Abstract
Mus pahari is a wild-derived, inbred mouse strain. M. pahari colony managers observed fragility of this strain’s skin resulting in separation of tail skin from the mouse if handled incorrectly. Tail skin tension testing of M. pahari resulted in significantly lowered force threshold for caudal skin rupture and loss in comparison to closely related inbred mouse species and subspecies and even more than a model for junctional epidermolysis bullosa. Histologically, the tail skin separated at the subdermal level with the dermis firmly attached to the epidermis, excluding the epidermolysis bullosa complex of diseases. The dermal collagen bundles were abnormally thickened and branched. Elastin fiber deposition was focally altered in the dermis adjacent to the hair follicle. Collagens present in the skin could not be differentiated between the species in protein gels following digestion with pepsin. Together these data suggest that M. pahari have altered extracellular matrix development resulting in separation of the skin below the level of the dermis with moderate force similar to the African spiny mouse (Acomys spp.).
Keywords: inbred, mouse, skin fragility, elastin fibers, collagen, wild-derived
Introduction
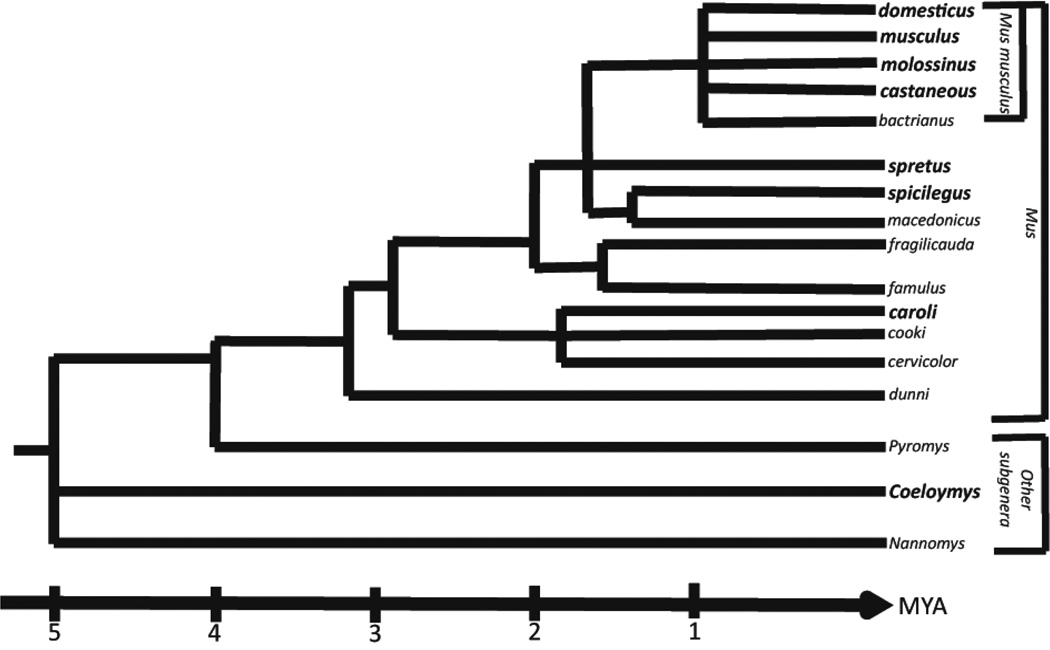
Mus pahari, also referred to as the Sikkim mouse or Gairdner’s shrewmouse, is a wild mouse species that was originally found in the Sikkim region of India, although it is wide ranging from northeastern India, through Bhutan, Myanmar, Thailand, and Vietnam (Lecompte et al., 2008; Srinivasulu, 2012; Thomas, 1916). This species was first described in 1881 by Oldfield Thomas, but was mistakenly identified as Mus nitidulus due to examination of “imperfect specimens”. In the early 1900’s, Thomas obtained four full specimens and was able to describe and delineate Mus pahari as a newly identified species (Thomas, 1916). The subgenus Coelomys, containing both Mus pahari and Mus mayori, diverged from other Mus genera approximately 4.5 million years ago (Boursot, 1993) (Figure 1). Species from these subgenera are distinguished by their shrew-like appearance, including long noses, small eyes, and velvet/spiny coats with species specific variances in the quantity of broad spines in the coat. These species are also geographically isolated, found preferentially in mountain forests (Marshall, 1977; Musser, 2005).
