Abstract
Objective:
To investigate a potential effect of riluzole on serum neurofilaments (Nf) compared to placebo and the relationship between longitudinal clinical and MRI outcomes and serum Nf levels.
Methods:
Serum samples were obtained from participants enrolled in a randomized double-blind trial of neuroprotection with riluzole vs placebo as an add-on to weekly interferon-β (IFN-β)–1a IM initiated 3 months after randomization. Nf measurements were performed by ELISA and electrochemiluminescence immunoassay.
Results:
Longitudinal serum samples were available from 22 riluzole and 20 placebo participants over 24 months. There was no observed treatment effect with riluzole. Nf light chain (NfL) levels decreased over time (p = 0.007 at 24 months), whereas the Nf heavy chain was unchanged (p = 0.997). Changes in NfL were correlated with EDSS change (p = 0.009) and neuropsychological outcomes. Brain volume decreased more rapidly in patients with high baseline NfL (p = 0.05 at 12 months and p = 0.008 at 24 months) and this relationship became stronger at 24 months (p = 0.024 for interaction). Higher and increasing NfL predicted higher number of gadolinium-enhancing lesions (p < 0.001 for both).
Conclusions:
Our findings support the potential value of serum NfL as a marker of neuroaxonal injury in early multiple sclerosis. Its reduction over time could represent regression to the mean, or a possible treatment effect of IFN-β-1a. The association with whole brain atrophy and the formation of acute white matter lesions has relevant implications to use serum NfL as a noninvasive biomarker of the overall consequences of brain damage and ongoing disease activity.
ClinicalTrials.gov identifier:
Neurofilaments (Nf) belong to the class IV family of intermediate filaments comprising light (NfL; 60–70 kDa), medium (130–170 kDa), and heavy chains (NfH; 180–200 kDa).1 They determine axonal caliber and transport, and during axonal injury can be measured in the CSF and blood. Immunoassays for both NfL and NfH have been extensively published and have short-term and long-term prognostic value in various neurologic disorders.2–5
The phase II riluzole trial in participants with early multiple sclerosis (MS) was a randomized, double-blind, placebo-controlled study assessing the putative neuroprotective effect of riluzole. The primary outcome of the trial, brain atrophy, was negative.6 Our goal was to investigate a potential treatment effect on serum NfL and NfH and the longitudinal relationship between serum Nf levels and clinical and MRI outcomes.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the institutional review boards of the University of California, San Francisco, and Oregon Health & Sciences University; all patients provided written, informed consent. This trial was registered with clinicaltrial.gov (NCT00501943).
Study design, participants, and procedures.
Participants had relapsing-remitting MS (RRMS) or a clinically isolated syndrome with less than 12 months duration, at least 2 silent T2-weighted hyperintense lesions on initial MRI, and no MS relapse or use of glucocorticosteroids within 4 weeks of baseline MRI scan.6 Patients were randomized (1:1) to riluzole 50 mg twice a day or placebo, and also initiated weekly interferon-β (IFN-β)–1a IM as an add-on therapy 3 months after initiating the study drug. Brain MRI scans were acquired on a 3T scanner according to a standardized protocol as described elsewhere.6 Participants considered to have a relapse were offered a 3-day course of IV methylprednisolone (1 g daily) or oral equivalent at the discretion of the treating neurologist. The next scheduled MRI was postponed for at least 28 days after the completion of steroid treatment.
Forty-three participants were randomized: 22 to riluzole and 21 to placebo. Five patients were lost to follow-up (2 on riluzole and 3 on placebo). Five participants switched to other disease-modifying therapy based on the treating physician's decision and clinical or MRI disease activity.6 Serum samples were available from 22 riluzole participants and 20 placebo participants (baseline samples were available from 41, month 3 samples from 38, month 6 samples from 40, month 9 samples from 14, month 12 samples from 37, month 15 samples from 10, month 18 samples from 8, month 21 samples from 2, and month 24 samples from 36).
