Abstract
Endobronchial ultrasound (EBUS) has gained importance for mediastinal lymph node staging. Contrast-enhanced EBUS is so far not a discussed technique including contrast-enhanced high mechanical index (MI)-EBUS and potentially contrast-enhanced low MI-EBUS. Possible use could include characterization of mediastinal lymph nodes for better selection of biopsies, differential diagnosis of the primary tumor, and evaluation of thrombosis or tumor in vein infiltration.
Keywords: Consolidation, guidelines, lung cancer, lymph node, lymphadenopathy, ultrasound contrast agents
INTRODUCTION
Endobronchial ultrasound (EBUS) has gained importance in the staging of mediastinal lymph nodes[1,2] and evaluation of primary lung cancer under various circumstances.[3,4,5] Recently published lung cancer staging guidelines recommend that endosonography (EBUS, endoscopic ultrasound [EUS]) should be the initial tissue-sampling procedure before surgical staging.[1,2,3,4] The value of conventional ultrasound technologies for mediastinal lymph node staging has been recently published.[3,4,6,7]
Beyond the well-recognized value of conventional EBUS, contrast-enhanced EBUS (CE-EBUS) has not been described to date. Herein, the potential uses of CE-EBUS are discussed. Acknowledging this method will not revolutionize EBUS; it may prove helpful in individual circumstances and improve understanding of the principles of CEUS techniques in the lung. We report on our limited experience using CE-EBUS and the potential value of this method.
CONTRAST-ENHANCED ENDOBRONCHIAL ULTRASOUND
Contrast-enhanced EUS
Contrast-enhanced EUS (CE-EUS) was introduced more than 10 years ago and its role has been extensively discussed.[8,9,10,11] CE-EUS has been incorporated into many guidelines.[12,13,14,15,16] In contrast to CE-EUS, the potential role of CE-EBUS has not been raised. Possible uses could include characterization of mediastinal lymph nodes for better selection of biopsy targets, clarification of the differential diagnosis of primary tumors, and evaluation of either thrombosis or tumor infiltration within vascular structures.
A short introduction into terminology
The acronym CEUS was introduced by the members of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) and is now accepted as the official term for all contrast-enhanced ultrasound techniques.[16,17,18,19,20,21,22,23,24,25,26] Accordingly, the term “CE-EUS” is used as a generic term for all contrast-enhanced techniques used with EUS, independent of the particular physical principles employed. Contrast enhancement of EUS examinations is possible using low or high mechanical index (MI). Therefore, the acronyms for high MI techniques have been defined (contrast-enhanced high MI-EUS [CEHMI-EUS], first published in 1997 and 2001[27,28,29,30] and contrast-enhanced low MI-EUS [CELMI-EUS], first applied in 2003 and published in 2005 and 2009).[31,32] CELMI-EUS was the original approach; however, the low MI techniques with either filters, wideband harmonic (phase or pulse) or cancellation techniques,[33] are not yet available for EBUS due to technical reasons.
Contrast-enhanced endoscopic Doppler techniques (color Doppler, power Doppler, others) use a high(er) MI and therefore are included within the CEHMI-EUS category or alternatively termed as CED-EUS (contrast-enhanced Doppler-EUS).[34] However, higher mechanical indices techniques are also used for (intermittent) harmonic imaging. These acronyms are independent from terminology created by specific manufacturers.
To summarize, there are two possible techniques to perform CE-EBUS; CEUS was originally designed to enhance Doppler signals[16,21,22] and CEHMI-EBUS is mainly discussed in this review [Figures 1–3]. CELMI-EBUS has not been introduced into daily routine. CELMI-EUS has only been used in very few and special cases, with conventional EBUS-scanners introduced into the bronchi.
