Abstract
Background
The aim of this study was to investigate the expression and the clinicopathologic significance of DNA methyltransferase 3B (DNMT3B), phosphatase and tensin homolog (PTEN) and human MutL homologs 1 (hMLH1) in endometrial carcinomas between Han and Uygur women in Xinjiang.
Material/Methods
The expression of DNMT3B, PTEN, and hMLH1 in endometrial carcinomas were assessed by immunohistochemistry, followed by an analysis of their relationship to clinical-pathological features and prognosis.
Results
There were a 61.7% (95/154) overexpression of DNMT3B, 50.0% (77/154) loss of PTEN expression and 18.2% (28/154) loss of hMLH1 expression. The expression of DNMT3B and PTEN in endometrial carcinomas was statistically significantly different between Uygur women and Han women (p=0.001, p=0.010, respectively). DNMT3B expression was statistically significant based on the grade of endometrial carcinomas (p=0.031). PTEN loss was statistically significant between endometrioid carcinomas (ECs) and non endometrioid carcinomas (NECs) (p=0.040). DNMT3B expression was statistically significant in different myometrial invasion groups in Uygur women (p=0.010). Furthermore, the correlation of DNMT3B and PTEN expression was significant in endometrial carcinomas (p=0.021). PTEN expression was statistically significant in the overall survival (OS) rate of women with endometrial cancers (p=0.041).
Conclusions
Our findings suggest that PTEN and DNMT3B possess common regulation features as well as certain ethnic differences in expression between Han women and Uygur women. An interaction may exist in the pathogenesis of endometrial carcinoma. DNMT3B was expressed differently in cases of myometrial invasion and PTEN was associated with OS, which suggested that these molecular markers may be useful in the evaluation of the biological behavior of endometrial carcinomas and may be useful indicators of prognosis in women with endometrial carcinomas.
MeSH Keywords: DNA Methyltransferase 3B (DNMT3B), Endometrial Neoplasms, Ethnic Groups, Medical Oncology, PTEN Phosphohydrolase, Survival Analysis
Background
Endometrial carcinoma is a common malignant tumor of women. There are more than 189,000 new cases and 45,000 deaths worldwide each year [1]. According to clinical pathologic characteristics, they were categorized into two subtypes. Type I tumors (about 70–80%) are endometrioid carcinomas (ECs), characterized by estrogen dependence, often accompanied by endometrial hyperplasia. On the contrary, type II (10–20%) tumors mainly include the serous adenocarcinoma and clear cell carcinomas which are non-endometrioid carcinomas (NECs) with worse biological behavior and poor prognosis [2,3]. However, little is known about the pathogenic mechanism and prognostic value of these two types of endometrial carcinomas.
The molecular mechanism of ECs is mainly PTEN inactivation (50–80%), microsatellite instability (MSI, 20–40%), PIK3CA (30%), K-ras gene mutations, and beta-catenin [4,5]. NECs molecular changes include the p53 gene inactivation, p16 gene mutations, and human epidermal growth factor receptor 2 (c-erbB2/Her-2) overexpression [6,7].
The population of Uygur women is slightly more than the population of Han women in the Xinjiang region. The diversity found in the pathogenesis and clinical characteristics of cervical carcinoma, lung cancer, and esophageal cancer among Han patients and Uygur patients indicates the presence of certain differences at the genetic level between these two ethnic groups [8,9].
Studies have shown that most PTEN gene expression is associated with the MMR genes like hMLH1 expression. In addition, DNMT3B and PTEN were identified as potential targets of both miR-145 and miR-143 by the algorithms of target prediction [10]. Our previous studies have indicated that co-downregulation of miR-145 and miR-143 may differ in Uygur women and Han women with regards to endometrial carcinomas. However, to our knowledge, DNMT3B, PTEN, and hMLH1 expressions and their role in endometrial carcinomas in Uygur women has not been extensively studied.
In this study, based on our earlier investigations of miRNA expression and follow-up studies of the clinical characteristics of endometrial carcinomas between Uygur women and Han women, we used immunohistochemistry to detect the expressions of DNMT3B, PTEN, and hMLH1 in endometrial carcinomas, then analyzed of their relationship to clinical-pathological features and prognosis.
Material and Methods
Patients and tissue samples
One hundred and fifty-four endometrial carcinomas tissue blocks (from 62 Uygur women and 92 Han women) based on formalin-fixed and paraffin-embedded tissue samples were obtained from the Affiliated Tumor Hospital of Xinjiang Medical University and the First Hospital of Peking University. All the patients had received no radiotherapy or chemotherapy prior to surgery.
