ABSTRACT
Oncogenic mutations of KRAS are the most frequent driver mutations in pancreatic cancer. Expression of an oncogenic allele of KRAS leads to metabolic changes and altered cellular signaling that both can increase the production of intracellular reactive oxygen species (ROS). Increases in ROS have been shown to drive the formation and progression of pancreatic precancerous lesions by upregulating survival and growth factor signaling. A key issue for precancerous and cancer cells is to keep ROS at levels where they are beneficial for tumor development and progression, but below the threshold that leads to induction of senescence or cell death. In KRas-driven neoplasia aberrantly increased ROS levels are therefore balanced by an upregulation of antioxidant genes.
KEYWORDS: KRas, mitochondria, oxidative stress, pancreatic cancer, PanIN, ROS
Epidemiological and animal studies suggest that supplementation of dietary antioxidants decreases cancer risk, which implies that increased ROS may play a role in carcinogenesis.1 Approximately 95% of all pancreatic ductal adenocarcinoma (PDA) show acquisition of activating KRAS mutations,2 which, due to oncogene-mediated alterations in the cell's metabolism, goes along with increased cellular oxidative stress levels.3-6 In mouse models for development of PDA, KRas-caused formation of ROS already is induced in acinar cells and gradually increased during ADM and PanIN formation and progression5 (Fig. 1).
Figure 1.
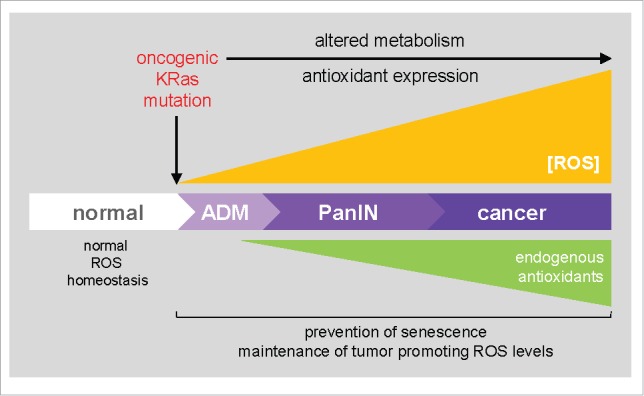
KRas-driven ROS homeostasis and its role in the development of pancreatic cancer. Acquisition of an oncogenic KRas mutation in pancreatic acinar cells leads to their transdifferentiation to duct-like cells. This process named acinar-to-ductal metaplasia (ADM) forms the precursor to PanIN lesions. KRas-induced formation of ROS, due to changes in the cell's metabolic programs, is involved in both ADM and growth and progression of PanIN lesions. A key issue for precancerous and cancer cells is to keep ROS at levels where they are beneficial for tumor development or progression, but below the threshold that leads to induction of senescence or cell death. In KRas-driven neoplasia aberrantly increased ROS levels are therefore accompanied by an upregulation of antioxidant genes.