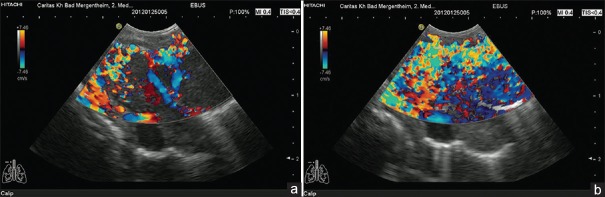
Figure 1.
Contrast-enhanced high mechanical index endobronchial ultrasound in a patient with lung carcinoma. The large lesion is shown with arterial signals in the surrounding vessels (a). The conventional color Doppler image showed only a large vessel in the surrounding (left lower part) of a partially necrotic lung carcinoma (b). Contrast-enhanced ultrasound using the contrast agent SonoVue® revealed significant enhancement of the Doppler signals after 16 and 17 s postinjectionem (c and d) and the decrease of enhancement 32 s postinjectionem (e). The clinical use was to avoid biopsy in the nonenhancing areas which finally have proven to be necrosis
Figure 3.
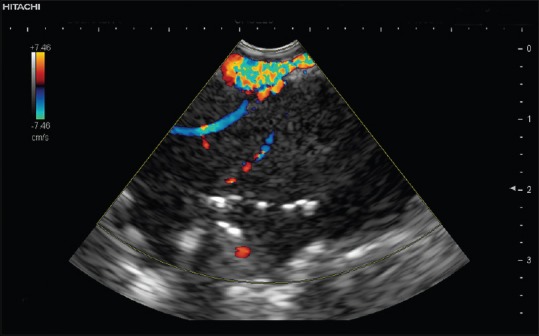
Contrast-enhanced high mechanical index endobronchial ultrasound with atelectasis. Atelectasis is characterized by the nonneoplastic straight vessels and aerobronchogram
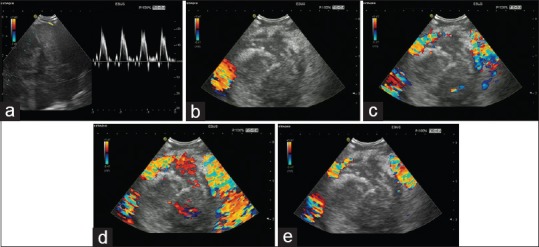
Figure 2.
Contrast-enhanced high mechanical index endobronchial ultrasound in a patient with lung carcinoma. Neoplastic vessels are only visualized using contrast-enhanced ultrasound (a). Biopsy was performed according to the guidance of vascularization to avoid necrosis. Blooming artifacts should be avoided which might occur directly after injection of the contrast agent (b)
Contrast-enhanced endobronchial ultrasound
Prerequisites for contrast-enhanced endobronchial ultrasound
Ultrasound imaging of the normal lung is not possible because ultrasound waves are reflected at the lung surface. Only the mediastinum and so-called “consolidations” (e.g., infiltration, atelectasis, and neoplasia) can be visualized when they abut the transducer. Peribronchial pathological processes may result in profound changes of tissue composition. Inflammatory and neoplastic processes may significantly improve acoustic transmission and allow for adequate EBUS evaluation. Visualization of deeper structures can be hampered by artifacts.[35,36]
Contrast-enhanced endobronchial ultrasound
CE-EBUS examination is, therefore, limited to the mediastinum including its vessels, lymph nodes, and mass lesions and to peribronchial parenchymal lung consolidations. The dual (pulmonary versus bronchial) arterial blood supply is important to consider when one utilizes CEUS to differentiate focal lung lesions by assessing the timing and extent of contrast enhancement.
Examination technique and dosage
CEUS is the application of ultrasound contrast agents (UCAs) using contrast adopted or specific modes. For further explanation, we refer to published guidelines. Currently used UCA is microbubbles stabilized by a shell, with a high degree of echogenicity. In Europe and Asia, the most commonly used UCA is SonoVue. SonoVue bubbles are just 1–4 µm in diameter (equal to or smaller than red blood cells). UCA allows depiction of both the macrovasculature and the microvasculature. A SonoVue® 4.8 mL bolus injection is recommended for imaging with a high-frequency probe in CE-EBUS.[10]
What should we know about contrast enhancement in the lung circulation?
