Abstract
PURPOSE
To assess the long-term quality of life (QoL) outcomes from a phase III trial comparing conventional (CIMRT) versus hypofractionated (HIMRT) IMRT in patients with localized prostate cancer.
METHODS AND MATERIALS
Between 2002 and 2006, 303 men with low- to high-risk prostate cancer were randomized to 76 Gy in 38 fractions (CIMRT) versus 70.2 Gy in 26 fractions (HIMRT). QoL was compared using the Expanded Prostate Cancer Index Composite (EPIC), International Prostate Symptom Score (IPSS), and EuroQoL (EQ5D) questionnaires. The primary outcome of the quality of life analysis was a minimum clinically important difference defined as a 0.5 standard deviation change from baseline for each respective QoL parameter. Treatment effects were evaluated using logistic mixed effects regression models.
RESULTS
A total of 286, 299, and 218 patients had baseline EPIC, IPSS, or EQ5D data available and were included in the analysis. Overall, there was no statistically significant difference between the two treatment arms in terms of EPIC, IPSS, or EQ5D scores over time although there was a trend towards lower EPIC urinary incontinence scores in the HIMRT arm. More patients in the HIMRT arm had a lower EPIC urinary incontinence score relative to baseline versus patients in the CIMRT arm with long-term follow-up. On multivariable analysis, there was no association between radiation fractionation scheme and any QoL parameter. When examining other clinical factors, lymph node radiation was associated with worse EPIC hormonal scores versus patients receiving no lymph node radiation. In general, QoL outcomes were generally stable over time with the exception of EPIC hormonal and EQ5D scores.
CONCLUSIONS
In this randomized prospective study, there were stable QoL changes in patients receiving HIMRT or CIMRT. Our results add to the growing body of literature suggesting that HIMRT may be an acceptable treatment modality in clinically localized prostate cancer.
Keywords: Hypofractionated, Intensity Modulated, Prostate Cancer, Quality of Life
INTRODUCTION
Prostate cancer is the most common type of invasive cancer in men in the United States with an estimated 220,800 cases and 27,540 deaths in 2015 [1]. Definitive radiotherapy is an acceptable standard of care in patients diagnosed with localized prostate cancer. The current standard definitive radiotherapy regimen consists of conventionally fractionated radiation (1.8–2 Gy per fraction) for approximately 8 weeks (76–80 Gy) [2, 3]. Hypofractionated radiation therapy delivers doses greater than 2 Gy per day with the potential advantages of reduced treatment cost and patient convenience, and a theoretical improvement in the therapeutic ratio for prostate cancer [4]. Early studies have demonstrated comparable clinical outcomes in patients undergoing conventional versus hypofractionated radiation [5, 6].
Due to the comparable efficacy of the various treatment regimens for prostate cancer, quality of life (QoL) outcomes play an important role in decision-making. In general, surgery and/or radiation result in distinct adverse effect profiles and thus QoL outcomes are used to help direct treatment according to baseline patient preference and function [7, 8]. To date, there is no long-term randomized prospective data comparing QoL outcomes of patients treated with conventional versus hypofractionated radiotherapy. Herein, we present long term patient reported outcomes from a contemporary phase III trial comparing conventionally fractioned intensity modulated radiation therapy (CIMRT) and hypofractionated intensity modulated radiotherapy (HIMRT) for prostate cancer.