Figure 1.
Phylogenetic tree of the genus Mus adapted from Boursot et al. 1993. The subgenus Coelomys, containing Mus pahari, diverged from the main Mus genera approximately 4.5 million years ago. The bolded species, subspecies, and subgenus indicate those that were compared to Mus pahari in these studies.
Dr. Eva Eicher imported a wild-derived inbred strain of this species to The Jackson in 1995 from Dr. Michael Potter of the National Cancer Institute, whereby the strain was designated as Mus pahari/EiJ. Genetically distinct from most laboratory mouse strains, M. pahari is a valuable research model for evolutionary, viral, and systems biology research (Sakuma et al., 2011). For example, M. pahari has been identified as having a functional xenotropic and polytropic retrovirus receptor 1 (Xpr1) gene not seen in other inbred laboratory strains making this mouse susceptible to infection by XMRV (Xenotropic murine leukemia virus-related virus), a gamma retrovirus found in human prostate cancers and implicated in chronic fatigue syndrome (Sakuma et al., 2011).
Wild mice in general can present with a number of husbandry challenges, usually associated with hyperactivity relative to commonly used inbred strains. Husbandry of this strain can be very challenging as mouse caretakers noted these mice have “fragile skin”, particularly affecting the tail, whereby the skin tears easily when the mice are handled. This aspect of the strain’s phenotype has been incidentally noted, although there was no mention of excessively fragile skin in the original phenotypic description of this species by Thomas. Fragile (tail) skin is also a feature of a spontaneous non-Hertlitz junctional epidermolysis bullosa mouse model also discovered at The Jackson Laboratory (Bubier et al., 2010). A comparative evaluation of the skin fragility in M. pahari mice is presented here to define the underlying cause.
Results
Confirming those observations made by the animal caretakers, skin was very easily removed from the M. pahari when the mice were picked up by their tails using forceps or by grasping the mice on the scruff of the neck. To avoid this problem, the mice were routinely handled by gathering them into a plastic cup for transfer between cages. Complete necropsies of two male M. pahari mice were performed. No obvious gross differences were noted between these two males and several other inbred mouse strains or species that were used for comparison, including M.m. domesticus (LEWES/EiJ and C57BL/6J), M.m. molossinus (MOLG/EiJ), M.m. castaneous (CAST/EiJ), M. spretus (SPRET/EiJ), M.caroli (CAROLI/EiJ), and M. spicilegus (PANCEVO/EiJ) (Figure 1, bold type, species, subspecies, and subgenus). Additional inbred strains were reviewed extensively in multiple replicates, ages, and both sexes for comparison (Sundberg et al., 2011; Sundberg et al., 2016). The Mus pahari had minor lesions including focal mineralization and fibrosis of abdominal fat (arrow, see Figure 1b in Ref (Pratt, 2017)), gall stones (see Figure 1c in Ref (Pratt, 2017)), and mild acanthosis of the forestomach (see Figure 1d in Ref (Pratt, 2017)). The intestines, especially the cecum, appeared to have a more open intercellular space in the submucosa suggesting that the changes seen in the skin maybe present in other organs (see Figure 1e in Ref (Pratt, 2017)). This was the impression of the prosector at the time of necropsy, that the skin and intestinal organs would tear more easily than in the control mice when handled.