The contrast agent arrives in the right heart and the pulmonary artery a few seconds (<4–7 s) after injection, indicating arterial pulmonary enhancement (“early arterial [pulmonary] enhancement”). It thereafter passes through the left heart, determining the start of systemic “late arterial bronchial” enhancement immediately. In patients with cardiac and/or pulmonary disease, the arrival time of pulmonary arterial supply may be longer than in cardio vascularly healthy subjects.
Typically, contrast enhancement is early and marked in pneumonia, followed by the combined supply from the pulmonary and bronchial arteries. Late arterial bronchial enhancement is seen in lung carcinoma.
Lymphadenopathy
EBUS is most importantly employed in the evaluation of mediastinal lymphadenopathy.
Color Doppler imaging (CDI) adds value in the differentiation of malignant from normal or inflammatory lymph nodes by displaying the macrovessel architecture. Normal lymph nodes generally show hilar-predominant normal vascularity. In benign lymph nodes, contrast enhancement within the cortex is homogeneous. Inflammatory lymph nodes are typically more vascularized without changes of the predominant hilar vessel architecture. In contrast, metastatic lymph nodes present peripheral or mixed vascularity with loss of the hilar-type vascularization.[10,37] Demonstration of malignant neovascularization, for example, vessels penetrating the lymph node capsule, has been used as the characteristic feature of lymph node metastases.[10]
Spectral Doppler ultrasound contributes to differentiation of malignant and benign lymph nodes. Normal and inflammatory lymph nodes show lower vascular resistance (resistive index) as compared to malignant lymph nodes,[38] but practicability and overall results are disappointing.[10]
Contrast-enhanced CDI (CEHMI-EBUS) improves the visualization of macrovessels (angioarchitecture)[39] and allows improved spectral Doppler ultrasound examination. Although Doppler ultrasound techniques have extended the opportunities for differentiation of malignant from benign lymph nodes by displaying changes of macrovascularity and the vascular resistance,[37,40,41] they do not improve lymph node detection rate and vascularity is often not detected in small lymph nodes.[42] Unfortunately, in general, Doppler techniques and contrast-enhanced Doppler techniques have not significantly improved the diagnostic workup of lymphadenopathy in daily routine.
There is a need for new imaging techniques using CELMI-EBUS for better characterization of lymph nodes, with the opportunity to assess also the internal microvessel architecture of lymph nodes. CELMI-EBUS could be also helpful identifying neoangiogenesis. Neoangiogenesis is characterized by peripherally located focal cortical thickening with arteriovenous shunts and caliber changes of the neoplastic vessels. This may lead to heterogeneous rim enhancement.[43] Focal hypoenhancement may be caused by high pressure in the lymph node. Nonenhancement (suggesting necrosis) is also an important imaging sign of malignant infiltration.[44,45] It is worth mentioning that nondestructive necrosis, which is reflected in avascular areas on CEUS, can be also found in granulomatous lymphadenitis, for example, cat-scratch disease (bartonellosis), tuberculosis, and sarcoidosis.[10]
Lymph node-specific UCAs have not been introduced so far.
In conclusion, criteria for carcinomatous lymph node infiltration on CEUS are centripetal inhomogeneous enhancement, changes in vascular architecture of microvessels and avascular areas as signs of malignant infiltration.[10]
Lymphoma
It is essential to consider lymphoma separately because of the different features to other lymph node disease.[37,46] EBUS is not a routine diagnostic procedure for the diagnosis and staging of lymphoma. Very few studies published to date have found lymphoma contrast enhancement patterns to be highly variable; the most often observed pattern is intense homogeneous enhancement, similar to reactive inflammatory lymph nodes. In conclusion, there is evidence that the vascular pattern of lymphomatous lymph node infiltration resembles that of nonmalignant nodes.[10]
Inflammatory lymphadenopathy
Most inflammatory processes do not change the hilar-predominant vessel architecture of lymph nodes. According to the majority of published papers, normal and inflammatory lymph nodes are characterized by a centrifugal and homogeneous enhancement pattern. Therefore, inflammation changes only the peak enhancement, not the pattern of distribution.