MATERIALS AND METHODS
Study Design and Radiation Technique
The patient population and methods have been previously described [9]. Between 2002 and 2006, 307 patients were enrolled, 303 were assessable, with 152 randomly assigned to receive CIMRT and 151 to receive HIMRT. Patients on the CIMRT arm received 76 Gy in 38 fractions at 2.0 Gy per fraction; patients on the HIMRT arm received 70.2 Gy in 26 fractions at 2.7 Gy per fraction. The hypofractionated arm of 70.2 Gy in 2.7 Gy fractions was hypothesized to be equivalent to 84.4 Gy in 2.0 Gy fractions based on an alpha/beta of 1.5 Gy for prostate cancer [10, 11]. Radiation techniques and dosimetric constraints have been previously described [9]. Briefly, all patients underwent supine CT and MRI simulation in an immobilization device. The first clinical target volume (CTV) included the prostate and proximal seminal vesicles, and this was the only CTV for low- to intermediate-risk patients. The distal seminal vesicles and pelvic lymph nodes were treated in those with high-risk disease. The dose of 95% of the PTV was to be the prescription dose or higher.
Eligible patients were required to have clinical T1-T3 disease and Gleason score ≥ 5 if they had intermediate or high-risk features. Intermediate risk was defined as Gleason score 7, pre-treatment PSA > 10–20 ng/mL, and Stage T1-T2 unless ≥ 4 biopsies classified as Gleason 7. High-risk was defined as Gleason score 8–10, Gleason score 7 in ≥4 cores, cT3 disease, or a pre-treatment PSA > 20 ng/mL. Up to 4 months of androgen deprivation therapy (ADT) with a luteinizing hormone releasing hormone (LHRH) agonist or anti-androgen before randomization was permitted. Patients with high-risk disease were planned to receive 24 months of ADT. Patients with less than high-risk were planned to receive up to four months ADT starting ≤ 4 months before random assignment. Stratification variables included PSA ≤ 10 versus ≥ 10 to 20 versus > 20 ng/mL, Gleason score 5 to 7 versus 8 to 10, and high risk versus lower risk disease.
Quality of Life Outcomes
Patients in each arm completed QoL questionnaires pre-treatment (used as baseline) and at each follow-up visit, patients continued to complete QoL after developing a treatment recurrence. The Expanded Prostate Cancer Index Composite (EPIC) is a 50-item questionnaire which is subdivided into five subscales including urinary incontinence, urinary irritative-obstructive, bowel, sexual, and hormonal summary scores. The questionnaire is scored from 0 to 100, with 100 being a perfect score with no symptoms. We additionally sub-classified EPIC distress scores as proposed by Talcott et al [12]. “No relevant problem” describes a patient with no distressful symptoms, “small to moderate problem” describes a patient reporting at least one distressful symptom, and “severe problem” describes a patient with at least one extremely distressful symptom. For example, “no relevant problems” in the EPIC bowel domain represents a patient with no distressful symptoms in any of the EPIC bowel domain items; “small to moderate problems” would represent a patient with at least one distressful symptom in the EPIC bowel domain subscale but no items marked very distressful. “Severe problems” would describe at least one very distressful symptom in the EPIC bowel domain subscale. The International Prostate Symptom Score (IPSS) is a seven-item questionnaire, which primarily measures obstructive and irritative urinary symptoms (frequency, nocturia, weak urinary stream, hesitancy, intermittence, incomplete emptying and urgency) [13]. Each sub-section is scored on a scale from 1 to 5 for a total maximum score of 35, with 0 being a perfect score with no symptoms. The IPSS also includes an additional QoL score rated from 1 to 6, with 0 being a perfect score, “delighted”.
The EuroQoL five-dimension questionnaire (EQ5D) is a method for obtaining valuations of health-related QoL; it is a two-part questionnaire that takes approximately 5 minutes to complete. The first part consists of 5-items covering 5 dimensions including: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension can be graded on 3 levels including: 1-no problems, 2- moderate problems and 3-extreme problems. The second part is a visual analogue scale (VAS) valuing current health state, measured on a 10 point-interval scale. Worst imaginable health state is scored as 0 at the bottom of the scale and best imaginable health state is scored as 100 at the top. Both the 5-item index score and the VAS score are transformed into a utility score between 0 “Worst health state” and 1 “Best health state” [14].