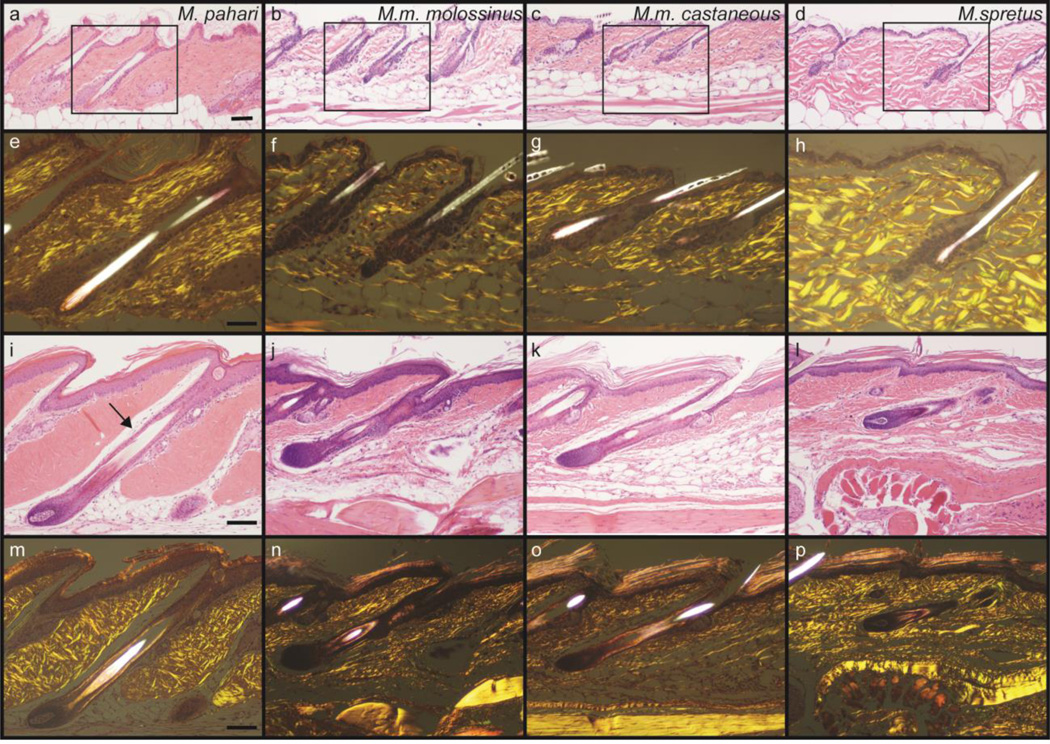
Tail skin was examined histologically from M. pahari mice and compared to tail skin sections from the aforementioned species and subspecies of the Mus genus (Figure 2, see Figure 1 in Ref (Pratt et al., 2017)). Sections from all the species were similar except for those from M. pahari. In the control species the collagen bundles of the tail skin dermis were dense, irregular, and relatively loosely packed (Figure 2b–d and n–p). In contrast to those in M. pahari mice, in which the collagen bundles were very densely packed and the dermis appeared to be thicker (Figure 2a,e,i,m). Using polarized light collagen bundles were thick and organized in a criss-crossed pattern, especially in the tail. Some of these variations are likely due to minor variations in orientation of the sections. In the tail, the dorsal space of very loose collagenous connective tissue immediately above the hair follicle was prominent. While this often overlooked feature was present in all other species, there was much looser connective tissue present. The same feature was evident below the dermis (Figure 2i, arrow). Gel separation of digested collagen was performed to determine if there were any major differences in the types and amounts of collagen between M. pahari and M. musculus (C57BL/6J). Again, no obvious differences were found (see Figure 1a in Ref (Pratt, 2017)).
Figure 2.
Tail skin from Mus pahari, Mus musculus molossinus, Mus musculus castaneous, and Mus spretus. Dorsal thoracic skin is shown in the top panels and tail skin in the lower panels. Note the densely compacted collagen in the dermis compared to the other Mus species. The same H&E stained sections under polarized light reveals a similar network of interlacing collagen bundles, although they appear to be larger and more organized in Mus pahari. Also note in the tail skin sections the clear space dorsal to paralleling the hair follicle. While present in all the other species, it is less prominent due to the presence of fine collagen fibers. H&E Stain. Scale bar: Panels a-d= 100um, Panels e-p=50um.
