Abstract
Background and Objectives:
Gastrointestinal stromal tumors (GISTs) represent the largest group of subepithelial tumors (SET) of the upper gastrointestinal (GI) tract. They may show malignant behavior, in contrast to other SET. Endoscopic ultrasound (EUS) is frequently used to characterize SET. With the introduction of contrast-enhanced ultrasound (CEUS) into EUS (CE-EUS), distinct enhancement patterns can be detected. In the presented study, the characteristic features of CE-EUS in GIST are analyzed and compared with those of other SET.
Materials and Methods:
Consecutive patients from four centers with SET of the upper and middle GI tract were included and received endoscopic or transcutaneous CEUS. The results were compared with EUS-guided tissue acquisition, forceps biopsy, or surgical resection.
Results:
Forty-two out of 62 (68%) patients had SET of the stomach, 17/62 (27%) of the small intestine, 2/62 (3%) of the esophagus, and 1/62 (2%) extraintestinal. Eighty-one percent underwent surgery. Leiomyoma was found in 5/62 (8%) and GIST in 57/62 patients (92%). Thirty-nine out of 57 (68%) patients had GIST lesions in the stomach, 17/57 (30%) had GIST of the small intestine, and 1/57 (2%) patients had extraintestinal GISTs. GIST size was 62.6 ± 42.1 (16–200) mm. Hyperenhancement had a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 98%, 100%, 100%, 93%, and 98% for the diagnosis of GIST. Fifty out of 57 patients with GIST (88%) showed avascular areas in the center of the lesions.
Conclusion:
CE-EUS and CEUS show hyperenhancement and avascular areas in a high percentage of GIST but not in leiomyoma. Thus, GIST and leiomyoma can be discriminated accurately.
Keywords: BR1 (Sonovue), contrast-enhanced ultrasound, endoscopic ultrasound, gastrointestinal stromal tumor, sonovue, subepithelial tumors
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) represent the largest group of subepithelial tumors (SET) of the stomach, duodenum and small intestine but occur only very seldom in the esophagus and large intestine. In contrast to leiomyomas, lipomas and most other SETs, they may have a malignant behavior. Therefore, after detection of a subepithelial lesion in the upper gastrointestinal (GI) tract, correct characterization is crucial to start an assessment whether surgery would be an appropriate approach to the patient.[1]
In contrast-enhanced computed tomography (CECT), GIST has necrotic or unenhancing areas in about 50%, if larger than 50 mm. Therefore, “necrotic areas is a sign with a high positive predictive value for the diagnosis of GIST, but the negative predictive value is low.[2] In short, exclusion of GIST is frequently not possible.
Endoscopic ultrasound (EUS) has become the method of choice for the characterization of upper GI tract disease, especially in subepithelial lesions as an adjunct to endoscopy. However, endosonographic discrimination of GISTs from benign subepithelial GI tumors, in particular leiomyoma, may be difficult and is based on minor differences of shape, echogenicity and homogeneity.[3,4,5] In particular, necrotic areas cannot be displayed properly with conventional EUS. Thus, contrast-enhanced ultrasound (CEUS) is used to display microperfusion on the capillary level with a high specificity for the contrast signal due to software reasons and the strict intravascular behavior of the contrast agent.[6]
Recently, real-time contrast-enhanced EUS (CE-EUS) with low mechanical index imaging based on contrast-specific harmonic imaging modes has become available.[7,8] A second advanced imaging technique used with EUS is real-time EUS elastography.[9,10] Based on previous experience in other tumors, we postulated that CE-EUS and EUS elastography might be helpful for discrimination of hypoechoic subepithelial GI tumors, in particular for discrimination of GIST and leiomyoma.
MATERIALS AND METHODS
All consecutive patients from four tertiary referral centers with SETs of the upper and middle GI tract submitted for US and EUS evaluation were analyzed and their data prospectively evaluated. All patients gave informed consent, and institutional board approval had been received for the study.
All patients with SETs of the upper GI tract were evaluated using EUS. In patients with SETs not within the reach of upper GI EUS, in particular SETs of the small intestine, transabdominal US was performed. EUS was performed using Hitachi Preirus and Hitachi Ascendus with Pentax EG UTK 3870 or Pentax EG URK 3670 scanners. First, the lesion was identified using the endoscopic image. Consecutively, EUS examination was performed. The size, shape, layer of origin and vascularization with conventional color or power Doppler ultrasound were analyzed. CE-EUS was performed after injection of 4.8 mL of Sonovue (Bracco, Italy). Using real-time imaging, the enhancement pattern was analyzed with special focus on the enhancement pattern and possible foci without contrast uptake. Real-time elastography has been also performed, using a region of interest that included at least 50% of the tumor, as well as surrounding structures. Percutaneous US was performed using Toshiba Aplio, GE Logiq E9, or Hitachi Ascendus.
