisition procedures.[12] This scope has a distal tip diameter of 12 mm, with 2.8 mm of working channel [Figure 9].
Figure 8.

Image presents the EG-387OUTK therapeutic echoendoscopes from Pentax Medical (courtesy of Pentax Medical), suitable for Hitachi-Aloka HIVISON series, and the new Hitachi-Aloka Arietta V70
Figure 9.

Detailed image from the tip of the diagnostic slim linear echoendoscope from Pentax-Medical, EG-3270UK (courtesy of Pentax Medical), suitable for Hitachi-Aloka HI VISON series, and the new Hitachi-Aloka Arietta V70
All Pentax echoendoscopes allow performance of elastography, spatial compounding, tissue harmonics, Doppler/power Doppler, B-mode, and M-mode imaging.
The Fujifilm linear platform consists of one echoendoscope:
EG-580UT with a working channel of 3.8 mm and equipped with an Albarran lever [Figure 10]. EG-530UT2 is the former scope, which also allows passage of therapeutic devices and needle position guide on the ultrasound image.
Figure 10.

Detailed image from the tip of the EG-580UT echoendoscopes from Fujifilm (courtesy of Fujifilm España, Fujifilm Europe GmbH) to be used with Fujifilm echoproccesors
The scanning modes include color Doppler, power Doppler, pulse wave, B-mode, and M-mode.
FINAL CONSIDERATIONS
The beginning of an EUS unit, and thus, the initial costs for establishing an EUS program are not negligible. In fact, a complete system cost may be around 200,000–300,000 euros. When facing this, important cost is of crucial value to determine the nature of the practice, the anticipated volume, and anticipated types of cases (diagnostic alone or diagnostic and therapeutic procedures), which will determine the number and type of echoendoscopes that will need to be purchased. Maintenance costs for an EUS program are also considerable. This is mainly due to damage that may occur from needle punctures of the echoendoscope, breaking of the elevator, and other general repairs. Data available in the literature estimated a mean maintenance cost of $41 per procedure.[13]
However, taking into account all the accepted and recent indications of EUS, it has demonstrated to be a cost-effective technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.ASGE Technology Committee. Murad FM, Komanduri S, et al. Echoendoscopes. Gastrointest Endosc. 2015;82:189–202. doi: 10.1016/j.gie.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Luthra AK, Evans JA. Review of current and evolving clinical indications for endoscopic ultrasound. World J Gastrointest Endosc. 2016;8:157–64. doi: 10.4253/wjge.v8.i3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglesias-García J, Lindkvist B, Lariño-Noia J, et al. The role of EUS in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma. Rev Esp Enferm Dig. 2012;104:315–21. doi: 10.4321/s1130-01082012000600006. [DOI] [PubMed] [Google Scholar]
- 4.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: Comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479–89. doi: 10.1111/den.12149. [DOI] [PubMed] [Google Scholar]
- 5.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 6.Fujii-Lau LL, Bamlet WR, Eldrige JS, et al. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc. 2015;82:46–56.e2. doi: 10.1016/j.gie.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman CG, Siddiqui UD. New scopes, new accessories, new stents for interventional endoscopic ultrasound. Clin Endosc. 2016;49:41–6. doi: 10.5946/ce.2016.49.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, et al. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8–16. doi: 10.7178/eus.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrani M, Lisotti A, Caletti G, et al. Contrast enhancement and elastography in endoscopic ultrasound: An update of clinical applications in pancreatic diseases. Minerva Med. 2016;107:217–22. [PubMed] [Google Scholar]
- 10.Pedrosa MC, Barth BA, Desilets DJ, et al. Enhanced ultrasound imaging. Gastrointest Endosc. 2011;73:857–60. doi: 10.1016/j.gie.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV-EUS-guided interventions: General aspects and EUS-guided sampling (short version) Ultraschall Med. 2016;37:157–69. doi: 10.1055/s-0035-1553788. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias-García J, Lariño-Noia J, Vallejo-Senra N, et al. Feasibility of endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) and biopsy (FNB) with a new slim linear echoendoscope. Rev Esp Enferm Dig. 2015;107:359–65. [PubMed] [Google Scholar]
- 13.Schembre D, Lin O. Frequency and costs of echo endoscope repairs: Results of a survey of endosonographers. Endoscopy. 2004;36:982–6. doi: 10.1055/s-2004-825862. [DOI] [PubMed] [Google Scholar]