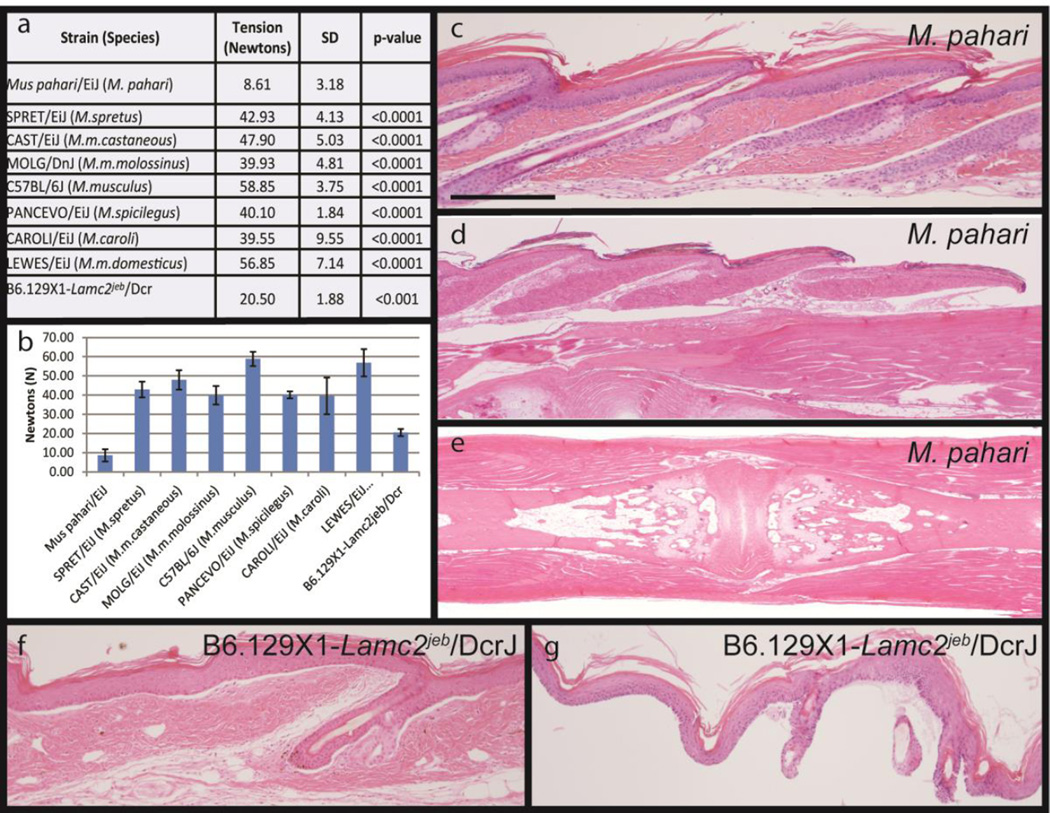
To quantify the skin fragility, a tail skin tension assay was used immediately post-mortem (Sproule et al., 2012). Tension testing failed to remove skin from SPRET/EiJ, CAST/EiJ, MOLG/DnJ, PANCEVO/EiJ, CAROLI/EiJ, LEWES/EiJ or C57BL/6J within the limits of the equipment. Mus pahari skin consistently separated at a low force, typically 7–12 Newtons, with no apparent age or sex difference (Figure 3a,b). For comparison, B6.129X1-Lamc2jeb/jeb/Dcr, mutant mice with non-Herlitz junctional epidermolysis bullosa, 12 week old female skin was removed at 19–23 Newtons (Bubier et al., 2010; Sproule et al., 2014; Sproule et al., 2012). Histologic evaluation of the tails that required a great deal of tension, often without success, had normal attachment of the epidermis and dermis. The mice with non-Herlitz junctional epidermolysis bullosa had separation of the epidermis from the dermis at the level of the basement membrane (Figure 3f,g). By contrast, tail skin of M. pahari mice separated below the dermis, at the layer of the hypodermal fat and subcutaneous tissues from underlying ligments, bone, and cartilage (Figure 3c–e).
Figure 3.