Statistical Analysis
The primary outcome of this study was freedom from biochemical failure with the original study consisting of a sample size of 300 assessable patients with 90% power to detect a hazard ratio of 0.46 when the proportions free of failure for the two arms at 4 years after the last patient is entered (2 years after the completion of androgen deprivation) are 70% and 85% at a significance level of 0.05 using a two-sided log-rank test. QoL outcomes were a pre-specified secondary endpoint, reported herein, examining differences by treatment arm at 1 year, 2 years, 3 years, 4 years, and 5 years. These secondary outcomes were considered hypothesis-generating and the trial was not powered to detect QoL differences between groups.
The primary outcome of the quality of life analysis was a minimum clinically important difference (MCID) defined as a 0.5 standard deviation change from baseline for each respective quality of life parameter [15, 16]. The patient’s demographic and clinical characteristics were compared between treatment groups via Chi-square tests or Wilcoxon tests. Treatment effects on QoL outcomes were evaluated using logistic mixed effects regression models with random intercepts to account for within-subject correlation. Baseline QoL scores were included as a baseline dependent variable, no treatment by time interaction was included. A multivariate cox proportional hazards model was constructed to assess for predictors of QoL outcomes, all available time points (1 year, 2 years, 3 years, 4 years, and 5 years) were included in the model in addition to clinical and treatment characteristics identified by clinical expertise. For the IPSS QoL outcomes, there were sufficient patients completing the questionnaire at year 6 and 7, this data was also included in the IPSS overall and IPSS quality of life analysis. The follow-up time was identified from the time of treatment completion to the time of last QoL assessment completion. P-values less than 0.05 were considered statistically significant. As recommended, if more than 20% of the items that comprised a domain summary score or subscale score were missing a response, the corresponding domain summary or subscale score was excluded from analysis [17]. All analyses were completed using SAS 9.2.
RESULTS
Patient Characteristics
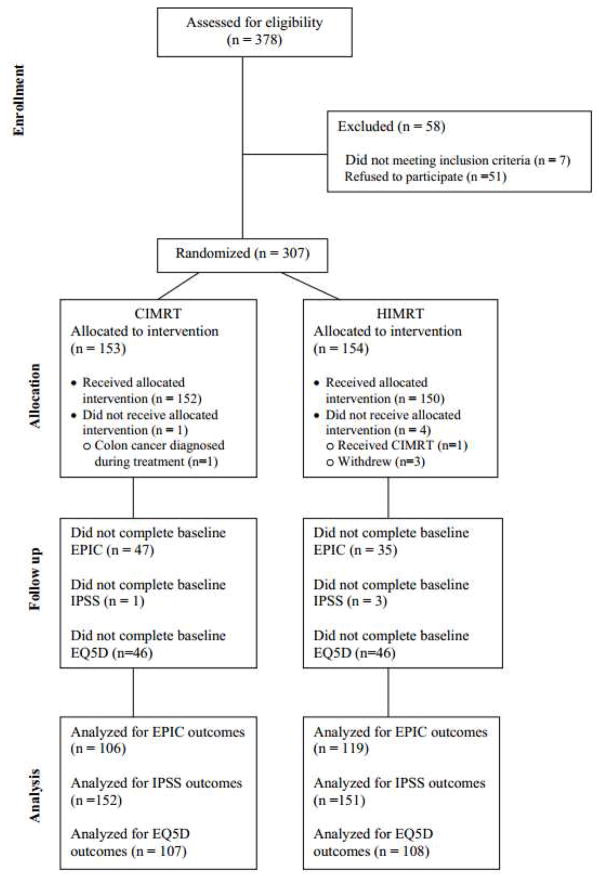
The median follow-up of the entire cohort was 69 months (range 7–136) with no significant differences in age, risk group, stage, Gleason score, or receipt of ADT between the two treatment arms (Table 1). At baseline, a total of 299 patients completed the IPSS, 286 completed the EPIC, and 218 completed the EQ5D questionnaire (Figure 1). There were no significant differences between the groups in terms of baseline EPIC summary scores, IPSS scores, or EQ5D scores (Table 1).