The primary endpoint was change in brain volume, measured with the use of the Structural Image Evaluation using Normalization of Atrophy. Secondary and tertiary endpoints included T2 lesion volume, new contrast-enhancing lesions, Expanded Disability Status Scale score (EDSS), Multiple Sclerosis Functional Composite (MSFC), relapse rate, and neuropsychometric assessments: Judgement of Line Orientation (visuospatial functioning); California Verbal Learning Test, second edition (CVLT-II, verbal episodic learning and recall); and Brief Visuospatial Memory Test–revised (BVMT-R, nonverbal episodic learning and recall).
Serum sampling and Nf measurements.
Serum samples were centrifuged at 1,000 g for 10 minutes at room temperature and stored at −80°C within 2 hours of collection.
NfH analyses were performed using a previously reported immunoassay (Encorbio.com; Gainesville, FL2). We measured serum NfL concentrations using a slightly modified (calibrators and samples were diluted in ready-to-use ELISA diluent, Mabtech AB, Nacka Strand, Sweden) and previously described electrochemiluminescence (ECL) immunoassay.7 Samples below the lowest calibrator that met acceptance criteria (calibrator: accuracy 80%–120%, coefficient of variation [CV] for duplicate back-calculated concentrations below 20%) but above the signal of the blank were extrapolated from the standard curve, otherwise assigned a concentration of 0 pg/mL (41/226 NfL measurements [18.1%] below the lowest calibrator 7.8 pg/mL and 117/226 NfH measurements [52%] below the lowest calibrator 260 pg/mL). Interassay CVs for both assays were below 15% for a low, medium, and high concentration quality control measured in duplicates on every plate. All samples were measured in duplicate and the analysts had no access to the diagnostic code or the clinical and imaging data. The mean intra-assay CVs for NfL and NfH were 7.8% and 8.3%, respectively.
Statistical methods.
Baseline and 12- and 24-month follow-up data were analyzed. For missing data, the next available measurement was used provided it was less than a year later. Nonparametric methods were used to compare NfL and NfH between groups (sex, age, and treatment) and to test for changes over time.
We used mixed effect models to estimate treatment effects on MRI and clinical measures and to investigate the relationship between MRI and clinical measures and Nf (log transformed to achieve approximate normality). We included terms for an interaction between baseline NfL or NfH and month of follow-up. If the interaction was not significant (p > 0.05), the interaction term was dropped and we tested for a relationship between Nf and the outcome averaged over all time points. In separate analyses, we evaluated change in NfL and NfH, as well as their baseline values, as potential predictors of change in each outcome. The number of enhancing lesions was modeled as a Poisson variable. The occurrence of a relapse was modeled as binomial outcomes. All other measures were assumed to have normal error distributions. T1 and T2 lesion volumes required log transformation to achieve approximate normality. Regression coefficients (β) are presented as rates of change per 10-fold change in Nf levels, transformed (exp [β]) to odds ratios for binomial outcomes or n-fold differences for Poisson outcomes.
RESULTS
Changes of Nf over time, riluzole effect, and demographic data.
Median NfH and NfL serum levels at baseline were 335 pg/mL and 14 pg/mL in placebo and 285 pg/mL and 25 pg/mL in the riluzole group (table). At 24 months, the median change for NfH in placebo was −90 pg/mL and riluzole −10 pg/mL (p = 0.247). For NfL, it was −7 pg/mL and −1 pg/mL, respectively (p = 0.590). Since there was no treatment effect, the 2 treatment groups were combined for further analyses.
Table.