The tail tension assay removes the tail skin and provides a quantifiable phenotype for comparison between Mus species and different mutant mouse strains that have somewhat similar fragile skin (a,b). Tail tension testing was used to measure the force, in Newtons, required to strip the skin from the mouse tail. Tension testing failed to remove skin from SPRET/EiJ, CAST/EiJ, MOLG/DnJ, PANCEVO/EiJ, CAROLI/EiJ, LEWES/EiJ or C57BL/6J within the limits of the equipment. Mus pahari skin consistently separated at a low force, typically 7–12 Newtons, with no apparent age or sex differential. Histology is required to determine where in the skin the separation occurs. For Mus pahari the separation occurs below the hypodermal fat layer. Skin removed from the tail (c) has an intact epidermis and dermis but the separation occurred below the fat layer (arrows). At the point where the full thickness skin separates as it is pulled off (d, arrow) a space forms between the dermis and underlying ligaments surrounding the coccygeal vertebrae. The remaining coccygeal vertebrae have only muscle and ligaments remaining (e). By contrast, mice with junctional epidermolysis bullosa (B6.129-Lamc2jeb/jeb) have separation at the level of the basement membrane (f). In the tail tension assay there is complete dermal-epidermal separation (g). H&E stain. p>value was calculated by Student’s t-test. Scale bar=200uM
These features were further emphasized using Masson’s trichrome stain. There was no sexual dichotomy (see Figure 1a–t in Ref (Pratt et al., 2017)). No differences were observed using Verhoeff-Van Gieson (VVG) stain for elastic fibers (see Figure 1u–y in Ref (Pratt et al., 2017)). Sirius red stain was used because differential anisotropic responses indicate the presence of collagen type I or III (see Figure 1z–ax in Ref (Pratt et al., 2017)) (Hadid et al., 2014; Seifert et al., 2012; Walls et al., 2012). There were no differences between M. pahari and any of the other species. Immunohistochemistry to evaluate smooth muscle actin (blood vessels and arrector pili muscles), CD31 (endothelial cells of blood vessels), or LYVE1 (lymphatics) did not reveal any differences in the vasculature of the dermis between the species (data not shown).
Discussion
The evolution of mice and related species has been a focus of attention because mice are so heavily used in research today. The subgenus Coelomys, containing Mus pahari, separated from typical laboratory mouse strains approximately 4.5 million years ago. It is interesting to note another wild species, the African spiny mouse, (Acomys spp.), is also a member of this subgenus and was recently the subject of investigation because of its easily torn truncal skin that healed and regrew hair (Hadid et al., 2014; Seifert et al., 2012; Walls et al., 2012).
The clinical feature suggested that the tail skin fragility in these mice might be due to abnormalities in the basement membrane as was the case for non-Herltiz junctional epidermolysis due to a hypomorphic mutation in laminin gamma 2 (Lamc2jeb). Modifier genes, such as collagen type XVII, alpha 1 (Col17a1), can affect this fragility (Bubier et al., 2010; Sproule et al., 2014). Other genetic or autoimmune based defects in keratins and adhesion molecules between keratinocytes (epidermolysis bullosa complex of diseases and various forms of pemphigus and pemphigoid, can also cause blistering and loss of skin on the tail). By contrast, M. pahari, if handled carefully, show no outward signs of tail disease or damage to >33 weeks of age, and the force required to remove the tail skin does not change with age. The separation below the level of the dermis is unusual with no apparent human disease equivalent. Whether this occurred as a spontaneous mutation within the inbred M. pahari colony or is a species or genus specific characteristic, as may be the case with the related wild Acomys spp., remains to be determined. However, as with lizards that can lose and regrow their tails to escape capture by predators, this may be an adaptive advantage to these rodents.
For many years, M. pahari colony managers have known about the fragility of this strain’s skin. Tail skin tension testing resulted in significantly lowered force threshold, while histologically the dermal collagen bundles were abnormally thickened and elastin fiber deposition was focally altered in the dermis adjacent to the hair follicle. Together this data suggests that M. pahari have altered extracellular matrix development resulting in separation of the skin below the level of the dermis with moderate force.