Lung consolidation
In contrast to the pleural and subpleural tissues, the ventilated lungs cannot be assessed by ultrasound examination. Pathologies of the lung can be only investigated by ultrasound when they are closely located and in physical contact to the transducer.[7] The following statements are deduced from CEUS applications of the lung in general.
Pneumonia
Using EBUS, the differentiation of pneumonic infiltration from other consolidations might be difficult and fine needle aspiration biopsy and cytological (histological) assessment are necessary. CEUS allows demarcation of abscesses and necrosis formation, as regions of absent enhancement.
Atelectasis
Contrast enhancement in atelectasis is similar to pneumonia [Figure 3], with early and marked enhancement, followed by a plateau. Centrally located obstructive lung carcinoma may be differentiated from atelectasis by later enhancement and also by other techniques.[7,47,48]
Pulmonary embolism
In pulmonary infarcts caused by pulmonary emboli, there is a reduced contrast enhancement in the first 30 s after UCA administration and, therefore, delayed enhancement.[7]
Lung carcinoma (and metastasis)
Arteries supplying lung carcinoma show late onset and a variable degree of contrast enhancement (due to their bronchial origin). Most importantly, CEUS allows improved targeting of enhancing tissue compared to nonenhancing necrotic zones, which should be avoided at biopsy.
Vascular infiltration
CE-EBUS may differentiate between vascular, neoplastic infiltration (enhancing) and appositional thrombus (nonenhancing).
POTENTIAL ADDITIONAL TECHNIQUES AND INDICATIONS
Dynamic contrast-enhanced ultrasound
Dynamic contrast-enhanced ultrasound (DCE-US) is an imaging technique utilizing microbubble contrast agents combined with quantification of tissue perfusion over time including parametric imaging[23] and has been adopted by EFSUMB as the term describing time-intensity curve analysis.[23,49] Its role in conventional EUS and other applications have been recently published.[49,50,51] DCE-US has not been applied to date in CE-EBUS and so needs further evaluation.
Functional assessment of lung tumor response
Due to recent advances in angiogenesis and its use for therapeutic indications, for example, tyrosine kinase inhibitory therapies, it is apparent that new imaging modalities are needed for this purpose. The commonly used Response Evaluation Criteria in Solid Tumors criteria based on the diameter of lesions do not fulfill the requirements for functional assessment of tumor response to the targeted therapies mentioned. The role of functional assessment of tumor response in CE-EBUS is a so far not applied technique and needs further evaluation.