The final diagnosis was established by EUS-guided tissue acquisition, bite-on-bite forceps biopsy, or surgical resection with pathological examination and immunohistochemistry.
RESULTS
Sixty-two patients met the inclusion criteria. Forty-two out of 62 (68%) patients had SETs of the stomach, 17/62 (27%) of the small intestine, 2/62 (3%) of the esophagus, and 1/62 (2%) extraintestinal. Fifty out of 62 patients (81%) underwent surgery, in 11/62 (18%) cytology was obtained by EUS-guided biopsy and in 1/62 (2%) histology was obtained by endoscopic forceps biopsy. Histology revealed leiomyoma in 5/62 (8%) and GIST in 57/62 patients (92%).
Major results
All patients but 1 (61/62, 98%) had GISTs with blue pattern in elastography, 13 of them (13/61, 21%) had heterogeneous patterns, and 48/61 (79%) had homogenous patterns. Four of five leiomyoma showed a blue pattern in elastography (80%).
All patients except 1 (56/57, 98%) had a hyperenhancing GIST in CE-EUS. The hypoenhancing lesion was a 30-mm low-risk GIST in the stomach. All leiomyomas showed hypoenhancement [Figure 1]. In our cohort, hyperenhancement had a sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 98%, 100%, 100%, 93% and 98%.
Figure 1.
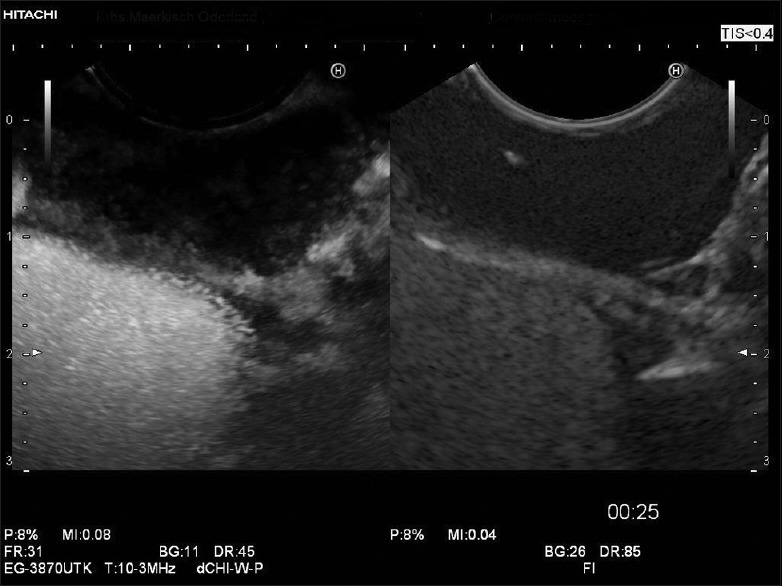
Typical enhancement pattern of leiomyoma; leiomyomas show frequently very low contrast uptake
Fifty out of 57 patients with GIST (88%) showed avascular areas in the center of the lesions which we interpreted as necrosis [Figures 2–4]. There was a trend toward smaller size for GIST without avascular areas (65.8 ± 43 [16–200] vs. 39.6 ± 26.9 [22–90], P = 0.062). In the current group, lesions with avascular areas developed no metastasis and were never classified as high risk. Lesions without avascular areas were all located in the stomach. The findings are presented in Table 1.
Figure 2.
