Abstract
Background and Objectives:
Imaging of the pancreas for detection of neuroendocrine tumors is indicated as surveillance in multiple endocrine neoplasia type 1 (MEN1) or if typical clinical symptoms combined with hormone production raise the suspicion of a neuroendocrine tumor. Endoscopic ultrasound (EUS) is considered the best imaging modality to detect small pancreatic tumors. However, little is known about how small pancreatic neuroendocrine tumors (pNETs) present on EUS.
Patients and Methods:
In this multicenter study, we retrospectively analyzed the endosonographic characteristics of small pNETs which had been detected due to typical biochemistry and clinical symptoms or during surveillance of MEN 1. Only small pancreatic tumors ≤15 mm with histological confirmation as pNET were included. B-mode and contrast-enhanced ultrasound- and EUS patterns were analyzed.
Results:
Among 32 patients with histologically proven small pNETs, 7 patients had known MEN1. Among the pNETs, 20 were insulinoma, 2 gastrinoma, 3 glucagonoma, 6 nonfunctional in MEN1, and one PPoma. 94% of the pNET appeared hypoechogenic, only 1 isoechogenic and 1 hyperechogenic. After contrast injection, 90% of the pNETS showed hyperenhancement compared to the surrounding pancreatic parenchyma.
Conclusion:
The high spatial resolution of EUS allows detection and even cytological confirmation of pNET <7 mm diameter. Hypoechogenicity in B-mode and hyperenhancement after injection of contrast agents are endosonographic characteristics of small pNET and present in >90% of pNETs.
Keywords: Contrast-enhanced endoscopic ultrasound, endoscopic ultrasound, fine needle aspiration, guidelines, pancreatic ductal adenocarcinoma, solid pancreatic lesions
INTRODUCTION
Due to its high spatial resolution, endoscopic ultrasound (EUS) is recommended as the method of choice for detection of very small pancreatic tumors; it is also useful to obtain cytopathology by fine needle aspiration (FNA).[1] The diagnostic potential of cross-sectional imaging, especially of magnetic resonance imaging (MRI) is well-known, but the accuracy of EUS still remains superior.[2]
Pancreatic neuroendocrine tumors (PNETs) are a rare entity with an incidence of 1–10 per million in the general population.[3,4] However, the prevalence in patients with multiple neuroendocrine neoplasia type 1 (multiple endocrine neoplasia type 1 [MEN-1]) is up to 75%, while pNETs are the main cause of MEN1-related death.[5] Therefore, early detection and surveillance is mandatory as surgery is the only curative treatment.
Imaging of the pancreas for detection of neuroendocrine tumors is indicated as surveillance in MEN1 or if typical clinical symptoms combined with hormone production raise the suspicion of a neuroendocrine tumor. EUS is considered the best imaging modality to detect small pancreatic tumors in particular in MEN1.[6,7,8,9,10,11,12] Moreover, EUS is increasingly used for active surveillance of very small nonfunctioning pNET, which carry a low oncological risk.[13,14,15]
However, little is known about how small pNETs present on EUS.
In a previous multicenter study, we could show that contrast enhanced transabdominal ultrasound (CE-US) and contrast-enhanced endoscopic ultrasound (CE-EUS) are helpful in characterizing small pancreatic tumors which are incidentally found on other imaging; however, in this study hormone-active tumors and pNETS in patients with MEN1 were excluded from this study.[12] The aim of the present study was to characterize small pancreatic tumors found in patients with known MEN1 or with clinical symptoms combined with biochemical elevation of typical neuroendocrine hormones such as insulin, glucagon, or gastrin.
PATIENTS AND METHODS
From 30 tertiary referral centers invited to participate in the study, 7 centers sent their cases of small pNETs found on annual surveillance of patients with MEN type 1 or due to typical hormone alterations and clinical symptoms.
Symptom evaluation, computer tomography (CT) and MRI or octreotide scans were performed as part of the clinical workup but not for the purpose of this study.
All patients included underwent transabdominal US and EUS of the pancreas. CE-US or CE-EUS, as well as EUS elastography, were performed depending on the availability of these imaging in the different centers. EUS was performed using radial (EG-3670URK) and/or linear (EG-3870UTK) echoendoscopes (Pentax Medical, Hamburg, Germany) with high-end ultrasound systems HI Vision Preirus and HI Vision Ascendus (Hitachi Medical Systems, Wiesbaden, Germany).