Median (interquartile range) for neurofilament (Nf) light chain (NfL, pg/mL) and heavy chain (NfH, pg/mL) at baseline for changes from baseline to 12 and 24 months and according to sex and age
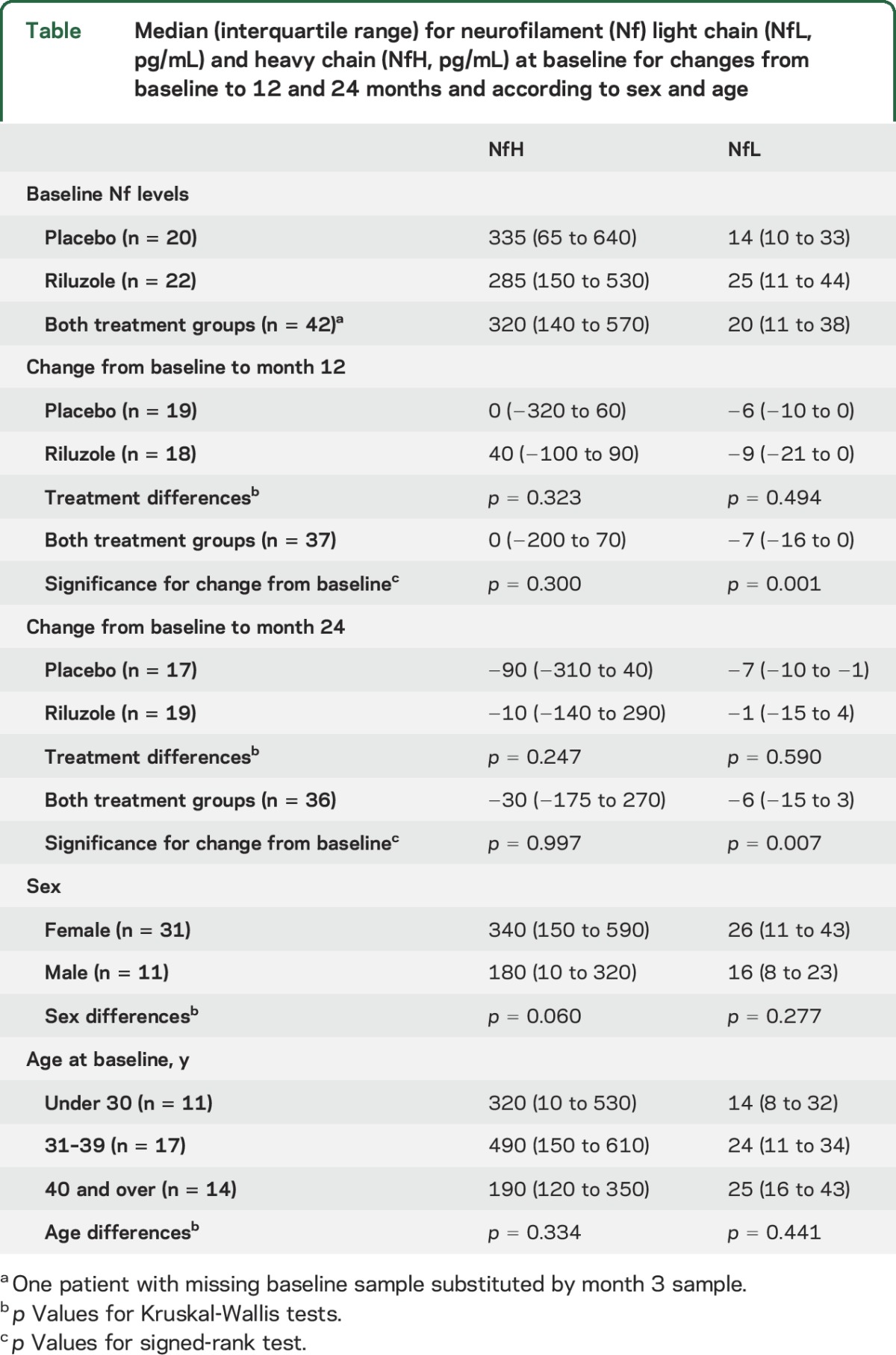
NfL decreased relative to baseline at 12 months (median change −7 pg/mL, p = 0.001) and 24 months (median change −6 pg/mL, p = 0.007); this reduction was already visible at month 3 (median change −5 pg/mL, p = 0.051) and month 6 (median change −5 pg/mL, p = 0.008) of the study, whereas NfH was unchanged (p = 0.300 at 12 months, p = 0.997 at 24 months).