All of the cases were histopathologically diagnosed as endometrial carcinomas. The information on age, ethnic group, histological type, grade of ECs, myometrial invasion, lymph node metastases, vessel invasion, tumor stage and hormone receptor (ER, PR) status were collected for each patient (Table 1). The histopathological type and clinical staging of endometrial carcinoma were classified accorded to the 2014 World Health Organization criteria [2] and 2009 International Federation of Gynecology and Obstetrics (FIGO) guidelines [11]. In the selected 154 cases for immunohistochemistry analysis, follow-up information was received for 115 patients; 98 patients (85.2%) were alive without clinical evidence of tumor (ranged from 4 to 128 months), and the median interval was 30.93 months. Survival time was defined as time from operation to time to last follow-up or death. The study was approved by the Ethical Committee.
Table 1.
Clinicopathological features of endometrial carcinomas in 154 patients.
| Variable | No. of cases n | |
|---|---|---|
| Age | Median 55.01 years, range 33–80 | |
| Ethnic group | Uygur | 62 (40.3%) |
| Han | 92 (59.7%) | |
| Histological type | ECs | 102 (66.2%) |
| NECs | 52 (33.8%) | |
| Grade (ECs) | low | 89 (87.3%) |
| high | 13 (12.7%) | |
| Myometrial invasion* | <1/2 | 99 (72.8%) |
| ≥1/2 | 37 (27.2%) | |
| Lymph node metastases** | No | 74 (85.1%) |
| Yes | 13 (14.9%) | |
| Vessel invasion* | No | 110 (80.9%) |
| Yes | 26 (19.1%) | |
| Stage* | I–II | 113 (83.1%) |
| III–IV | 23 (16.9%) | |
| ER | Negative | 57 (37.0%) |
| Positive | 97 (63.0%) | |
| PR | Negative | 60 (39.0%) |
| Positive | 94 (61.0%) | |
136 patients received hysterectomy;
87 patients received lymphadenectomy.
Immunohistochemistry
Formalin-fixed, paraffin-embedded primary tissue blocks were cut 4-μm thick by the Dako EnVisionTM system. Slides were incubated with the primary antibodies at 4°C overnight against DNMT3B (52A1018, Abcam, 1: 250), hMLH1 (ES05, Novocastra, 1: 200), and PTEN (138G6, Cell signaling, 1: 50).
According to our experiences and other studies [12,13], the DNMT3B, PTEN, and hMLH1 staining was evaluated based on semi-quantitative scoring using an immunoreactive score based on the combination of staining intensity and the percentage of positive tumor cells.
The intensity score of DNMT3B, hMLH1, and PTEN were evaluated as follows: 0, negative; 1, weak staining; 2, moderate staining; 3, strong staining. DNMT3B percentage of positive tumor cells was scored as: 0, 0–1%; 1, 2–10%; 2, 11–33%; 3, 34–66%; 4, >66%. DNMT3B overexpression was defined as the score of the combination of staining intensity and the percentage of positive tumor cells ≥6. hMLH1 and PTEN percentage of positive tumor cells was scored as 0=0–1%; 1=1–10%; 2=11–50%; 3=51–80%; and 4 >80% positive cells. PTEN loss was defined as the score of the combination of staining intensity and the percentage of positive tumor cells ≤3. hMLH1 loss were defined as the score of combination of staining intensity and the percentage of positive tumor cells ≤1. Endometrial mesenchymal cells exhibiting strong hMLH1 and PTEN staining were used as positive controls itself. The negative control was run without the addition of the primary antibodies.
Statistical analyses
All statistical analyses were performed using the software package from SPSS version 17.0 for Windows (SPSS Inc., IL, USA). The associations between DNMT3B, PTEN, and hMLH1 expression and different clinical characteristics were estimated using χ2 test. The correlation coefficients of DNMT3B and PTEN were calculated using the Pearson correlation. The patients were routinely followed and clinically overall survival (OS) was defined as time between date of surgery and date of death or the date of last follow-up. OS was calculated using the Kaplan-Meier method. Differences were indicated as statistically significant when p was less than 0.05 and all p values were two-tailed.
Results
Clinical characteristics of endometrial carcinomas patients
The group of 154 patients consisted of 62 (40.3%) Uygur women and 92 Han women (59.7%). The median age at diagnosis was 55.01 years, range from 33–80 years. The 154 patients were classified according to the grade of ECs, myometrial invasion, lymph node metastases, and vessel invasion. Patients detailed characteristic are summarized in Table 1.