The use of EUS-guided FNA (EUS-FNA) and the particular EUS-guided sampling technique, as well as the choice of echoendoscope (radial or linear), were at the discretion of the endoscopist in the participating centers. Furthermore, the indication for surgery and the preferred surgical techniques for management of pNET varied between the centers. As some of the centers were only referral centers for EUS, they did not perform cross-sectional imaging for follow-up.
The localization in caput (head), corpus (body), or cauda (tail) was defined according to tumor node metastasis classification: cauda between the left side of the aorta and the splenic hilum; the corpus between the left lateral border of the superior mesenteric vein and left side of the aorta and the caput between the left lateral border of the mesenteric vein and right side of the head/duodenum.
Evaluation criteria
The anonymized data collection over a 10-year period included age, sex, final diagnosis (etiology of the lesion[s]), benign or malignant nature, size, location (head, body, tail), and echogenicity (hypoechoic, isoechoic, mixed echogenicity, hyperechoic).
Contrast enhancement was assessed after intravenous injection of 4.8 mL SonoVue® (Bracco SpA, Milan, Italy); hyper-, iso-, or hypo-enhancement compared to the surrounding pancreatic parenchyma was documented. All contrast enhanced examinations were performed as described in the guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology.[16]
Data analysis and statistics
All data were analyzed according to the pathological diagnosis. Dimensions were given as median (minimum – maximum) as appropriate. Institutional Board approval according to the ethical guidelines from Helsinki was obtained. Only patients older than 18 years were included into the study.
RESULTS
Histological or cytological confirmation
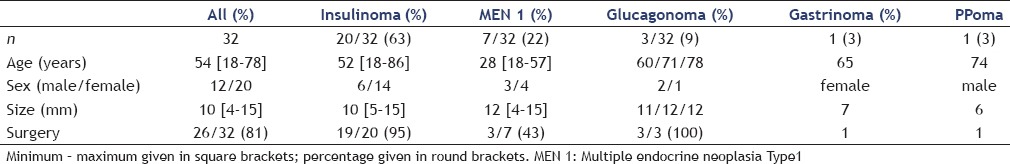
Among 32 patients with histologically or cytologically proven small pNETs, 7 patients had known MEN1. Among the pNETs, 20 were insulinoma, 2 gastrinoma, 3 glucagonoma, 6 nonfunctional in MEN1, and one PPoma [Table 1].
Table 1.
Clinical and pathologic characterization of 32 patients with small neuroendocrine pancreatic lesions
In 13 patients, the diagnosis of a pNET had been obtained by EUS-FNA and cytology.[17] In this subgroup, the median tumor size was 10 mm ranging from 5 to 15 mm. Five of the pNETs diagnosed by EUS-FNA were ≤7 mm.
In 26 patients, the diagnosis of pNET was confirmed after surgery; thereof, seven patients had already positive cytology by EUS-FNA before surgery.
Location of the tumor in the pancreas
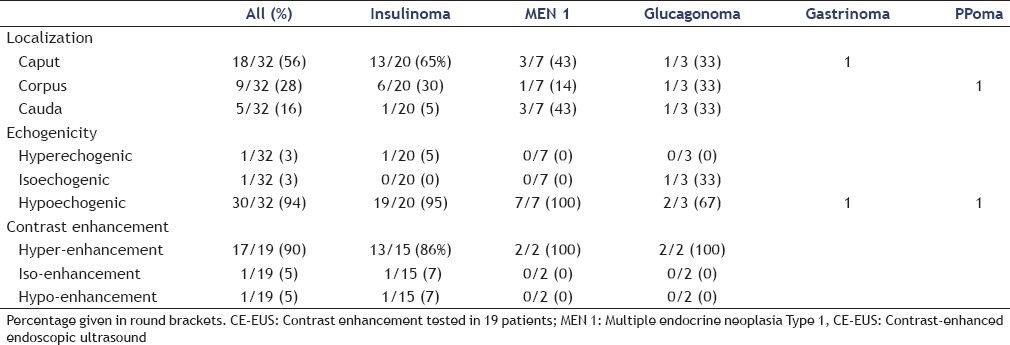
Most of the neuroendocrine tumors were found in the pancreatic head (56%), this was also true for insulinoma (65%) [Table 2].