In pancreatic cancer, oncogenic KRas induces the generation of ROS through multiple mechanisms. Typical metabolic changes initiated by tumor cells are, for example, an increase in aerobic glycolysis (Warburg effect) to support growth under hypoxic conditions7 or altered mitochondrial metabolic activity.5,6,8-10 Oncogenic KRas can modulate mitochondrial metabolism and ROS generation by regulating hypoxia-inducible factors (HIFs) HIF-1α and HIF-2α,8 or through regulation of the transferrin receptor (TfR1), which is highly expressed in pancreatic cancers.11 In addition, KRas can induce suppression of respiratory chain complex I and III to cause mitochondrial dysfunction.6,12 Decreased mitochondrial efficiency then results in an increased production of ROS.5 A possible cause is the ROS-mediated occurrence of 4-hydroxy-2-nonenal (4HNE) and 4HNE-adduct formation with macromolecules, which can lead to inhibition of mitochondrial proteins or damage of mtDNA.5
KRas-induced increases in intracellular ROS levels can also occur via altered NADPH oxidase activities,1 i.e. due to activation of Rac1-NOX4 signaling.13 For example, Rac1 in KrasG12D-expressing PanIN1B/PanIN2 is increasingly active when the tumor protein p53-induced nuclear protein 1 (TP53INP1) is knocked out or decreasingly expressed.14 Other mechanisms by which increases in intracellular ROS can be achieved include enhanced growth factor signaling,15,16 KRasG12D-induced induction of autophagy-specific genes 5 and 7 (ATG5, ATG7),17 repression of SESN3, which controls the regeneration of peroxiredoxins,18 or expression of micro RNAs such as miR-155.19
In vivo in KC mice the depletion of ROS using NAC or the mitochondrially-targeted antioxidant mitoQ leads to a dramatic decrease in formation and progression of precancerous lesions.5,14 KRasG12D-induced mitochondrial ROS (mROS) engages key-signaling pathways that previously have been linked to development and progression of pancreatic cancer. These include activation of the ERK1/2 signaling pathway,6 upregulation of epidermal growth factor receptor (EGF-R) signaling,5 as well as induction of canonical and alternative activation pathways for nuclear factor κ-B (NF-κB),5 which both have been implicated in the progression of PDA.20,21
The serine/threonine kinase Protein Kinase D1 (PKD1) is a major mediator of KRas-mROS signaling,5,22 however, its activation by mROS most likely is indirect. Previously, it was shown that in response to mROS PKD1 can be activated via Src-mediated phosphorylation events.23-25 Src is a redox-regulated kinase and its activation involves the oxidation of cysteine residues which then results in intramolecular disulfide bond formation and increased kinase activity.26 This can be further potentiated by ROS-mediated oxidation and inactivation of regulatory phosphotyrosine phosphatases.27 Although it remains to be tested, arguments for an involvement of Src in the KRas-mROS-PKD1 signaling cascade are recent findings showing cooperation of Src and oncogenic KRas in driving pancreatic neoplasia,28 metastatic growth and therapy resistance in pancreatic cancer.29
PKD1 can activate NF-κB downstream of ROS,24,25 and during development of PDA, KRas-mROS-PKD1-NF-κB signaling upregulates the expression of EGF-R, its ligands TGFα and EGF as well as their sheddase ADAM17.5 Overexpression of EGFR and its ligands occurs frequently in the early development process of PDA.15 It is required to elevate overall KRas activity (oncogenic and wildtype KRas) to pathological levels by additionally activating the wildtype allele.30-32 An emerging key-role of PKD1 for the initiation of pancreatic cancer is indicated by its additional involvement in the activation of Notch signaling downstream of mutant and wildtype KRas.22,33 Notch and NF-κB signaling pathways can co-operate to mediate formation of pre-neoplastic lesions.34 Thus PKD1 brings together 2 important pathways that drive the formation of precancerous lesions.