Table 1.
Patient Characteristics and Baseline Quality of Life Scores
| CIMRT n=152 | HIMRT n=151 | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | |||||
| Median (Range) | 67 (45–86) | 67 (49–86) | 0.79 | ||
| Pre-treatment PSA | |||||
| < 10 | 99 | 65.1 | 95 | 62.9 | |
| 10–20 | 40 | 26.3 | 41 | 27.2 | |
| > 20 | 13 | 8.6 | 15 | 9.9 | |
| T-stage | |||||
| T1 | 59 | 38.8 | 61 | 40.4 | 0.77 |
| T2 | 77 | 50.7 | 71 | 47 | |
| T3 | 16 | 10.5 | 19 | 12.6 | |
| NCCN Risk Group | |||||
| Low | 24 | 15.8 | 22 | 14.6 | 0.96 |
| Intermediate | 85 | 55.9 | 86 | 57 | |
| High | 43 | 28.3 | 43 | 28.5 | |
| Gleason Score | |||||
| 6 | 51 | 33.5 | 53 | 35.1 | 0.96 |
| 7 | 72 | 47.4 | 70 | 46.4 | |
| ≥ 8 | 29 | 19.1 | 28 | 28 | |
| ADT | |||||
| No | 81 | 53.3 | 83 | 55 | 0.77 |
| Yes | 71 | 46.7 | 68 | 45 | |
| Lymph Node Radiation | |||||
| No | 104 | 68.4 | 103 | 68.2 | 0.97 |
| Yes | 48 | 31.6 | 48 | 31.8 | |
| Race | |||||
| Black | 27 | 17.8 | 11 | 7.3 | 0.02 |
| White | 119 | 78.3 | 134 | 88.7 | |
| Other | 6 | 3.9 | 6 | 4 | |
| Baseline EPIC, Median (Range) | |||||
| Urinary Irritative/Obstructive | 92.86 (39.29–100) | 91.67 (64.29–100) | 0.90 | ||
| Urinary Incontinence | 92.35 (39.5–100) | 100 (52–100) | 0.40 | ||
| Hormone | 93.18 (56.82–100) | 93.18 (54.44–100) | 1.00 | ||
| Sexual | 47.77 (0–93.75) | 53.23 (0–94.23) | 0.70 | ||
| Bowel | 95.54 (58.93–100) | 98.21 (60.71–100) | 0.09 | ||
| Baseline IPSS, Median (Range) | |||||
| Overall | 6 (0–28) | 6 (0–26) | 0.8 | ||
| QoL score | 2 (0–6) | 2 (0–6) | 0.3 | ||
| Baseline EQ5D, Median (Range) | |||||
| EQ5D Index | 1 (0.4–1.0) | 1 (0.51–1.0) | 0.57 | ||
| EQ5D VSAS | 85 (50–100) | 85 (30–100) | 0.17 | ||
Figure 1.