Expression of DNMT3B, PTEN and hMLH1 in endometrial carcinomas and their relationship with clinicopathological variables
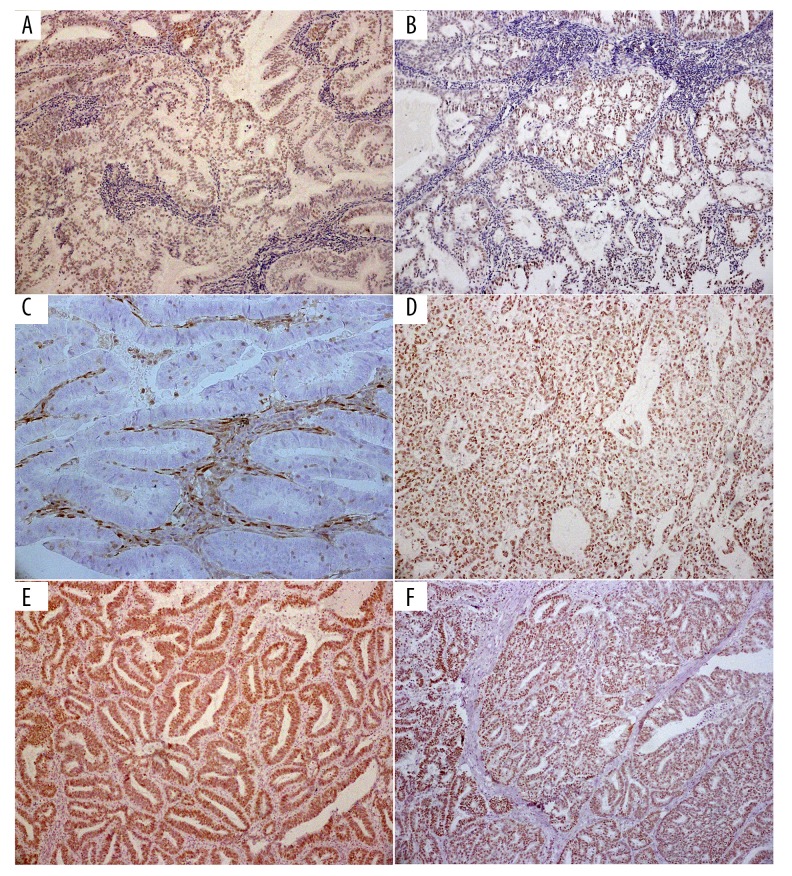
The DNMT3B protein exhibited nuclear localization (Figure 1A in ECs, Figure 1B in NECs,). The PTEN protein exhibited both cytoplasmic and nuclear localization (Figure 1C in ECs, Figure 1D in NECs). The hMLH1 protein exhibited nuclear localization (Figure 1E in ECs, Figure 1F in NECs).
Figure 1.
(A) DNMT3B overexpression exhibits nuclear localization in ECs. (B) DNMT3B overexpression exhibits nuclear localization in NECs. (C) PTEN expression exhibits neither cytoplasmic nor nuclear localization in ECs. (D) PTEN expression exhibits nuclear localization in NECs. (E) hMLH1 protein exhibits nuclear localization in ECs. (F) hMLH1 protein exhibits nuclear localization in NECs.
DNMT3B overexpression was 61.7% (95/154), PTEN loss expression was 50.0% (77/154) and hMLH1 loss of expression was 18.2% (28/154) in the 154 endometrial carcinomas.
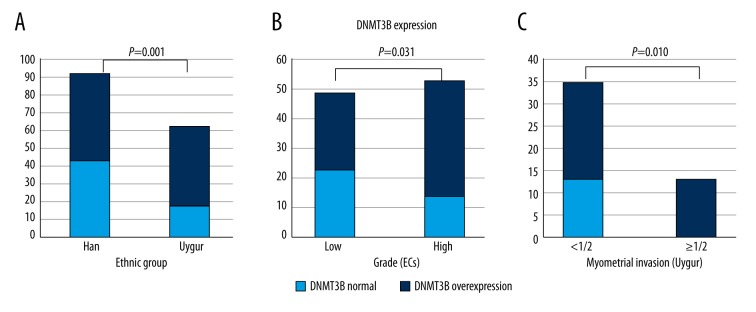
DNMT3B overexpression occurred more often in Uygur women (71.0%, 44/62) than in Han women (55.4%, 51/92), p=0.001, and was found more frequently in high-grade ECs (73.6%, 39/53) than in low-grade ECs (53.1%, 26/49), p=0.031 (Figure 2A, 2B). While DNMT3B expressions in different tumor types (ECs/NECs), hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, and FIGO staging appear obviously different, the differences had no statistical significance (p>0.05).
Figure 2.