During development of PDA, the excess of ROS caused by oncogenic KRas needs to be counterbalanced by an increased expression of antioxidant molecules (Fig. 1) in order to generate the pathophysiological conditions under which ROS can mediate cell proliferation35 and down-regulate tumor suppressors such as INK4/p16 and SMAD4.9 Otherwise ROS can increase to levels where they induce senescence or cell death.36 Fine tuning to mitigate the damaging effects of ROS in KRas-driven tumors occurs by upregulation of ROS detoxifying enzymes. This can be mediated via activation of nuclear respiratory factor 2 (Nrf2), a transcription factor that regulates a panel of antioxidant genes. KrasG12D mutations increase the transcription of Nrf2 in vivo in KC mice and PDA.37 Induction of Nrf2 expression is a response to mitochondrial ROS (mROS), since its expression is lost in pancreata of KC mice that were treated with a mitochondrial antioxidant.5 Acquisition of Nrf2 expression results in low, but pro-tumorigenic levels of ROS in pre-neoplastic pancreatic cells and cancer cells.14 A similar relationship between ROS and Nrf2 signaling has been described for development and progression of other cancers.38 Nrf2 activity also can be further increased in pancreatic neoplasia due to somatic mutations that disrupt the interaction with its inhibitor Keap1.39
Besides Nrf2, Pim kinases also contribute to KRasG12V-driven modulation of cellular ROS levels by regulating levels of glutathione peroxidase 4 and peroxiredoxin 3 or expression of manganese superoxide dismutase (MnSOD), which is encoded by the SOD2 gene.40 If Pims are absent, c-Myc can compensate by regulating expression of the SOD2 gene.40 Similarly, upregulation of SOD2 expression is mediated in response to mROS via PKD1-NF-κB signaling.24 The product of MnSOD activity is an increase in hydrogen peroxide, a bona fide signaling molecule that is important for tumor cell proliferation.16 It should be noted that besides upregulation of antioxidant systems, in pancreatic cancer, KRas activates multiple other mechanisms that contribute to prevent cell death and senescence. These include upregulation of the transcription factor Twist41 and signaling that bypasses retinoblastoma (Rb) protein.42
In summary, during the development and progression of PDA, oncogenic KRas causes metabolic changes that lead to increased generation of mitochondrial reactive oxygen species. Oncogenic KRas also upregulates antioxidant systems to balance ROS to levels at which they drive major signaling pathways that contribute to oncogenic transformation and tumor progression.5,30,32 A key question now is if the knowledge can be applied for developing novel therapeutic approaches. One possibility is to inhibit expression or activity of Nrf2, with the overall goal to reduce induction of antioxidant systems and to drive KRas-generated ROS to levels where they induce senescence or cell death. Such an approach may be most effective in combination with chemotherapeutic drugs that additionally increase cellular ROS production. Another possibility is to administer mitochondrially-targeted antioxidants such as mitoQ, with the overall goal to suppress KRas-induced mROS formation to target development and progression of PDA. While approaches aiming on increasing ROS levels may be more efficient in targeting progressed PDA, approaches aiming on decreasing ROS levels may be more efficient as a cancer prevention strategy.
Abbreviations
- ADM
acinar-to-ductal metaplasia
- EGF
epidermal growth factor
- EGF-R
epidermal growth factor receptor
- KC
mice expressing oncogenic KRas under a pancreatic acinar cell-specific promoter
- KRas
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- Kras
mouse gene encoding KRas
- KRAS
human gene encoding KRas
- mROS
mitochondrial reactive oxygen species
- NAC
N-acetyl-L-cysteine
- NF-κB
nuclear factor κ-B
- Nrf2
nuclear respiratory factor 2
- PanIN
pancreatic intraepithelial neoplasia
- PDA
pancreatic ductal adenocarcinoma
- PKD1
protein kinase D1
- ROS
reactive oxygen species
- TGFα
transforming growth factor-α.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Pancreatic Cancer Action Network (PanCAN) for their support.
Funding
This work was supported by the NIH grants CA200572 and CA140182 (to PS). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funders had no role in decision to publish, or preparation of the manuscript.