We found no significant differences in NfH or NfL across sexes or age groups. There was a weak correlation between serum NfH and NfL levels at baseline (r = 0.31, p = 0.047), but not at 12 months (p = 0.563) or 24 months (p = 0.820).
Association between Nf serum levels and clinical measures.
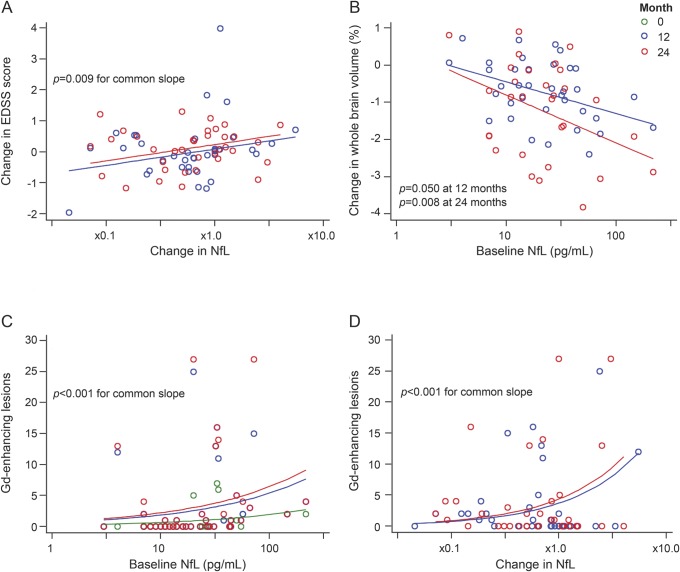
Longitudinal changes in NfL levels but not NfH were positively correlated with EDSS change (figure, A): EDSS score increased by 0.53 (95% confidence interval [CI] 0.14 to 0.91, p = 0.009) points with a 10-fold increase in NfL. Increase in NfL within patients was correlated with poorer judgement of line orientation (β −0.644, 95% CI −1.14 to −0.15, p = 0.013), a lower CVLT-II score (total recall) (β −4.390, 95% CI −7.99 to −0.79, p = 0.020), and a lower BVMT-R score (β −3.130, 95% CI −5.78 to −0.48, p = 0.025) over the study period. Higher baseline NfL and an increase in NfL appeared to be associated with better Paced Auditory Serial Addition Test (PASAT) scores (odds ratio exp [β]: 2.1, 95% CI 1.7 to 2.5, p < 0.001; 1.5, 95% CI 1.2 to 1.9, p < 0.001, respectively), but in general a majority of the patients scored >50 out of 60 possible answers (mean score 54), leading to a clustering of values. There were no significant associations between Nf at baseline or change over time and the Nine-Hole Peg Test or the Timed 25-foot Walk Test.
Figure. Associations between serum neurofilament light chain (NfL) and clinical and MRI parameters.
(A) Changes in NfL levels over the study period were positively correlated with changes in Expanded Disability Status Scale (EDSS) score (p = 0.009) (blue circles: change in EDSS and serum NfL between baseline and month 12; red circles: changes in EDSS and serum NfL between baseline and month 24). (B) Brain volume decreased more rapidly in patients with high baseline NfL (regression coefficient β −0.85, 95% confidence interval [CI] −0.04 to −1.66, p = 0.050 at month 12, and −1.29, 95% CI −0.42 to −2.15, p = 0.008 at 24 months), and the negative relationship between brain volume and NfL at 12 months became stronger at 24 months (p = 0.024 for the difference). (C) Patients with higher NfL levels at baseline had more enhancing lesions with 10-fold higher NfL baseline levels being associated with 2.9-fold (95% CI 2.2 to 3.8, p < 0.001) more enhancing lesions over time. (D) Patients with increasing NfL had a higher number of gadolinium (Gd)-enhancing lesions, with a 10-fold increase in NfL being associated with a 4.7-fold (95% CI 3.3 to 6.9, p < 0.001) increase in new enhancing lesions over the study period.