Methods and Materials
Mice
Stocks of Mus pahari/EiJ (stock #002655), C57BL/6J (stock #000664), SPRET/EiJ (stock #001146), CAROLI/EiJ (stock #000926), LEWES/EiJ (stock #002798), CAST/EiJ (stock #000928), PANCEVO/EiJ (stock #001384), and MOLG/DnJ (stock #000555) (The Jackson Laboratory; Bar Harbor, ME) were maintained in a humidity, temperature, and light cycle (12hr:12hr) controlled vivarium under specific pathogen-free conditions (http://jaxmice.jax.org/genetichealth/health_program.html). Mice were housed in double-pen polycarbonate cages (330 cm2 floor area) at a maximum capacity of four mice per pen. Mice were allowed free access to autoclaved food (NIH 31, 6% fat; LabDiet 5K52, Purina Mills, St. Louis, MO) and acidified water (pH 2.8–3.2). All work was done with the approval of The Jackson Laboratory Animal Care and Use Committee. For each comparison conducted in this study at least one female and one male adult (66–340 days of age) from each strain had their tails removed, decalcified, and histological sections produced of both cross and longitudinal sections. Three M. pahari females (200–238 days of age) and two males (40 and 200 days of age) had their tail skin evaluated and two 98 day-old males underwent complete necropsies (Silva, 2012). In addition, skin from M. Pahari mice used to measure tail skin adhesion had both the skin removed in the assay and the remaining tail and bones processed and evaluated histologically.
Quantitative Measurement of Tail Skin Adhesion
A mechanical test was developed to provide quantitative data to assess skin fragility, specifically; force (in Newtons) needed to remove tail skin from a euthanized mouse with a form of junctional epidermolysis bullosa. The device and its use are described in detail elsewhere (Sproule et al., 2012). Briefly, mice are euthanized and immediately thereafter, their tails are clamped in a cantilever device attached to a meter (a pull test). This test was applied to all mice in this study which consisted of at least two males and two females between 9 and 30 weeks of age of SPRET/EiJ, CAST/EiJ, MOLG/EiJ, and M. pahari/EiJ, as well as at least one male and one female between 9 and 50 weeks of age of C57BL/6J, PANCEVO/EiJ, CAROLI/EiJ, LEWES/EiJ, and B6.129X1-Lamc2jeb/Dcr mice. The skin removed and remaining tail tissues were examined histologically to determine the anatomic location of separation.
Statistics
Tail tension testing data from all species was statistically analyzed using JMP v11.2.1 software (SAS Institute Inc, Cary, NC). A Shapiro-Wilk test was used to determine whether the data was normally distributed, whereby the data was found to have a p-value >0.05 indicative of normally distributed data. Utilizing a Levene’s test we also determined that the data had equal variance allowing for the use of a Students t-test for further analysis.
Collagen screen
Dorsal skin (200 mg) from age matched M. pahari and C57BL/6J was dissected, minced, and incubated overnight in 4ml of digestion buffer (0.5M acetic acid, 0.2M NaCl, and 1mg/ml Pepsin). Samples were centrifuged at 13,000×g for 10 min at 4°C and supernatant transferred to a clean tube. Protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo-Scientific, Rockville, IL). Samples were diluted to 10ug/ml then combined 1:1 in 2x Lammeli Sample Buffer (Bio-Rad, Hercules, CA). Samples were then heated in a 95°C waterbath for 5 minutes before running on a 4–15% Mini-PROTEAN TGX Gel (Bio-Rad, Hercules, CA). Protein was stained with coomasie blue and imaged with a Syngene GBOX F3 (Syngene, Fredrick, MD).
Histological examination
Mice were euthanized by CO2 asphyxiation and necropsies. Skin was removed as previously described or complete necropsies were performed (Silva, 2012). All tissues were fixed in Fekete’s acid alcohol formalin solution for 12 hours and then processed routinely. Tail was removed and oriented in longitudinal and cross sections after decalcification. Tissues were serially sectioned and stained with either hematoxylin and eosin (H&E), Masson’s trichrome, Verhoeff-Van Geison, or Sirius red. H&E and Sirius red stained sections were reviewed using both white and polarized light microscopy. Antibodies used for immunohistochemistry included CD31 (for endothelial cells; Abcam, Cambridge MA, cat# ab28364, 1:100), LYVE1 (for lymphatics, Abcam, Cambridge MA, cat# ab14917, 1:100), and SMA (for vascular smooth muscle: 1:200, NB600-531, Novus, Littleton, CO, USA or 1:400, A 2547, Sigma). Details are available on http://tumor.informatics.jax.org/html/antibodies.html. The immunohistochemistry was done using a Ventana autostainer (Tuscon, AZ). Diaminobenzidine (Sigma, St. Louis, MO) was used as chromogen.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R21 AR063781 to JPS). The Jackson Laboratory Shared Scientific Services were supported in part by a Basic Cancer Center Core Grant from the National Cancer Institute (CA034196, to The Jackson Laboratory).