References
- [1].Kong B, Qia C, Erkan M, Kleeff J, Michalski CW. Overview on how oncogenic Kras promotes pancreatic carcinogenesis by inducing low intracellular ROS levels. Front Physiol 2013; 4:246; PMID:24062691; http://dx.doi.org/ 10.3389/fphys.2013.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J 2001; 7:251-8; PMID:11561601 [PubMed] [Google Scholar]
- [3].Kodydkova J, Vavrova L, Stankova B, Macasek J, Krechler T, Zak A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas 2013; 42:614-21; PMID:23558240; http://dx.doi.org/ 10.1097/MPA.0b013e318288360a [DOI] [PubMed] [Google Scholar]
- [4].Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al.. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013; 496:101-5; PMID:23535601; http://dx.doi.org/ 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liou GY, Doppler H, DelGiorno KE, Zhang L, Leitges M, Crawford HC, Murphy MP, Storz P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep 2016; 14:2325-36; PMID:26947075; http://dx.doi.org/ 10.1016/j.celrep.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010; 107:8788-93; PMID:20421486; http://dx.doi.org/ 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/ 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chun SY, Johnson C, Washburn JG, Cruz-Correa MR, Dang DT, Dang LH. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1alpha and HIF-2alpha target genes. Mol Cancer 2010; 9:293; PMID:21073737; http://dx.doi.org/ 10.1186/1476-4598-9-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mishra PK, Raghuram GV, Jain D, Jain SK, Khare NK, Pathak N. Mitochondrial oxidative stress-induced epigenetic modifications in pancreatic epithelial cells. Int J Toxicol 2014; 33:116-29; PMID:24563415; http://dx.doi.org/ 10.1177/1091581814524064 [DOI] [PubMed] [Google Scholar]
- [10].Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab 2015; 3:1; PMID:25621173; http://dx.doi.org/ 10.1186/s40170-015-0128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeong SM, Hwang S, Seong RH. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem Biophys Res Commun 2016; 471:373-9; PMID:26869514; http://dx.doi.org/ 10.1016/j.bbrc.2016.02.023 [DOI] [PubMed] [Google Scholar]
- [12].Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y, Ogasawara M, Trachootham D, Feng L, Pelicano H, et al.. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res 2012; 22:399-412; PMID:21876558; http://dx.doi.org/ 10.1038/cr.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, Gorgoulis VG, d'Adda di Fagagna F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ 2014; 21:998-1012; PMID:24583638; http://dx.doi.org/ 10.1038/cdd.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Al Saati T, Clerc P, Hanoun N, Peuget S, Lulka H, Gigoux V, Capilla F, Beluchon B, Couvelard A, Selves J, et al.. Oxidative stress induced by inactivation of TP53INP1 cooperates with KrasG12D to initiate and promote pancreatic carcinogenesis in the murine pancreas. Am J Pathol 2013; 182:1996-2004; PMID:23578383; http://dx.doi.org/ 10.1016/j.ajpath.2013.02.034 [DOI] [PubMed] [Google Scholar]
- [15].Korc M. Role of growth factors in pancreatic cancer. Surg Oncol Clin N Am 1998; 7:25-41; PMID:9443985 [PubMed] [Google Scholar]
- [16].Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010; 44:479-96; PMID:20370557; http://dx.doi.org/ 10.3109/10715761003667554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim MJ, Woo SJ, Yoon CH, Lee JS, An S, Choi YH, Hwang SG, Yoon G, Lee SJ. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem 2011; 286:12924-32; PMID:21300795; http://dx.doi.org/ 10.1074/jbc.M110.138958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zamkova M, Khromova N, Kopnin BP, Kopnin P. Ras-induced ROS upregulation affecting cell proliferation is connected with cell type-specific alterations of HSF1/SESN3/p21Cip1/WAF1 pathways. Cell Cycle 2013; 12:826-36; PMID:23388456; http://dx.doi.org/ 10.4161/cc.23723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang P, Zhu CF, Ma MZ, Chen G, Song M, Zeng ZL, Lu WH, Yang J, Wen S, Chiao PJ, et al.. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget 2015; 6:21148-58; PMID:26020803; http://dx.doi.org/ 10.18632/oncotarget.4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doppler H, Liou GY, Storz P. Downregulation of TRAF2 mediates NIK-induced pancreatic cancer cell proliferation and tumorigenicity. PLoS One 2013; 8:e53676; PMID:23301098; http://dx.doi.org/ 10.1371/journal.pone.0053676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, Lopez-Berestein G, Vivas-Mejia PE, Sood AK, McConkey DJ, et al.. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res 2008; 14:8143-51; PMID:19088029; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liou GY, Doppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, Crawford HC, Fields AP, Murray NR, Wang QJ, et al.. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun 2015; 6:6200; PMID:25698580; http://dx.doi.org/ 10.1038/ncomms7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol 2004; 24:2614-26; PMID:15024053; http://dx.doi.org/ 10.1128/MCB.24.7.2614-2626.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol 2005; 25:8520-30; PMID:16166634; http://dx.doi.org/ 10.1128/MCB.25.19.8520-8530.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J 2003; 22:109-20; PMID:12505989; http://dx.doi.org/ 10.1093/emboj/cdg009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 2005; 25:6391-403; PMID:16024778; http://dx.doi.org/ 10.1128/MCB.25.15.6391-6403.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal 2005; 7:560-77; PMID:15890001; http://dx.doi.org/ 10.1089/ars.2005.7.560 [DOI] [PubMed] [Google Scholar]
- [28].Shields DJ, Murphy EA, Desgrosellier JS, Mielgo A, Lau SK, Barnes LA, Lesperance J, Huang M, Schmedt C, Tarin D, et al.. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene 2011; 30:2123-34; PMID:21242978; http://dx.doi.org/ 10.1038/onc.2010.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kelber JA, Reno T, Kaushal S, Metildi C, Wright T, Stoletov K, Weems JM, Park FD, Mose E, Wang Y, et al.. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res 2012; 72:2554-64; PMID:22589274; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, et al.. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 2012; 22:304-17; PMID:22975374; http://dx.doi.org/ 10.1016/j.ccr.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Navas C, Hernandez-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell 2012; 22:318-30; PMID:22975375; http://dx.doi.org/ 10.1016/j.ccr.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, Logsdon CD. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 2014; 33:532-5; PMID:23334325; http://dx.doi.org/ 10.1038/onc.2012.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liou GY, Leitges M, Storz P. Pancreatic oncogenic signaling cascades converge at Protein Kinase D1. Cell Cycle 2015; 14:1489-90; PMID:25928263; http://dx.doi.org/ 10.1080/15384101.2015.1032646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maniati E, Bossard M, Cook N, Candido JB, Emami-Shahri N, Nedospasov SA, Balkwill FR, Tuveson DA, Hagemann T. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest 2011; 121:4685-99; PMID:22056382; http://dx.doi.org/ 10.1172/JCI45797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shidara Y, Yamagata K, Kanamori T, Nakano K, Kwong JQ, Manfredi G, Oda H, Ohta S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res 2005; 65:1655-63; PMID:15753359; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-2012 [DOI] [PubMed] [Google Scholar]
- [36].Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, Yu ZX, Ferrans VJ, Howard BH, Finkel T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem 1999; 274:7936-40; PMID:10075689; http://dx.doi.org/ 10.1074/jbc.274.12.7936 [DOI] [PubMed] [Google Scholar]
- [37].DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al.. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475:106-9; PMID:21734707; http://dx.doi.org/ 10.1038/nature10189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Perera RM, Bardeesy N. Cancer: when antioxidants are bad. Nature 2011; 475:43-4; PMID:21734699; http://dx.doi.org/ 10.1038/475043a [DOI] [PubMed] [Google Scholar]
- [39].Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 2009; 34:176-88; PMID:19321346; http://dx.doi.org/ 10.1016/j.tibs.2008.12.008 [DOI] [PubMed] [Google Scholar]
- [40].Song JH, An N, Chatterjee S, Kistner-Griffin E, Mahajan S, Mehrotra S, Kraft AS. Deletion of Pim kinases elevates the cellular levels of reactive oxygen species and sensitizes to K-Ras-induced cell killing. Oncogene 2015; 34:3728-36; PMID:25241892; http://dx.doi.org/ 10.1038/onc.2014.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 2010; 18:448-58; PMID:21075310; http://dx.doi.org/ 10.1016/j.ccr.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carriere C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS, Korc M. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology 2011; 141:1091-101; PMID:21699781; http://dx.doi.org/ 10.1053/j.gastro.2011.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]