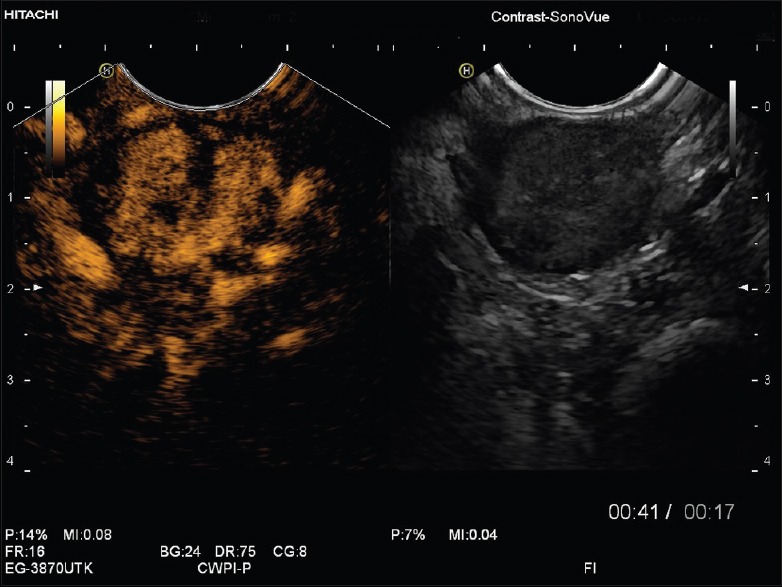
Gastrointestinal stromal tumors in contrast-enhanced endoscopic ultrasound showing hypervascularization and displaying avascular areas
Figure 4.
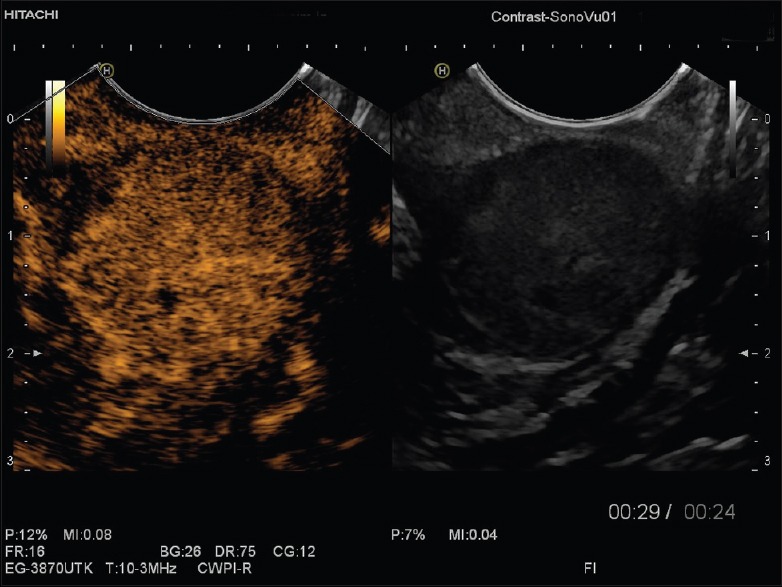
Gastrointestinal stromal tumors in contrast-enhanced endoscopic ultrasound showing pronounced hypervascularization and displaying a tiny avascular area in the center
Table 1.
Characteristics of patients and lesions
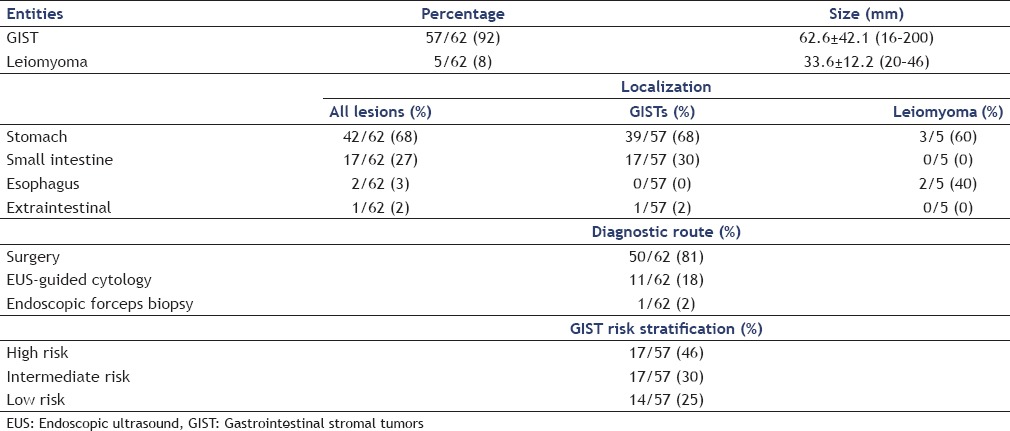
Figure 3.