(A) DNMT3B expression occurred more often in Uygur women (71.0%, 44/62) than in Han women (55.4%, 51/92) (p=0.001). (B) DNMT3B was found more frequently in high-grade ECs (73.6%, 39/53) than in low-grade ECs (53.1%, 26/49), p=0.031. (C) DNMT3B expression occurred more frequently in <1/2 myometrial invasion subgroups than >1/2 myometrial invasion subgroups in Uygur women (p=0.010).
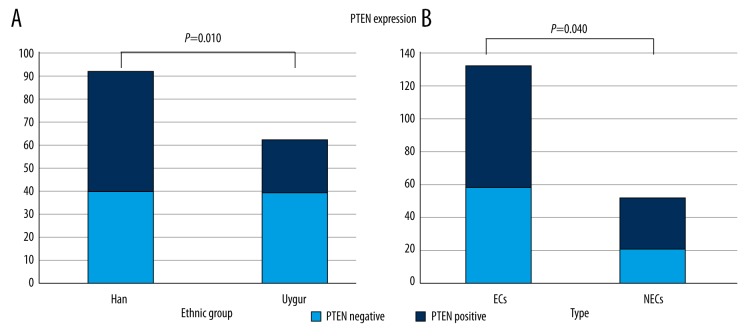
PTEN loss occurred more often in Uygur women (64.5%, 40/62) than in Han women (40.2%, 37/92) p=0.010, (Table 2, Figure 2) and was found more frequently in ECs (57.8%, 59/102) than NECs (34.6%, 18/52), p=0.040 (Table 2, Figure 3). PTEN expression values were obvious differently between groups with different hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, grade of ECs, and FIGO staging, but the differences were not statistically significant (p>0.05).
Table 2.
Clinicopathological features and expression levels of PTEN, MLH1, DNMT3B in endometrial carcinoma.
| Variable | DNMT3B | PTEN | hMLH1 | ||||
|---|---|---|---|---|---|---|---|
| Normal | Overexpress | Loss | Normal | Loss | Normal | ||
| Ethnic group | Han | 43 | 49 | 40 | 52 | 23 | 69 |
| Uygur | 18 | 44 | 40 | 22 | 10 | 52 | |
| P | 0.001 | 0.010 | 0.188 | ||||
| Histological type | ECs | 37 | 65 | 59 | 74 | 23 | 79 |
| NECs | 24 | 28 | 21 | 31 | 10 | 42 | |
| P | 0.236 | 0.040 | 0.635 | ||||
| Grade (ECs) | Low | 23 | 26 | 30 | 19 | 13 | 36 |
| High | 14 | 39 | 29 | 24 | 10 | 42 | |
| P | 0.031 | 0.506 | 0.355 | ||||
| Myometrial invasion* | <1/2 | 43 | 56 | 53 | 46 | 24 | 75 |
| ≥1/2 | 11 | 26 | 18 | 19 | 7 | 30 | |
| P | 0.146 | 0.612 | 0.510 | ||||
| Lymph node metastases** | No | 28 | 46 | 35 | 39 | 14 | 60 |
| Yes | 3 | 10 | 8 | 5 | 2 | 11 | |
| P | 0.305 | 0.344 | 0.762 | ||||
| Vessel invasion* | No | 45 | 65 | 56 | 54 | 27 | 83 |
| Yes | 9 | 17 | 15 | 11 | 4 | 22 | |
| P | 0.555 | 0.533 | 0.317 | ||||
| Stage* | I–II | 46 | 67 | 59 | 54 | 26 | 87 |
| III–IV | 8 | 15 | 12 | 11 | 5 | 18 | |
| P | 0.597 | 0.997 | 0.895 | ||||
| ER | Negative | 20 | 37 | 28 | 29 | 11 | 46 |
| Positive | 41 | 56 | 52 | 45 | 22 | 75 | |
| P | 0.379 | 0.591 | 0.621 | ||||
| PR | Negative | 22 | 38 | 29 | 31 | 15 | 45 |
| Positive | 39 | 55 | 51 | 43 | 18 | 76 | |
| P | 0.551 | 0.473 | 0.388 | ||||
136 patients received hysterectomy;
87 patients received lymphadenectomy.
Figure 3.
(A) PTEN loss occurred more often in Uygur women (64.5%, 40/62) than in Han women (40.2%, 37/92), p=0.010). (B) PTEN loss was found more frequently in ECs (57.8%, 59/102) than NECs (34.6%, 18/52) (p=0.040).
hMLH1 expression in different ethnic groups, hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, grade of ECs, and FIGO staging were obviously different, but the differences were not statistically significant (p>0.05).