SUMMARY AND CONCLUSIONS
This review concludes the potential role of CE-EBUS in:
Characterization of mediastinal lymphadenopathy
Characterization of mediastinal and lung masses
Characterization of invasion of vascular structures (neoplasia versus appositional thrombus).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 2.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, Part I: Endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311–25. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenssen C, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, Part 2: Mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis. 2015;7:E439–58. doi: 10.3978/j.issn.2072-1439.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:c1. doi: 10.1055/s-0034-1392453. [DOI] [PubMed] [Google Scholar]
- 6.De Leyn P. Clinical value of ESTS guidelines on preoperative lymph node staging for NSCLC. Eur J Cardiothorac Surg. 2011;40:280–1. doi: 10.1016/j.ejcts.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Mathis G, Cui XW, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol. 2015;41:351–65. doi: 10.1016/j.ultrasmedbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Saftoiu A, Vilmann P, Dietrich CF, et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2015;82:59–69. doi: 10.1016/j.gie.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Kitano M, Kamata K, Imai H, et al. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27(Suppl 1):60–7. doi: 10.1111/den.12454. [DOI] [PubMed] [Google Scholar]
- 10.Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850–60. doi: 10.3748/wjg.v19.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich CF, Sharma M, Hocke M. Contrast-enhanced endoscopic ultrasound. Endosc Ultrasound. 2012;1:130–6. doi: 10.7178/eus.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich CF, Lorentzen T, Sidhu PS, et al. An introduction to the EFSUMB Guidelines on Interventional Ultrasound (INVUS) Ultraschall Med. 2015;36:460–3. doi: 10.1055/s-0035-1553462. [DOI] [PubMed] [Google Scholar]
- 13.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General aspects (long Version) Ultraschall Med. 2015;36:E1–14. doi: 10.1055/s-0035-1553593. [DOI] [PubMed] [Google Scholar]
- 14.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV – EUS-guided interventions: General aspects and EUS-guided sampling (Long Version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 15.Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V. Ultraschall Med. 2016;37:77–99. doi: 10.1055/s-0035-1553738. [DOI] [PubMed] [Google Scholar]
- 16.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich CF, Ignee A, Trojan J, et al. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401–5. doi: 10.1136/gut.2003.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich CF, Schuessler G, Trojan J, et al. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–7. doi: 10.1259/bjr/88181612. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht T, Blomley M, Bolondi L, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249–56. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 20.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) – Update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 21.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver – Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 22.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver – Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich CF, Averkiou MA, Correas JM, et al. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–51. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich CF. Signalverstärkte Lebersonographie zur verbesserten Detektion und Charakterisierung von Leberraumforderungen. Dtsch Arztebl. 2002;24:1666–1672. [Google Scholar]
- 25.Dietrich CF. 3D real time contrast enhanced ultrasonography, a new technique. Rofo. 2002;174:160–3. doi: 10.1055/s-2002-20102. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich CF, Brunner V, Braden B, et al. Erste Erfahrungen mit einem neuen Signalverstärker bei der Untersuchung der Leber. Ultraschall Med. 1998;19:S21. (Abstract) [Google Scholar]
- 27.Bhutani MS, Hoffman BJ, van Velse A, et al. Contrast-enhanced endoscopic ultrasonography with galactose microparticles: SHU508 A (Levovist) Endoscopy. 1997;29:635–9. doi: 10.1055/s-2007-1004270. [DOI] [PubMed] [Google Scholar]
- 28.Hirooka Y, Naitoh Y, Goto H, et al. Usefulness of contrast-enhanced endoscopic ultrasonography with intravenous injection of sonicated serum albumin. Gastrointest Endosc. 1997;46:166–9. doi: 10.1016/s0016-5107(97)70067-1. [DOI] [PubMed] [Google Scholar]