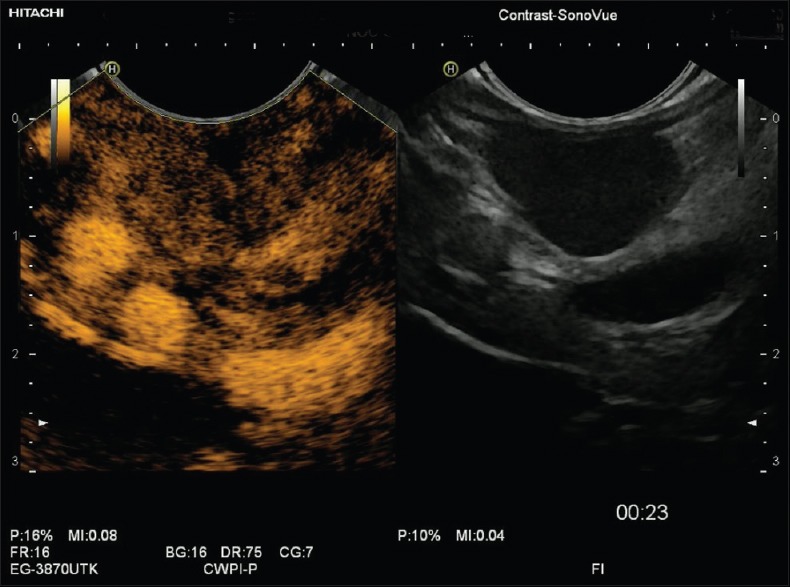
Gastrointestinal stromal tumors in contrast-enhanced endoscopic ultrasound showing less pronounced hypervascularization and displaying small avascular areas
Additional results
Two out of five leiomyoma (40%) were found in the esophagus, and no GIST was found in the esophagus.
Thirty-nine out of 57 (68%) patients had GIST lesions in the stomach, 17/57 (30%) had GIST of the small intestine, and 1/57 (2%) patients had extraintestinal GISTs. GIST size was 62.6 ± 42.1 [16–200] mm. There were 26/57 (46%) patients with high-risk GISTs, 17/57 (30%) with intermediate-risk GIST, 14/57 (25%) patients with low-risk GISTs, and 0/62 (0%) with very low-risk GISTs.
Seven out of 26 (27%) patients with high-risk GIST had simultaneous liver metastases. The majority of patients with liver metastases (9/62, 15%) had high-risk GIST (7/9, 78%), but 2/9 (22%) patients with liver metastases had intermediate histology [Table 1].
There was a trend toward smaller size in leiomyoma compared with GIST, but it did not reach statistical significance (P = 0.066) [Table 1]. All lesions were hypoechoic compared to submucosal layer and isoechoic compared to the muscularis propria layer. In 49/57 (86%) of all lesions, the layer of origin could be identified as proper muscular layer. The remaining 8/57 (14%) lesions were located extraintestinally (1/8, 13%) or in the small intestine (5/8, 50%), where layer association was difficult due to the low thickness of the bowel wall. Two out of eight lesions (38%) were located in the stomach, and the reasons for failing layer association could not be identified.
DISCUSSION
Our findings show that contrast-enhanced low mechanical index EUS displays avascular areas and hyperenhancement in a high percentage of GISTs. A small control group consisting of GI leiomyoma constantly displayed hypoenhancement, thus suggesting CE-EUS to be an appropriate method to distinguish both entities. No leiomyoma showed circumscribed avascular areas.
Another finding was the hard character (low strain) of GIST in real-time EUS-elastography. Nevertheless, also four of five leiomyomas displayed a hard elastographic pattern. Therefore, in contradiction to recently published data,[11] EUS elastography might not be helpful for differential diagnosis of GIST from GI leiomyoma.