CONSORT Diagram
Change in functioning according to treatment arm
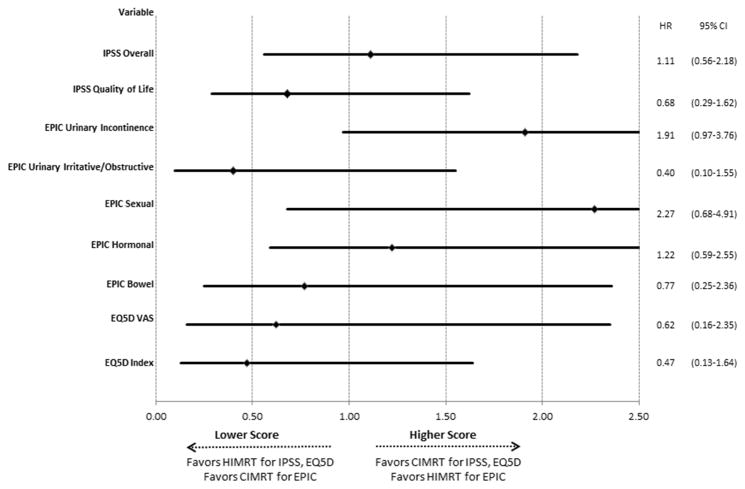
Figure 2 demonstrates the unadjusted hazard ratios for each QoL outcome according to treatment arm. Overall, there was no statistically significant difference between the two treatment arms in terms of EPIC, IPSS, or EQ5D scores although there was a trend towards lower EPIC urinary incontinence scores in the HIMRT arm. There was an initial decrease in the EPIC bowel, EPIC sexual and EPIC hormonal scores for both treatment arms although this stabilized with further follow-up. More patients in the HIMRT arm had a lower EPIC urinary incontinence score relative to baseline versus patients in the CIMRT arm with long-term follow-up. Supplementary Figure 1 demonstrates the mean QoL scores by treatment group at each time point.
Figure 2.
Forest Plot Demonstrating Quality of Life Outcomes (minimum clinically important difference) According to Radiation Fractionation
In order to further analyze the patients with improved, unchanged, or worsening EPIC scores, we examined three-year outcomes according to baseline status (no relevant problems at baseline, small to moderate problems at baseline, severe problems at baseline). In general, patients presenting with no problems at baseline generally had low rates of developing severe problems at 36 months across all subgroups while patients with severe problems at baseline continued to have severe problems at 36 months. Further analysis is demonstrated in Supplementary Figure 2.
Change in function over time
Table 2 demonstrates the change in QoL scores according to treatment time. There was no significant difference in the overall IPSS score, IPSS QoL, EPIC urinary irritative/obstructive, EPIC urinary incontinence, EPIC sexual, or EPIC Bowel score at any time point. There was a worsening in the EPIC Hormonal score at years 2–5. In addition there was a worsening of the EQ5D VAS score at year 1 and EQ5D index at year 2, both of which subsequently resolved.
Table 2.
Mixed Model Demonstrating Quality of Life (minimum clinically important difference) Outcomes According to Baseline Status
| Variable | OR | 95% CI | |
|---|---|---|---|
| IPSS Overall | |||
| Year 1 | 0.78 | 0.50 | 1.23 |
| Year 2 | 0.89 | 0.56 | 1.42 |
| Year 3 | 0.85 | 0.51 | 1.40 |
| Year 4 | 1.14 | 0.68 | 1.90 |
| Year 5 | 1.12 | 0.63 | 1.98 |
| Year 6 | 0.57 | 0.23 | 1.40 |
| Year 7 | 0.83 | 0.35 | 1.94 |
| IPSS Quality of Life | |||