Table 2.
Localization, B-mode characteristics and contrast enhancement pattern of patients with small solid neuroendocrine pancreatic lesions
B-mode echogenicity
Only one glucagonoma showed isoechogenicity compared to the surrounding pancreatic parenchyma, and one insulinoma was reported as hyperechogenic, while 94% of the pNETs appeared hypoechogenic on B-mode ultrasound examination [Table 2].
Contrast enhancement
Contrast enhancement studies were performed in 19 patients with small pNETs. CE - EUS was performed in 10 patients, CE-US in 9 patients and both methods in 5 patients. In one of the five patients, who underwent both, CE - US and CE-EUS, the pNET in the pancreatic tail has only been visualized endosonographically.
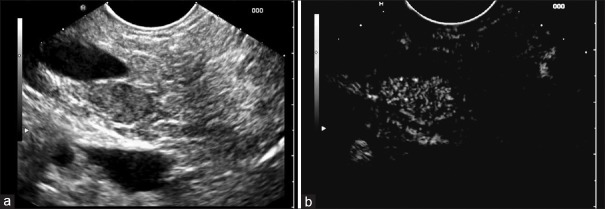
After intravenous injection of contrast agents, 90% of the pNETS showed hyperenhancement compared to the enhancement of the surrounding pancreatic parenchyma [Figure 1 and Table 2]. Only one 11 mm sized glucagonoma appeared hypoenhancing on CE-EUS and a 5 mm sized insulinoma was isoenhancing.
Figure 1.
Insulinoma in the pancreatic head using B-mode (a) and contrast-enhanced endoscopic ultrasound with early hyperenhacnement in comparison to the surrounding pancreatic parenchyma (b). Portal vein and inferior vena cava do not show enhancement during this very early contrast phase
DISCUSSION
The detection and exact localization of hormone-producing pNETs which are often only minuscule is crucial for further organ-preserving surgical management,[18] active surveillance[13,14,15] or modern ablative methods. In the hands of an experienced operator, EUS has a high sensitivity to detect even minute pancreatic tumors which is also pivotal in the surveillance of high-risk patients such as patients with MEN type, von Hippel-Lindau disease, neurofibromatosis type 1 or tuberous sclerosis.[10,13,14,15,19,20] As soon as, the diagnosis of MEN type 1 has been confirmed in an individual by genetic testing and/or clinical manifestation and/or affected family members, an active surveillance program is commenced including annual pancreatic imaging with CT, MR or EUS depending on the local expertise and availability.[21]
Conventional imaging such as transabdominal ultrasound, CT, and MRI have a lower sensitivity than EUS[6,8,9,10,11] and have disappointing results in further characterization of small pancreatic lesions.
CE-EUS has proved to be a helpful new technique in the differential diagnosis of pancreatic masses,[12,22,23,24,25,26] but little is known about the EUS characteristics of small (<15 mm) pNET which are usually found due to their hormone production or during surveillance of patients with MEN type 1.
Recognition of the typical imaging features of pNETs is important for early detection and appropriate patient management. In this retrospective multicenter study, we could demonstrate that the vast majority of small pNET are predominantly hypoechogenic in B-mode (94%) and demonstrate hyperenhancement compared to the surrounding pancreatic parenchyma after contrast injection (90%). The hyperenhancement in the arterial phase is likely explained by the abundant arterial vascularization in neuroendocrine tumors.[22,27,28] and could be visualized even in diminutive tumors. Dilatation of the pancreatic duct or signs of vascular infiltration were not observed.
Currently, the best prognostic parameter for the behavior of a detected pNET is the tumor grade which underlines the important role of the cytology obtained by EUS-FNA.[11,29,30] pNET can be categorized in well-differentiated endocrine tumor of generally benign behavior or poorly differentiated endocrine carcinoma (high grade of malignancy).[31] In our study, even in minuscule pNETs as small as 5 mm, the cytological confirmation by EUS-FNA was possible, underlining the immense diagnostic potential of the EUS technique owing to its high spatial resolution which enables very precise targeting.[9,11,29,32,33]
An emerging role of EUS in the management of pNET is the EUS-guided tattooing or placement of fiducials which mark the exact localization of small pNET. This technique has proved useful for parenchyma-saving pancreatic surgery.[34,35,36] Moreover, in selected cases, EUS-guided ablation techniques have proven effective in symptomatic functional pNET.[37,38,39,40,41,42,43,44]
Potential limitations of our study are its retrospective nature and the limited number of patients undergoing contrast studies.