GIST represents the largest group of mesenchymal tumors of the GI tract. The most common locations of GIST are stomach (60%), small bowel (30%) and duodenum (5%).[12,13] The relative risk of malignant behavior of a GIST primarily depends on the number of mitoses per 50 high-power fields, on its size, on certain types of mutation, and on the location within the GI tract.[14,15] Nevertheless, GIST tumors have an indeterminate malignant potential and current diagnosis cannot rely on imaging alone.[16]
Current GIST guidelines recommend surgical resection when lesions are larger than 20 or 30 mm.[17,18,19] Resection can be problematic when patient's situation or tumor localization makes wedge resection or similar techniques problematic.[2] However, smaller tumors (<20–30 mm) can be safely considered for endoscopic resection, with or without laparoscopic control.[20,21] Consequently, NCCN guidelines recommend follow-up only in tumors <20 mm without high-risk EUS features while the rest should be resected.[22]
The most common imaging method is computed tomography (CT).[23] About 50% of all GISTs show cystic/necrotic areas in CECT examinations.[24] Ulceration, heterogeneous enhancement, lack of annular growth and lymphadenopathy, and demonstration of a supplying artery as well as draining veins are predictive for GISTs.[2,12] In SETs, EUS is the most accurate method to predict the layer of origin and to evaluate structural features.[25,26] However, differential diagnosis is challenging.[4,5,20,27,28,29,30,31] The yield of endoscopic techniques for tissue diagnosis is limited.[26,32] In prospective studies, a definite diagnosis was possible only in 34%–88% of cases using EUS-guided tissue acquisition.[33,34,35,36,37,38]
Since a few years, CEUS is now available for EUS techniques.[7,8,39,40,41] Preliminary data suggested that CE-EUS can discriminate GIST (hypervascular in all cases) from benign lesions.[42] Moreover, CE-EUS has been used to predict malignant behavior of GISTs.[43,44,45] EUS is further recommended in most of the guidelines for upper GI tract tumors.[46] For gastric stromal tumors, EUS easily demonstrates the submucosal origin of the lesion.[25] EUS has a high accuracy (99%) for localization, characterization of the lesion as GI mesenchymal tumors (83%) and for differentiating benign from malignant tumors (80%).[47]
Review of the literature
One study assessing the vascularity of GIST using CE-EUS showed that all 16 high-risk tumors had irregular vessels, whereas only 5 of 13 low-risk GISTs showed this feature. All tumors with homogeneous enhancement were low-risk GIST or benign spindle cell neoplasias (leiomyoma, Schwannoma). Seven high-risk GISTs displayed avascular areas on CE-EUS, whereas none of the low-risk tumors were found to have this feature. Interestingly, in one study, the sensitivity of CE-EUS for detecting intratumoral vessels (100%) was significantly higher than that for contrast-enhanced multidetector CT (31%) and for power Doppler-EUS (63%).[43]
CONCLUSION
Our data confirm preliminary evidence[42] that CE-EUS is an accurate technique for discrimination of GIST and benign GI mesenchymal tumors, in particular GI leiomyoma. Hypervascularity and the presence of avascular areas are highly predictive criteria of GIST as compared to GI leiomyoma and may be used in addition to B-mode features in the differential diagnosis of GI SET. Due to the fact that both types of GI mesenchymal tumors are relatively hard lesions, EUS elastography is not useful for differentiation of GIST and GI leiomyoma. However, we did not use quantification techniques in EUS elastography (strain ratio or histogram analysis), which might have the potential to better discriminate between various degrees of stiffness of GI SET. Further limitations of our study were its retrospective design and the small control group including only five GI leiomyomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jenssen C, Barreiros AP, Will U, et al. German survey on EUS-Guided diagnosis and management of gastrointestinal stromal tumors (GISTs) – Evidence or “Gut-Feeling”? Ultraschall Med. 2015;36:494–500. doi: 10.1055/s-0034-1398970. [DOI] [PubMed] [Google Scholar]
- 2.Choi YR, Kim SH, Kim SA, et al. Differentiation of large (>/= 5 cm) gastrointestinal stromal tumors from benign subepithelial tumors in the stomach: Radiologists’ performance using CT. Eur J Radiol. 2014;83:250–60. doi: 10.1016/j.ejrad.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647–53. doi: 10.1111/1751-2980.12099. [DOI] [PubMed] [Google Scholar]
- 4.Kim GH, Kim KB, Lee SH, et al. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. BMC Gastroenterol. 2014;14:7. doi: 10.1186/1471-230X-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376–81. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: A new technique. Z Gastroenterol. 2005;43:1219–23. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 8.Saftoiu A, Vilmann P, Dietrich CF, et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2015;82:59–69. doi: 10.1016/j.gie.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji Y, Kusano C, Gotoda T, et al. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors: A pilot study. Dig Endosc. 2016;28:173–8. doi: 10.1111/den.12569. [DOI] [PubMed] [Google Scholar]