| Year 1 | 1.07 | 0.69 | 1.65 |
| Year 2 | 1.29 | 0.82 | 2.02 |
| Year 3 | 1.04 | 0.64 | 1.70 |
| Year 4 | 1.34 | 0.81 | 2.21 |
| Year 5 | 1.61 | 0.91 | 2.82 |
| Year 6 | 1.44 | 0.54 | 3.79 |
| Year 7 | 0.87 | 0.32 | 2.33 |
| EPIC Urinary Incontinence | |||
| Year 1 | 1.13 | 0.55 | 2.35 |
| Year 2 | 1.20 | 0.55 | 2.62 |
| Year 3 | 0.86 | 0.39 | 1.91 |
| Year 4 | 1.08 | 0.49 | 2.36 |
| Year 5 | 0.70 | 0.29 | 1.66 |
| EPIC Urinary Irritative/Obstructive | |||
| Year 1 | 1.09 | 0.34 | 3.49 |
| Year 2 | 0.76 | 0.23 | 2.51 |
| Year 3 | 0.75 | 0.22 | 2.51 |
| Year 4 | 1.19 | 0.35 | 4.02 |
| Year 5 | 1.21 | 0.34 | 4.34 |
| EPIC Sexual | |||
| Year 1 | 0.18 | 0.04 | 0.77 |
| Year 2 | 0.87 | 0.24 | 3.13 |
| Year 3 | 0.59 | 0.15 | 2.31 |
| Year 4 | 0.64 | 0.17 | 2.38 |
| Year 5 | 0.97 | 0.24 | 3.94 |
| EPIC Hormonal | |||
| Year 1 | 1.72 | 0.74 | 3.99 |
| Year 2 | 3.14 | 1.33 | 7.45 |
| Year 3 | 3.06 | 1.24 | 7.53 |
| Year 4 | 3.17 | 1.29 | 7.76 |
| Year 5 | 4.08 | 1.62 | 10.25 |
| EPIC Bowel | |||
| Year 1 | 0.40 | 0.15 | 1.07 |
| Year 2 | 0.50 | 0.18 | 1.38 |
| Year 3 | 1.82 | 0.70 | 4.73 |
| Year 4 | 1.26 | 0.47 | 3.37 |
| Year 5 | 1.17 | 0.41 | 3.33 |
| EQ5D VAS | |||
| Year 1 | 4.26 | 1.56 | 11.63 |
| Year 2 | 2.25 | 0.79 | 6.40 |
| Year 3 | 2.04 | 0.70 | 5.93 |
| Year 4 | 1.59 | 0.55 | 4.64 |
| Year 5 | 2.67 | 0.88 | 8.14 |
| EQ5D Index | |||
| Year 1 | 1.53 | 0.51 | 4.56 |
| Year 2 | 3.69 | 1.21 | 11.26 |
| Year 3 | 1.51 | 0.48 | 4.80 |
| Year 4 | 2.95 | 0.92 | 9.45 |
| Year 5 | 0.99 | 0.28 | 3.46 |
Multivariable Analysis
Multivariable outcomes are demonstrated in Table 3. There was no association between radiation fractionation scheme and any QoL parameter although there was a trend towards worse EPIC urinary incontinence scores in the HIMRT group. When examining other clinical factors, lymph node radiation was associated with worse EPIC hormonal scores versus patients receiving no lymph node radiation. In addition, time after treatment was a predictor of worse EPIC hormonal scores.
Table 3.
Multivariable Model of Association Between Quality of Life Outcomes (minimum clinically important difference) and Clinical Variables
| OR | 95% CI | ||
|---|---|---|---|
| IPSS Overall | |||
| Fractionation (HIMRT vs CIMRT) | 0.97 | 0.57 | 1.65 |
| Year 1 | 0.82 | 0.53 | 1.25 |
| Year 2 | 0.89 | 0.57 | 1.38 |
| Year 3 | 0.85 | 0.53 | 1.37 |
| Year 4 | 1.12 | 0.69 | 1.82 |
| Year 5 | 1.09 | 0.64 | 1.87 |
| Year 6 | 0.57 | 0.24 | 1.36 |