CONCLUSION
Due to the high spatial resolution provided by EUS, even very small pNET of a few mm diameter can be detected. EUS also enables cytological confirmation of very small pNET <7 mm diameter. Typical endosonographic features of small pNETs are hypoechogenicity in B-mode and hyperenhancement after injection of ultrasound contrast agents; these characteristics are present in >90% of pNETs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV – EUS-guided interventions: General aspects and EUS-guided sampling (Long Version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 2.D’Onofrio M, Gallotti A, Pozzi Mucelli R. Imaging techniques in pancreatic tumors. Expert Rev Med Devices. 2010;7:257–73. doi: 10.1586/erd.09.67. [DOI] [PubMed] [Google Scholar]
- 3.Ehehalt F, Saeger HD, Schmidt CM, et al. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14:456–67. doi: 10.1634/theoncologist.2008-0259. [DOI] [PubMed] [Google Scholar]
- 4.Niederle MB, Hackl M, Kaserer K, et al. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909–18. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli F, Giudici F, Giusti F, et al. Gastroenteropancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1. Cancers (Basel) 2012;4:504–22. doi: 10.3390/cancers4020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khashab MA, Yong E, Lennon AM, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc. 2011;73:691–6. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Puli SR, Kalva N, Bechtold ML, et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: A systematic review and meta analysis. World J Gastroenterol. 2013;19:3678–84. doi: 10.3748/wjg.v19.i23.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James PD, Tsolakis AV, Zhang M, et al. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: A meta-analysis. Gastrointest Endosc. 2015;81:848–56.e1. doi: 10.1016/j.gie.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Mitra V, Nayar MK, Leeds JS, et al. Diagnostic performance of endoscopic ultrasound (EUS)/endoscopic ultrasound – fine needle aspiration (EUS-FNA) cytology in solid and cystic pancreatic neuroendocrine tumours. J Gastrointestin Liver Dis. 2015;24:69–75. doi: 10.15403/jgld.2014.1121.vmi. [DOI] [PubMed] [Google Scholar]
- 10.van Asselt SJ, Brouwers AH, van Dullemen HM, et al. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointest Endosc. 2015;81:159–67.e2. doi: 10.1016/j.gie.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Fujimori N, Osoegawa T, Lee L, et al. Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand J Gastroenterol. 2016;51:245–52. doi: 10.3109/00365521.2015.1083050. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF, Sahai AV, D’Onofrio M, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016 doi: 10.1016/j.gie.2016.04.034. pii: S0016-510730118-3. [DOI] [PubMed] [Google Scholar]
- 13.Kann PH, Balakina E, Ivan D, et al. Natural course of small, asymptomatic neuroendocrine pancreatic tumours in multiple endocrine neoplasia type 1: An endoscopic ultrasound imaging study. Endocr Relat Cancer. 2006;13:1195–202. doi: 10.1677/erc.1.01220. [DOI] [PubMed] [Google Scholar]
- 14.Kann PH, Kann B, Fassbender WJ, et al. Small neuroendocrine pancreatic tumors in multiple endocrine neoplasia type 1 (MEN1): Least significant change of tumor diameter as determined by endoscopic ultrasound (EUS) imaging. Exp Clin Endocrinol Diabetes. 2006;114:361–5. doi: 10.1055/s-2006-924322. [DOI] [PubMed] [Google Scholar]
- 15.D’souza SL, Elmunzer BJ, Scheiman JM. Long-term follow-up of asymptomatic pancreatic neuroendocrine tumors in multiple endocrine neoplasia type I syndrome. J Clin Gastroenterol. 2014;48:458–61. doi: 10.1097/MCG.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 16.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beger HG, Siech M, Poch B, et al. Limited surgery for benign tumours of the pancreas: A systematic review. World J Surg. 2015;39:1557–66. doi: 10.1007/s00268-015-2976-x. [DOI] [PubMed] [Google Scholar]
- 19.van Asselt SJ, Brouwers AH, van Dullemen HM, et al. Potential value of EUS in pancreatic surveillance of VHL patients. Eur J Endocrinol. 2016;174:611–20. doi: 10.1530/EJE-15-1012. [DOI] [PubMed] [Google Scholar]