Nineteen relapses occurred in 11 participants during the study period. Participants experiencing relapses during the study did not have higher serum NfH (at month 12: 100 vs 280 pg/mL, p = 0.436; month 24: 305 vs 315 pg/mL, p = 0.930) or NfL (at month 12: 22 vs 13 pg/mL, p = 0.257; month 24: 17 vs 13 pg/mL, p = 0.534) than participants who were relapse-free.
Association between NF and MRI measures.
Brain volume decreased more rapidly in patients with high baseline NfL (β −0.85, 95% CI −0.04 to −1.66, p = 0.05 at month 12, and −1.29, 95% CI −0.42 to −2.15, p = 0.008 at 24 months, p = 0.024 for difference between month 12 and 24) (figure, B). This means, for example, that while brain volume fell by 0.74% (95% CI 0.39 to 1.08) over 12 months and 1.23% (95% CI 0.86 to 1.59) over 24 months in patients with average NfL levels at baseline, in those with NfL levels 10 times greater than average it fell by 1.57% (95% CI 0.67 to 2.45) in 12 months and by 2.40% (95% CI 1.53 to 3.46) over 24 months. Similar associations were not seen for baseline NfH levels (p = 0.098 for 12 months, p = 0.408 for 24 months). Change in NfL and NfH were not correlated with brain atrophy, either alone (p = 0.598 and p = 0.703, respectively) or after taking account of their baseline values (p = 0.712 and p = 0.467, respectively).
Patients with higher NfL or NfH at baseline had more enhancing lesions over the study period (figure, C), with 10-fold higher NfL or NfH baseline levels being associated with exp(β): 2.9-fold (95% CI 2.2 to 3.8, p < 0.001) or 1.7-fold (95% CI 3.3 to 6.9, p < 0.001) more enhancing lesions over time, respectively. Patients with increasing NfL or NfH had a higher number of enhancing lesions (figure, D), with a 10-fold increase in NfL or NfH over time being associated with a 4.7-fold (95% CI 3.3 to 6.9, p < 0.001) or 1.3-fold (95% CI 1.1 to 1.7, p = 0.009) increase in new enhancing lesions, respectively. There were no associations between Nf and T2 lesion volume at baseline or over time.
DISCUSSION
We show that baseline serum NfL levels predict subsequent progression of brain atrophy, disability, and MS-relevant cognitive measures. This was not noted with serum NfH, the levels of which remained unchanged over 2 years. A previous study in secondary progressive MS reported serum NfH levels to correlate with brain atrophy and clinical measures such as EDSS and MSFC.4 Future studies will show if these discrepancies relate to differences in disease stage or analytical performance of bioassays with higher sensitivity like ECL or single molecule analysis technologies vs conventional ELISA.8
Analyses of changes in brain volume require considerable follow-up and are affected by different factors not directly related to axonal loss such as fluid shifts, aging, remyelination, and astrogliosis. In our study, baseline serum NfL predicted brain atrophy over the subsequent 12 and 24 months, the latter association being the strongest. Higher baseline and increase in serum NfL levels were also associated with the subsequent development of more enhancing lesions. We have reported higher concentrations of serum NfL in patients with early RRMS vs healthy controls and higher concentrations were cross-sectionally associated with higher MRI lesion load. This was also true for more advanced measures of brain tissue integrity like magnetization transfer and T1 and T2* relaxation times.7
We did not note a treatment effect of riluzole on Nf levels. This corroborates the lack of effect reported for brain atrophy measures, the primary outcome.6 We did observe a reduction of serum NfL levels at 12 and 24 months compared to baseline. This could represent regression to the mean, given that the study inclusion criteria allowed for the selection of patients with active disease, or possibly a treatment effect of IFN-β-1a or both. The observed nonsignificant (p = 0.051) reduction in serum NfL levels already at month 3 before initiation of active treatment supports the hypothesis of regression to the mean effects, while potential additional influences of treatment cannot be excluded. Recent studies in RRMS with high disease activity suggested an association of natalizumab or fingolimod treatment with a decrease in CSF NfL levels and corresponding improvements in clinical, MRI, and other laboratory measures.9,10 Serum NfL levels therefore may be influenced by immunomodulatory therapies, and this observation needs to be reproduced in larger prospectively acquired cohorts.