In addition, in Uygur women, DNMT3B overexpression occurred more frequently in <1/2 myometrial invasion subgroups than >1/2 myometrial invasion subgroups, and the correlation reached statistical significance (p=0.010) (Figure 2C). However, DNMT3B expression in other clinical-pathological subgroups, PTEN and HMLH1 expression in different ethnic groups, hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, grade of ECs, and FIGO staging were obviously different, but the differences were not statistically significant (p>0.05). (Table 3.)
Table 3.
Clinicopathological features and expression levels of PTEN, MLH1, DNMT3B in endometrial carcinoma of Uygurs.
| Variable | DNMT3B | PTEN | hMLH1 | ||||
|---|---|---|---|---|---|---|---|
| Normal | Overexpress | Loss | Normal | Loss | Normal | ||
| Histological type | ECs | 16 | 33 | 32 | 17 | 7 | 42 |
| NECs | 2 | 11 | 8 | 5 | 3 | 10 | |
| P | 0.223 | 0.801 | 0.444 | ||||
| Grade (ECs) | Low | 9 | 12 | 14 | 7 | 3 | 18 |
| High | 7 | 21 | 18 | 10 | 4 | 24 | |
| P | 0.187 | 0.862 | 1.000 | ||||
| Myometrial invasion* | <1/2 | 13 | 22 | 24 | 11 | 8 | 27 |
| ≥1/2 | 0 | 13 | 8 | 5 | 1 | 12 | |
| P | 0.010 | 0.646 | 0.232 | ||||
| Lymph node metastases** | No | 10 | 27 | 22 | 15 | 7 | 30 |
| Yes | 0 | 4 | 4 | 0 | 0 | 4 | |
| P | 0.556 | 0.110 | 0.339 | ||||
| Vessel invasion* | No | 12 | 26 | 24 | 14 | 8 | 30 |
| Yes | 1 | 9 | 8 | 2 | 1 | 9 | |
| P | 0.248 | 0.315 | 0.426 | ||||
| Stage* | I–II | 13 | 27 | 26 | 14 | 8 | 32 |
| III–IV | 0 | 8 | 6 | 2 | 1 | 7 | |
| P | 0.088 | 0.584 | 0.620 | ||||
| ER | Negative | 3 | 17 | 13 | 7 | 3 | 17 |
| Positive | 15 | 27 | 27 | 15 | 7 | 35 | |
| P | 0.093 | 0.956 | 0.868 | ||||
| PR | Negative | 6 | 18 | 15 | 9 | 5 | 19 |
| Positive | 12 | 26 | 25 | 13 | 5 | 33 | |
| P | 0.578 | 0.792 | 0.423 | ||||
48 Uyghur patients received hysterectomy;
41 Uyghur patients received lymphadenectomy.
Correlation of DNMT3B and PTEN expression in endometrial carcinomas
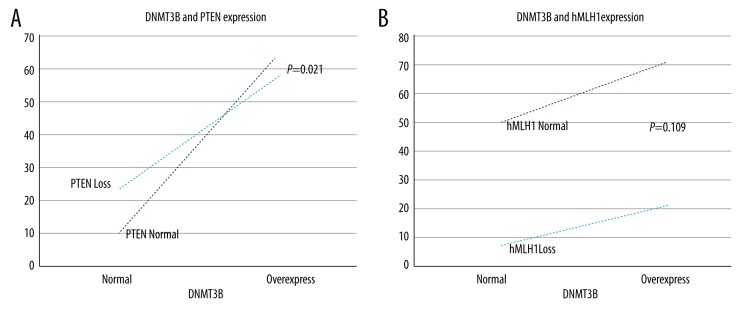
DNMT3B overexpression occurred most frequently in PTEN normal endometrial carcinomas (64/154, 41.6%) than in PTEN loss ones (57/154, 37.0%), and the correlation was statistically significant (p=0.021). DNMT3B overexpression occurred most often in hMLH1 normal endometrial carcinomas (74/154, 48.1%), followed by DNMT3B normal as well as hMLH1 normal groups (52/154, 33.8%); DNMT3B overexpression but hMLH1 loss ones (21/154, 13.6%) and DNMT3B normal but hMLH1 loss ones (7/154, 4.5%), but the correlation was not statistically significant (p>0.05). (Figure 4.) There was a trend of positive correlation between the expression of DNMT3B and PTEN in endometrial carcinoma patients (R=0.186) and a trend of negative correlation between the expression of DNMT3B and hMLH1 in endometrial carcinoma patients (R=−0.129) (Table 4).
Figure 4.