- 20.Thomas-Marques L, Murat A, Delemer B, et al. Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol. 2006;101:266–73. doi: 10.1111/j.1572-0241.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 21.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: A meta-analysis. Gastrointest Endosc. 2012;76:301–9. doi: 10.1016/j.gie.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio M, Biagioli E, Gerardi C, et al. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: A systematic review and meta-analysis. Ultraschall Med. 2014;35:515–21. doi: 10.1055/s-0034-1385068. [DOI] [PubMed] [Google Scholar]
- 25.Fusaroli P, Napoleon B, Gincul R, et al. The clinical impact of ultrasound contrast agents in EUS: A systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587–96.e10. doi: 10.1016/j.gie.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich CF, Sharma M, Hocke M. Contrast-enhanced endoscopic ultrasound. Endosc Ultrasound. 2012;1:130–6. doi: 10.7178/eus.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich CF, Braden B, Hocke M, et al. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J Cancer Res Clin Oncol. 2008;134:635–43. doi: 10.1007/s00432-007-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yazdani S, Kasajima A, Tamaki K, et al. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum Pathol. 2014;45:866–74. doi: 10.1016/j.humpath.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Unno J, Kanno A, Masamune A, et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol. 2014;49:1367–74. doi: 10.3109/00365521.2014.934909. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32–8. doi: 10.1055/s-0033-1344958. [DOI] [PubMed] [Google Scholar]
- 31.Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 32.Hijioka S, Hara K, Mizuno N, et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016;51:923–30. doi: 10.1007/s00535-016-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishna SG, Bhattacharya A, Li F, et al. Diagnostic differentiation of pancreatic neuroendocrine tumor from other neoplastic solid pancreatic lesions during endoscopic ultrasound-guided fine-needle aspiration. Pancreas. 2016;45:394–400. doi: 10.1097/MPA.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 34.Lennon AM, Newman N, Makary MA, et al. EUS-guided tattooing before laparoscopic distal pancreatic resection (with video) Gastrointest Endosc. 2010;72:1089–94. doi: 10.1016/j.gie.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Law JK, Singh VK, Khashab MA, et al. Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg Endosc. 2013;27:3921–6. doi: 10.1007/s00464-013-2975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuzono T, Kanno Y, Nakahori M, et al. Preoperative endoscopic ultrasonography-guided tattooing of the pancreas with a minuscule amount of marking solution using a newly designed injector. Dig Endosc. 2016;28:744–8. doi: 10.1111/den.12675. [DOI] [PubMed] [Google Scholar]
- 37.Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V. Ultraschall Med. 2016;37:E77–99. doi: 10.1055/s-0035-1553738. [DOI] [PubMed] [Google Scholar]
- 38.Paik WH, Seo DW, Dhir V, et al. Safety and efficacy of EUS-guided ethanol ablation for treating small solid pancreatic neoplasm. Medicine (Baltimore) 2016;95:e2538. doi: 10.1097/MD.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin SY, Lu XP, Jiang HX. EUS-guided ethanol ablation of insulinomas: Case series and literature review. Medicine (Baltimore) 2014;93:e85. doi: 10.1097/MD.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Inabnet WB, 3rd, Sarpel U, et al. EUS-guided ethanol ablation of symptomatic pancreatic insulinomas. Gastrointest Endosc. 2015;82:1127. doi: 10.1016/j.gie.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich CF, Fusaroli P, Jenssen C. European Federation of Societies for Ultrasound in Medicine and Biology guidelines 2015 on interventional endoscopic ultrasound. Endosc Ultrasound. 2016;5:143–8. doi: 10.4103/2303-9027.183968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V – EUS-guided therapeutic interventions (short version) Ultraschall Med. 2016;37:412–20. doi: 10.1055/s-0035-1553742. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III – Abdominal treatment procedures (Short Version) Ultraschall Med. 2016;37:27–45. doi: 10.1055/s-0035-1553965. [DOI] [PubMed] [Google Scholar]
- 44.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III – Abdominal treatment procedures (Long Version) Ultraschall Med. 2016;37:E1–32. doi: 10.1055/s-0035-1553917. [DOI] [PubMed] [Google Scholar]