With regard to the pathobiology of Nf isoforms in the adult nervous system, NfH may play a more important role in the development of large diameter axons contributing their size,11 while NfL expression is important for the development of dendritic growth in most neurons.12 It is possible that in the early stages of MS dendrites are more vulnerable to injury during relapses and are released in greater quantities into the surrounding tissues. There is also the stoichiometric contributions of NfL:NfH to consider, with approximately 4 times more NfL than NfH in brain tissue,1 which may make NfL an easier and more reproducible measurement than NfH. NfL levels in our study were measured by a more sensitive ECL immunoassay, whereas serum NfH was measured by conventional ELISA.
The strengths of our study include the deeply phenotyped cohort with concomitant samples and clinical and MRI measures. In addition, patients were seen very early after disease onset and treatments were homogeneous in most of the study, limiting confounding (no participant had received steroids for at least 4 weeks and all were treatment-naive before baseline). Finally, we used robust and conservative statistical analyses of longitudinal data that were acquired blindly by investigators. Limitations of our study include the relatively small sample size limiting the ability to detect a treatment effect of riluzole on various markers. Due to the design of the original trial, longitudinal samples from untreated patients or healthy controls were not available. Finally, analyses of Nf were not a priori defined in the original trial, hence not corrected for multiple comparisons and therefore exploratory in nature.
The reason for the associations between higher baseline serum NfL or an increase in levels over time and better PASAT scores is unclear. Given the potential limitations of PASAT and the clustering of values in the high normal range in our study, these findings should be interpreted with caution and need to be replicated independently including a broader range of patients with cognitive changes.13 The unexpected lack of association of NfH with clinical and most imaging outcomes needs to be investigated further to determine whether the difference relates to disease stage. We present initial data suggesting that serum NfL may be a promising prognostic marker of axonal injury in early MS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank M. Limberg for technical support and the patients for their participation in the study.
GLOSSARY
- BVMT-R
Brief Visuospatial Memory Test–revised
- CI
confidence interval
- CV
coefficient of variation
- CVLT-II
California Verbal Learning Test, second edition
- ECL
electrochemiluminescence
- EDSS
Expanded Disability Status Scale
- IFN-β
interferon-β
- MS
multiple sclerosis
- MSFC
Multiple Sclerosis Functional Composite
- Nf
neurofilament
- NfL
neurofilament light chain
- NfH
neurofilament heavy chain
- PASAT
Paced Auditory Serial Addition Test
- RRMS
relapsing-remitting multiple sclerosis
Footnotes
Editorial, page 816
AUTHOR CONTRIBUTIONS
J. Kuhle: study concept and design, acquisition of data, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content. B. Nourbakhsh: acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. D. Grant: acquisition of data, critical revision of manuscript for intellectual content. S. Morant: analysis and interpretation of data, critical revision of manuscript for intellectual content. C. Barro: acquisition of data, critical revision of manuscript for intellectual content. Ö Yaldizli: critical revision of manuscript for intellectual content. D. Pelletier: acquisition of data, critical revision of manuscript for intellectual content. G. Giovannoni: critical revision of manuscript for intellectual content. E. Waubant: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. S. Gnanapavan: study concept and design, acquisition of data, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content.