(A) DNMT3B overexpression occurred most frequently in PTEN normal ECs (64/154, 41.6%) than in PTEN loss ECs (57/154, 37.0%), p=0.021). (B) DNMT3B overexpression occurred differently in hMLH1 normal or loss groups, but the correlation was not statistically significant (p=0.109).
Table 4.
Correlation of DNMT3B, PTEN and hMLH1 in endometrial carcinomas.
| Variable | DNMT3B | ||
|---|---|---|---|
| Normal | Overexpress | ||
| PTEN | Loss | 23 | 57 |
| Normal | 10 | 64 | |
| P | 0.021 | ||
| R | 0.186 | ||
| hMLH1 | Loss | 7 | 21 |
| Normal | 52 | 74 | |
| P | 0.109 | ||
| R | −0.129 | ||
Significant prognostic value of PTEN expression patterns for endometrial carcinoma
Survival dates were analyzed for 115 follow-up patients during the follow-up periods of 4 to 128 months (median, 30.93 months). The endometrial carcinoma survival rate was 85.2% (98/115). OS curves based on DNMT3B, PTEN, and hMLH1 expressions were constructed using the Kaplan-Meier method.
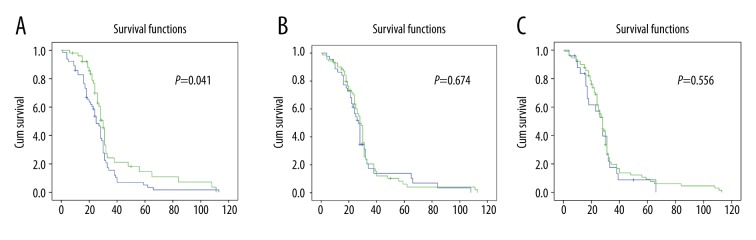
Compared with PTEN protein positive patients, patients who had lost PTEN protein expression might have better prognosis. The OS difference of PTEN expression between negative and positive groups was statistically significant (p=0.041). In addition, there was no obvious difference in OS between the DNMT3B and hMLH1 overexpression group and the loss of expression groups (p=0.674, p=0.556, respectively) (Figure 5).
Figure 5.
(A) The overall survival (OS) differences of PTEN expression between negative and positive groups were statistically significant (p=0.041). (B) OS differences between the DNMT3B overexpression or normal groups was not statistically significant (p=0.674). (C) OS differences between hMLH1 loss or no loss groups were not statistically significant (p=0.556).
Discussion
In our study, we found that the expression levels of DNMT3B and PTEN were significantly different in Uygur women compared to Han women.
Some research has shown that the molecular mechanisms of endometrial cancer, as well as prognosis, are different based on hormone-dependent status. Despite recent improvements in treatment, the clinical outcomes for ECs and NECs patients remains different and unsatisfactory [14]. There is an urgent needed to investigate the underlying molecular markers to improve the outcome of patients with endometrial carcinomas.
To our knowledge, reproductive factors are important in the carcinogenesis of endometrial carcinomas [15]. In Xinjiang, Uygur women have more children, marry earlier, and are more likely to experience premature menopause compared to Han women. In a retrospective review of clinical characteristics of endometrial carcinomas in the Affiliated Tumor Hospital of Xinjiang Medical University between 2009 and 2014, we found that Han women had about 5.4 to 6 times the incidence compared to Uygur women, every year. Uygur women mainly present with well differentiation type I carcinoma (ECs), while poorly differentiated tumors (NECs) are mainly found in Han women. There are no previous reports on the reasons and characteristics of molecular mechanism for these differences.
Numerous studies have reported that PTEN inactivation is the most frequent molecular mechanisms of ECs. While most of PTEN gene expression has been associated with MMR genes such as hMLH1 expression [16]. However, DNMT3B is expressed in endometrioid carcinomas and was identified as one of the potential targets of both miR-145 and miR-143 by the algorithms of target prediction [10].
Our previous studies have shown that co-downregulation of miR-145 and miR-143 and its targets DNMT3B and PTEN may differ in ECs and NECs. DNMT3B overexpression and miR-145 or miR-143 downregulation was been reported to be more powerful in predicting shorter survival [12]. The upregulation of miR-200a/miR-141 and miR-205 have been shown to be different in ECs and NECs, which may be associated with hormone receptor status (ER, PR) of women with endometrial cancer and may be a predictor of prognosis [13].