- 12.Cai PQ, Lv XF, Tian L, et al. CT characterization of duodenal gastrointestinal stromal tumors. AJR Am J Roentgenol. 2015;204:988–93. doi: 10.2214/AJR.14.12870. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 14.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–15. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 15.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Saftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy for the molecular diagnosis of gastrointestinal stromal tumors: Shifting treatment options. J Gastrointestin Liver Dis. 2008;17:131–3. [PubMed] [Google Scholar]
- 17.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March, 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–78. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 18.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–30. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 19.American Gastroenterological Association Institute. American Gastroenterological Association Institute medical position statement on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2215–6. doi: 10.1053/j.gastro.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc. 2015;7:192–205. doi: 10.4253/wjge.v7.i3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mino JS, Guerron AD, Monteiro R, et al. Long-term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. Surg Endosc. 2016;30:1747–53. doi: 10.1007/s00464-015-4416-2. [DOI] [PubMed] [Google Scholar]
- 22.von Mehren M, Randall RL, Benjamin RS, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014;12:853–62. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 23.Barrios CH, Blackstein ME, Blay JY, et al. The GOLD ReGISTry: A global, prospective, observational registry collecting longitudinal data on patients with advanced and localised gastrointestinal stromal tumours. Eur J Cancer. 2015;51:2423–33. doi: 10.1016/j.ejca.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Baheti AD, Shinagare AB, O’Neill AC, et al. MDCT and clinicopathological features of small bowel gastrointestinal stromal tumours in 102 patients: A single institute experience. Br J Radiol. 2015;88:20150085. doi: 10.1259/bjr.20150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236–56. doi: 10.1055/s-2008-1027388. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt AJ, Jenssen C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: Optimal or optional? Ann Gastroenterol. 2015;28:160–172. [PMC free article] [PubMed] [Google Scholar]
- 27.Onishi M, Tominaga K, Sugimori S, et al. Internal hypoechoic feature by EUS as a possible predictive marker for the enlargement potential of gastric GI stromal tumors. Gastrointest Endosc. 2012;75:731–8. doi: 10.1016/j.gie.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah P, Gao F, Edmundowicz SA, et al. Predicting malignant potential of gastrointestinal stromal tumors using endoscopic ultrasound. Dig Dis Sci. 2009;54:1265–9. doi: 10.1007/s10620-008-0484-7. [DOI] [PubMed] [Google Scholar]
- 30.Jeon SW, Park YD, Chung YJ, et al. Gastrointestinal stromal tumors of the stomach: Endosonographic differentiation in relation to histological risk. J Gastroenterol Hepatol. 2007;22:2069–75. doi: 10.1111/j.1440-1746.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen TH, Hsu CM, Chu YY, et al. Association of endoscopic ultrasonographic parameters and gastrointestinal stromal tumors (GISTs): Can endoscopic ultrasonography be used to screen gastric GISTs for potential malignancy? Scand J Gastroenterol. 2016;51:374–7. doi: 10.3109/00365521.2015.1095350. [DOI] [PubMed] [Google Scholar]
- 32.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV – EUS-guided Interventions: General aspects and EUS-guided sampling (Long Version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 33.Eckardt AJ, Adler A, Gomes EM, et al. Endosonographic large-bore biopsy of gastric subepithelial tumors: A prospective multicenter study. Eur J Gastroenterol Hepatol. 2012;24:1135–44. doi: 10.1097/MEG.0b013e328356eae2. [DOI] [PubMed] [Google Scholar]
- 34.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 35.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–5. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 37.Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39–45. doi: 10.1055/s-0033-1344895. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich C, Hartung E, Ignee A. The use of contrast-enhanced ultrasound in patients with GIST metastases that are negative in CT and PET. Ultraschall Med. 2008;29(Suppl 5):276–7. doi: 10.1055/s-2008-1027878. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–597.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich CF, Jenssen C, Hocke M, et al. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques – A pictorial essay. Z Gastroenterol. 2012;50:457–67. doi: 10.1055/s-0031-1282076. [DOI] [PubMed] [Google Scholar]
- 42.Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–20. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound. 2015;43:89–97. doi: 10.1002/jcu.22195. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Qian L, Li P, et al. The diagnostic value of endoscopic ultrasonography and contrast-enhanced harmonic endoscopic ultrasonography in gastrointestinal stromal tumors. Endosc Ultrasound. 2016;5:111–7. doi: 10.4103/2303-9027.180475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grenacher L, Schwarz M, Lordick F, et al. S3 guideline – Diagnosis and treatment of gastric carcinoma: Relevance for radiologic imaging. Rofo. 2012;184:706–12. doi: 10.1055/s-0031-1299371. [DOI] [PubMed] [Google Scholar]
- 47.Ji F, Wang ZW, Wang LJ, et al. Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography. J Gastroenterol Hepatol. 2008;23(8 Pt 2):E318–24. doi: 10.1111/j.1440-1746.2008.05322.x. [DOI] [PubMed] [Google Scholar]