- 29.Hirooka Y, Goto H, Ito A, et al. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: A preliminary study. Am J Gastroenterol. 1998;93:632–5. doi: 10.1111/j.1572-0241.1998.179_b.x. [DOI] [PubMed] [Google Scholar]
- 30.Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–9. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: A new technique. Z Gastroenterol. 2005;43:1219–23. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich CF. Contrast-enhanced low mechanical index endoscopic ultrasound (CELMI-EUS) Endoscopy. 2009;41(Suppl 2):E43–4. doi: 10.1055/s-0028-1119491. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich CF, Fusaroli P, Jenssen C. European Federation of Societies for Ultrasound in Medicine and Biology guidelines 2015 on interventional endoscopic ultrasound. Endosc Ultrasound. 2016;5:143–8. doi: 10.4103/2303-9027.183968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: Contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–50. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Tuma J, Jenssen C, Möller K, et al. Ultrasound artifacts and their diagnostic significance in internal medicine and gastroenterology – Part 1: B-mode artifacts. Z Gastroenterol. 2016;54:433–50. doi: 10.1055/s-0042-103247. [DOI] [PubMed] [Google Scholar]
- 36.Jenssen C, Tuma J, Möller K, et al. Ultrasound artifacts and their diagnostic significance in internal medicine and gastroenterology – Part 2: Color and spectral Doppler artifacts. Z Gastroenterol. 2016;54:569–78. doi: 10.1055/s-0042-103248. [DOI] [PubMed] [Google Scholar]
- 37.Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005;184:1691–9. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 38.Ying M, Ahuja A. Sonography of neck lymph nodes. Part I: Normal lymph nodes. Clin Radiol. 2003;58:351–8. doi: 10.1016/s0009-9260(02)00584-6. [DOI] [PubMed] [Google Scholar]
- 39.Schmid-Wendtner MH, Partscht K, Korting HC, et al. Improved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast-enhanced color Doppler sonography. Arch Dermatol. 2002;138:491–7. doi: 10.1001/archderm.138.4.491. [DOI] [PubMed] [Google Scholar]
- 40.Ahuja A, Ying M. Sonographic evaluation of cervical lymphadenopathy: Is power Doppler sonography routinely indicated? Ultrasound Med Biol. 2003;29:353–9. doi: 10.1016/s0301-5629(02)00759-7. [DOI] [PubMed] [Google Scholar]
- 41.Tschammler A, Heuser B, Ott G, et al. Pathological angioarchitecture in lymph nodes: Underlying histopathologic findings. Ultrasound Med Biol. 2000;26:1089–97. doi: 10.1016/s0301-5629(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 42.Moritz JD, Ludwig A, Oestmann JW. Contrast-enhanced color Doppler sonography for evaluation of enlarged cervical lymph nodes in head and neck tumors. AJR Am J Roentgenol. 2000;174:1279–84. doi: 10.2214/ajr.174.5.1741279. [DOI] [PubMed] [Google Scholar]
- 43.Rubaltelli L, Beltrame V, Tregnaghi A, et al. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR Am J Roentgenol. 2011;196:W8–12. doi: 10.2214/AJR.10.4711. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi T, Yamashita Y, Katahira K, et al. Differential diagnosis of small round cervical lymph nodes: Comparison of power Doppler US with contrast-enhanced CT and pathologic results. Radiat Med. 2001;19:119–25. [PubMed] [Google Scholar]
- 45.King AD, Tse GM, Ahuja AT, et al. Necrosis in metastatic neck nodes: Diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004;230:720–6. doi: 10.1148/radiol.2303030157. [DOI] [PubMed] [Google Scholar]
- 46.Nakase K, Yamamoto K, Hiasa A, et al. Contrast-enhanced ultrasound examination of lymph nodes in different types of lymphoma. Cancer Detect Prev. 2006;30:188–91. doi: 10.1016/j.cdp.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound. 2016;5:233–8. doi: 10.4103/2303-9027.187866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich CF, Mathis G, Blaivas M, et al. Lung B-line artefacts and their use. J Thorac Dis. 2016;8:1356–65. doi: 10.21037/jtd.2016.04.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fröhlich E, Muller R, Cui XW, et al. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34:179–96. doi: 10.7863/ultra.34.2.179. [DOI] [PubMed] [Google Scholar]
- 50.Cui XW, Ignee A, Jedrzejczyk M, et al. Dynamic Vascular Pattern (DVP), a quantification tool for contrast enhanced ultrasound. Z Gastroenterol. 2013;51:427–31. doi: 10.1055/s-0032-1325371. [DOI] [PubMed] [Google Scholar]
- 51.Ignee A, Jedrejczyk M, Schuessler G, et al. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-reliability and potential sources of errors. Eur J Radiol. 2010;73:153–8. doi: 10.1016/j.ejrad.2008.10.016. [DOI] [PubMed] [Google Scholar]