Our results found 61.7% (95/154) DNMT3B overexpression, 50.0% (77/154) loss of PTEN expression, and 18.2% (28/154) loss of hMLH1 expression. Our results were consistent with other studies [17,18]. In our study, DNMT3B overexpression occurred differently in Uygur women than in Han women and differently in high-grade compared to low-grade ECs. Moreover, DNMT3B expression was associated with myometrial invasion subgroups in Uygur women. However, DNMT3B expression was not distinct based on different types (ECs/NECs), hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, and FIGO staging subgroups. This was a different finding from our smaller previous survey. However, due to the large number of patient samples and regional differences, the results of this study were significant and may represent different expression of DNMT3B in endometrial cancer subtype.
Another reason for the differences between this data and our previous study might be because the former study included more Han patients and some old tissue blocks, and the longer time of storage of the formalin-fixed, paraffin-embedded tissue may have decreased the staining efficiency in immunohistochemistry process.
However, in this study, PTEN loss occurred differently in different ethnic groups as well as in different histological types, but no significant difference based on hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, grade of ECs, or FIGO staging subgroups. This suggests that patients that have a loss of PTEN protein expression might have a better prognosis. These findings were consistent with our earlier results. Our results were not concordant with the findings by Yao et al. [19]. They found that the expression of PTEN and p53 had little connection with prognosis prediction of patients with endometrial carcinomas. However, in our study we confirmed the postoperative specimens were more representative, due to the limitation of diagnosis of endometrial curettage specimen.
In cases of sporadic endometrial carcinoma, about 30% of patients have at least one kind of MMR protein expression missing, and most of them (70%) are caused by the hMLH1 gene methylation [20]. However, for hMLH1 expression, there were no statistical difference in different ethnic groups, hormone receptor (ER, PR) status, myometrial invasion, lymph node metastases, vessel invasion, grade of ECs, or FIGO staging subgroups, and no distinction in Uygur women. The possible reasons may be related to regional or group differences, or other molecular changes.
More interestingly, we found that the expression status of DNMT3B was closely associated with PTEN. DNMT3B overexpression occurred most frequently in PTEN normal endometrial carcinomas. There may be a trend of a positive correlation between DNMT3B overexpression and PTEN loss. We wonder if the overexpression of DNMT3B may precipitate the loss of expression of PTEN gene, or PTEN loss may lead to DNMT3B overexpression in the progression of endometrial carcinoma. The co-effect and mechanism is unpredictable. Loss of the PTEN product by DNA methylation-mediated transcription silencing predicts poor prognosis. It is possible that DNMT3B acts like a bridge between PTEN expression and miRNA regulation. Further studies on the relationship between the expression of DNMT3B and PTEN could be very rewarding.
However, in this study, no statistically correlations between DNMT3B and hMLH1 or PTEN and hMLH1 were found in endometrial cancers. Studies have indicated that silencing of the tumor suppressor genes by DNA methylation mechanism plays an important role in the pathogenesis of endometrial cancer. DNMT3B, as a key enzyme in DNA methylation, act as a tumor suppressor in endometrial carcinoma [21]. Promoter hypermethylation is one of the most common mechanisms of PTEN, DNA mismatch repair (MMR) gene inactivation. In addition, hMLH1 can also be inactivated by hypermethylation, and as such, the genes preferentially mutated [22]. We plan in future studies to detect the methylation status of DNMT3B, PTEN, and hMLH1 in endometrial carcinoma to explore these relationships in-depth.
Together, all these findings imply that there may be certain interaction between DNMT3B, PTEN, and hMLH1 in endometrial carcinomas. We reconfirmed that DNMT3B overexpression and loss of PTEN and hMLH1 expression are different in Uygur women compared to Han women. In addition, DNMT3B overexpression significantly correlated with myometrial invasion, and PTEN loss significantly correlated with ECs or NECs.
Conclusions
Based on our previous research, our findings suggest that the function of DNMT3B and PTEN may differ in Han women compared to Uygur women. These two ethnic groups possess common expression regulation as well as certain ethnic differences. The interaction between DNMT3B and PTEN may exist in the pathogenesis of endometrial carcinoma. Furthermore, the evaluation of DNMT3B and PTEN expression may plays a vital role in predicting the biological behavior and prognosis of patients with endometrioid carcinomas. Joint detection of the expression of DNMT3B, PTEN, and hMLH1 in endometrial carcinomas might be potential targets for clinical-pathological features and prognosis indicators. Therefore, progress in the understanding of the pathogenesis mechanism of endometrial carcinoma could result in comprehension new anticancer treatments.