STUDY FUNDING
This study was funded in part by the US National MS Society (RG 3692A, PI Waubant).
DISCLOSURE
J.K.'s institution (University Hospital Basel) received and used exclusively for research support: consulting fees from Novartis and Protagen AG; speaker fees from the Swiss MS Society, Biogen, Novartis, Roche, and Genzyme; travel expenses from Merck Serono and Novartis; and grants from ECTRIMS Research Fellowship Programme, University of Basel, Swiss MS Society, Swiss National Research Foundation, Bayer (Schweiz) AG, Genzyme, Novartis, and Roche. B. Nourbakhsh is a grantee of the National MS Society. This research was conducted while B.N. was an American Brain Foundation and a Biogen Idec Postdoctoral Fellow. D. Grant and S. Morant report no disclosures relevant to the manuscript. C. Barro has received travel support from Teva and Novartis. O. Yaldizli received honoraria for lectures from Teva and Bayer Schering (paid to University Hospital Basel). D. Pelletier has received research support and/or consulting fees from Biogen, Novartis, Roche, Sanofi Genzyme, Vertex, National Institute of Health, and the US NMSS. G. Giovannoni is a steering committee member for AbbVie's daclizumab trials, Biogen-Idec's BG12 and daclizumab trials, Novartis' fingolimod and siponimoid trials, Teva's laquinimod trials, and Roche's ocrelizumab trials. He has received consultancy fees from Biogen-Idec, Merck-Serono, Novartis, and Genzyme-Sanofi advisory board meetings, GSK's Phase 3 MS trial programme, and Synthon BV DSMB activities. He has received honoraria for speaking at Genzyme-Sanofi and Biogen-Idec meetings. E. Waubant is funded by the NIH, the NMSS, and the Race to Erase MS. She volunteers on an advisory board for a Novartis trial. She has received honorarium or travel support from ACTRIMS, ECTRIMS, and the AAN. She is site PI for clinical trials with Roche, Novartis, and Biogen. S. Gnanapavan has received grant support from the National Multiple Sclerosis Society; speaker's fees from the Australian MS Society, Genzyme, and Novartis; and travel support from Teva and Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Scott D, Smith KE, O'Brien BJ, Angelides KJ. Characterization of mammalian neurofilament triplet proteins: subunit stoichiometry and morphology of native and reconstituted filaments. J Biol Chem 1985;260:10736–10747. [PubMed] [Google Scholar]
- 2.Shaw G, Yang C, Ellis R, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun 2005;336:1268–1277. [DOI] [PubMed] [Google Scholar]
- 3.Salzer J, Svenningsson A, Sundstrom P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 2010;16:287–292. [DOI] [PubMed] [Google Scholar]
- 4.Gnanapavan S, Grant D, Morant S, et al. Biomarker report from the phase II lamotrigine trial in secondary progressive MS: neurofilament as a surrogate of disease progression. PLoS One 2013;8:e70019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petzold A. The prognostic value of CSF neurofilaments in multiple sclerosis at 15-year follow-up. J Neurol Neurosurg Psychiatry 2015;86:1388–1390. [DOI] [PubMed] [Google Scholar]
- 6.Waubant E, Maghzi AH, Revirajan N, et al. A randomized controlled phase II trial of riluzole in early multiple sclerosis. Ann Clin Transl Neurol 2014;1:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016;22:1550–1559. [DOI] [PubMed] [Google Scholar]
- 8.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 2011;69:83–89. [DOI] [PubMed] [Google Scholar]
- 10.Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 2015;84:1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elder GA, Friedrich VL Jr, Kang C, et al. Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol 1998;143:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Casey DM, Julien JP, Xu Z. Normal dendritic arborization in spinal motoneurons requires neurofilament subunit L. J Comp Neurol 2002;450:144–152. [DOI] [PubMed] [Google Scholar]
- 13.Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 2006;21:53–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.