Abbreviations
- M.
Mus
- M.m.
Mus mus
- spp
species
- MYA
million years ago
- Xpr1
xenotropic and polytropic retrovirus receptor 1
- XMRV
Xenotropic murine leukemia virus-related virus
- Lamc2
laminin, gamma 2
- jeb
junctional epidermolysis bullosa
- H&E
Hematoxylin and Eosin
- VVG
Verhoeff-Van Gieson
- Col17a1
collagen type XVII, alpha 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest CHP, CSP, and RVK state no conflicts of interest. Dr. Sundberg has a research contract with Bioniz, LLC, for preclinical trials unrelated to this project.
References
- Boursot P, Auffray J-C, Britton-Davidian J, Bonhomme F. The Evolution of the House Mouse. Annu. Rev. Ecol. Syst. 1993;24:119–152. [Google Scholar]
- Bubier JA, et al. A mouse model of generalized non-Herlitz junctional epidermolysis bullosa. J Invest Dermatol. 2010;130:1819–1828. doi: 10.1038/jid.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadid Y, et al. Sympatric incipient speciation of spiny mice Acomys at "Evolution Canyon," Israel. Proc Natl Acad Sci U S A. 2014;111:1043–1048. doi: 10.1073/pnas.1322301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecompte E, et al. Phylogeny and biogeography of African Murinae based on mitochondrial and nuclear gene sequences, with a new tribal classification of the subfamily. BMC Evol Biol. 2008;8:199. doi: 10.1186/1471-2148-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JT. A synopsis of Asian species of Mus (Rodentia, Muridae) Bulletin of the American Museum of Natural History. 1977;158:173–220. [Google Scholar]
- Musser GGaC, M D. Rodentia:Myomorpha:Muroidea:Murinae. Baltimore, MD: JHU Press; 2005. [Google Scholar]
- Pratt CH, et al. Examination of Collagen Structure in the skin of Mus pahari/EiJ. Data In Brief. 2017 [Google Scholar]
- Pratt CH, Potter CS, Sproule TJ, Kuiper RV, Karst SY, Dadras SS, Roopenian DC, Sundberg JP. Strain specific phenotypic findings in the inbred mouse strain, Mus pahari/EiJ. Data In Brief. 2017 In Press. [Google Scholar]
- Sakuma T, et al. Early events in retrovirus XMRV infection of the wild-derived mouse Mus Pahari. J Virol. 2011;85:1205–1213. doi: 10.1128/JVI.00886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) . Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva KAaS, J P. Necropsy Methods. London: Academic Press; 2012. [Google Scholar]
- Sproule TJ, et al. Molecular identification of collagen 17a1 as a major genetic modifier of laminin gamma 2 mutation-induced junctional epidermolysis bullosa in mice. PLoS Genet. 2014;10:e1004068. doi: 10.1371/journal.pgen.1004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproule TJ, et al. A direct method to determine the strength of the dermal-epidermal junction in a mouse model for epidermolysis bullosa. Exp Dermatol. 2012;21:453–455. doi: 10.1111/j.1600-0625.2012.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu CaSB. Checklist of South Asian Mammals. New York, NY: Springer Science+Buisness Media; 2012. [Google Scholar]
- Sundberg JP, et al. The mouse as a model for understanding chronic diseases of aging: the histopathologic basis of aging in inbred mice. Pathobiol Aging Age Relat Dis. 2011;1 doi: 10.3402/pba.v1i0.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP, et al. Approaches to Investigating Complex Genetic Traits in a Large-Scale Inbred Mouse Aging Study. Vet Pathol. 2016;53:456–467. doi: 10.1177/0300985815612556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. Mus Pahari. J. Bombay Nat. Hist. Soc. 1916;24:415. [Google Scholar]
- Walls AC, et al. African spiny mouse: Real skin shedding meets mythology. Nature. 2012;491:527. doi: 10.1038/491527e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.