Footnotes
Declaration of conflict of interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (81360381) and Postgraduate Research Projects of Xinjiang Medical University (CXCY038)
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014;15:e268–78. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Carcangiu ML, Simon HC, Young RH. WHO Classification of tumours of female reproductive organs. Lyon, France: IARC Press; 2014. pp. 122–35. [Google Scholar]
- 4.Ioffe YJ, Chiappinelli KB, Mutch DG, et al. Phosphatase and tensin homolog (PTEN) pseudogene expression in endometrial cancer: A conserved regulatory mechanism important in tumorigenesis? Gynecol Oncol. 2012;124(2):340–46. doi: 10.1016/j.ygyno.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nout RA, Bosse T, Creutzberg CL, et al. Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/beta-catenin and P53 pathway activation. Gynecol Oncol. 2012;126(3):466–73. doi: 10.1016/j.ygyno.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Yu CG, Jiang XY, Li B, et al. Expression of ER, PR, C-erbB-2 and Ki-67 in endometrial carcinoma and their relationships with the clinicopathological features. Asian Pac J Cancer Prev. 2015;16(15):6789–94. doi: 10.7314/apjcp.2015.16.15.6789. [DOI] [PubMed] [Google Scholar]
- 7.Smolle MA, Bullock MD, Ling H, et al. Long non-coding RNAs in endometrial carcinoma. Int J Mol Sci. 2015;16:26463–72. doi: 10.3390/ijms161125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Hong Y, Lu C, et al. The occurrence of cervical cancer in Uygur women in Xinjiang Uygur Autonomous Region is correlated to microRNA-146a and ethnic factor. Int J Clin Exp Pathol. 2015;8(8):9368–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Abdurahman A, Anwar J, Turghun A, et al. Epidermal growth factor receptor gene mutation status and its association with clinical characteristics and tumor markers in non-small-cell lung cancer patients in Northwest China. Mol Clin Oncol. 2015;3(4):847–50. doi: 10.3892/mco.2015.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Adila S, Zhang X, et al. MicroRNA expression signature profile and its clinical significance in endometrioid carcinoma. Zhonghua Bing Li Xue Za Zhi. 2014;43:88–94. [PubMed] [Google Scholar]
- 11.Werner HM, Trovik J, Marcickiewicz J, et al. Revision of FIGO surgical staging in 2009 for endometrial cancer validates to improve risk stratification. Gynecol Oncol. 2012;125:103–8. doi: 10.1016/j.ygyno.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XM, Dong Y, Ti HJ, et al. Down-regulation of miR-145 and miR-143 might be associated with DNA methyltransferase 3B overexpression and worse prognosis in endometrioid carcinomas. Hum Pathol. 2013;44(11):2571–80. doi: 10.1016/j.humpath.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Si JW, Li WT, et al. miR-200a/miR-141 and miR-205 upregulation might be associated with hormone receptor status and prognosis in endometrial carcinomas. Int J Clin Exp Pathol. 2015;8(3):2864–75. [PMC free article] [PubMed] [Google Scholar]
- 14.Banno K, Nogami Y, Kisu I, et al. Candidate biomarkers for genetic and clinicopathological diagnosis of endometrial cancer. Int J Mol Sci. 2013;14:12123–37. doi: 10.3390/ijms140612123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014;24(3):384–93. doi: 10.1097/IGC.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Dong Y, Zhang X, et al. [Significance of phosphoinositide 3 kinase/AKT pathway alterations in endometrial carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2011;40(12):799–804. [in Chinese] [PubMed] [Google Scholar]
- 17.Lee H, Choi HJ, Kang CS, et al. Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma. Mod Pathol. 2012;25:1508–15. doi: 10.1038/modpathol.2012.111. [DOI] [PubMed] [Google Scholar]
- 18.Jin F, Dowdy SC, Xiong Y, et al. Up-regulation of DNA methyltransferase 3B expression in endometrial cancers. Gynecol Oncol. 2005;96(2):531–38. doi: 10.1016/j.ygyno.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Xu W, Wang Y, et al. [Relationships between the molecular biomarkers and the clinicopathologic features and prognosis in endometrial carcinoma]. Beijing Da Xue Xue Bao. 2011;43(5):743–48. [in Chinese] [PubMed] [Google Scholar]
- 20.Garg K, Soslow RA. Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma. J Clin Pathol. 2009;62(8):679–84. doi: 10.1136/jcp.2009.064949. [DOI] [PubMed] [Google Scholar]
- 21.Bai X, Song Z, Fu Y, et al. Clinicopathological significance and prognostic value of DNA methyltransferase 1, 3a, and 3b expressions in sporadic epithelial ovarian cancer. PLoS One. 2012;7:e40024. doi: 10.1371/journal.pone.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvesen HB, MacDonald N, Ryan A, et al. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int J Cancer. 2001;91:22–26. doi: 10.1002/1097-0215(20010101)91:1<22::aid-ijc1002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]