| Year 7 | 0.79 | 0.35 | 1.79 |
| Age (Continuous) | 0.94 | 0.91 | 0.98 |
| LN Radiation (Yes vs No) | 1.12 | 0.48 | 2.58 |
| ADT Use (No vs Yes) | 1.21 | 0.56 | 2.63 |
| IPSS Quality of Life | |||
| Fractionation (HIMRT vs CIMRT) | 0.77 | 0.43 | 1.38 |
| Year 1 | 1.05 | 0.70 | 1.57 |
| Year 2 | 1.23 | 0.81 | 1.86 |
| Year 3 | 1.02 | 0.65 | 1.60 |
| Year 4 | 1.27 | 0.80 | 2.01 |
| Year 5 | 1.48 | 0.88 | 2.48 |
| Year 6 | 1.38 | 0.57 | 3.37 |
| Year 7 | 0.87 | 0.35 | 2.14 |
| Age (Continuous) | 0.99 | 0.96 | 1.03 |
| LN Radiation (Yes vs No) | 1.36 | 0.52 | 3.54 |
| ADT Use (No vs Yes) | 1.55 | 0.65 | 3.74 |
| EPIC Urinary Incontinence | |||
| Fractionation (HIMRT vs CIMRT) | 1.88 | 0.95 | 3.72 |
| Year 1 | 1.14 | 0.55 | 2.38 |
| Year 2 | 1.22 | 0.56 | 2.68 |
| Year 3 | 0.87 | 0.39 | 1.94 |
| Year 4 | 1.08 | 0.49 | 2.38 |
| Year 5 | 0.71 | 0.30 | 1.69 |
| Age (Continuous) | 0.97 | 0.92 | 1.01 |
| LN Radiation (Yes vs No) | 1.57 | 0.54 | 4.59 |
| ADT Use (No vs Yes) | 0.89 | 0.33 | 2.36 |
| EPIC Urinary Irritative/Obstructive | |||
| Fractionation (HIMRT vs CIMRT) | 0.45 | 0.12 | 1.69 |
| Year 1 | 1.02 | 0.32 | 3.30 |
| Year 2 | 0.73 | 0.22 | 2.40 |
| Year 3 | 0.73 | 0.22 | 2.47 |
| Year 4 | 1.14 | 0.34 | 3.88 |
| Year 5 | 1.20 | 0.33 | 4.32 |
| Age (Continuous) | 0.99 | 0.92 | 1.07 |
| LN Radiation (Yes vs No) | 1.59 | 0.21 | 11.90 |
| ADT Use (No vs Yes) | 0.19 | 0.03 | 1.37 |
| EPIC Sexual | |||
| Fractionation (HIMRT vs CIMRT) | 3.31 | 0.61 | 17.82 |
| Year 1 | 0.19 | 0.05 | 0.76 |
| Year 2 | 0.80 | 0.23 | 2.76 |
| Year 3 | 0.62 | 0.17 | 2.30 |
| Year 4 | 0.60 | 0.17 | 2.16 |
| Year 5 | 0.92 | 0.24 | 3.56 |
| Age (Continuous) | 0.10 | 0.01 | 0.73 |
| LN Radiation (Yes vs No) | 50.48 | 4.01 | 634.76 |
| ADT Use (No vs Yes) | 0.93 | 0.83 | 1.04 |
| EPIC Hormonal | |||
| Fractionation (HIMRT vs CIMRT) | 1.08 | 0.51 | 2.29 |
| Year 1 | 1.76 | 0.75 | 4.12 |
| Year 2 | 3.28 | 1.37 | 7.86 |
| Year 3 | 3.27 | 1.31 | 8.15 |
| Year 4 | 3.28 | 1.32 | 8.13 |
| Year 5 | 4.36 | 1.71 | 11.12 |
| Age (Continuous) | 0.44 | 0.17 | 1.16 |
| LN Radiation (Yes vs No) | 2.70 | 0.99 | 7.38 |
| ADT Use (No vs Yes) | 0.97 | 0.93 | 1.02 |
| EPIC Bowel | |||
| Fractionation (HIMRT vs CIMRT) | 0.78 | 0.25 | 2.41 |
| Year 1 | 0.41 | 0.15 | 1.10 |
| Year 2 | 0.52 | 0.19 | 1.44 |
| Year 3 | 1.83 | 0.71 | 4.77 |
| Year 4 | 1.29 | 0.48 | 3.45 |
| Year 5 | 1.19 | 0.42 | 3.38 |
| Age (Continuous) | 0.99 | 0.92 | 1.07 |
| LN Radiation (Yes vs No) | 0.56 | 0.09 | 3.33 |
| ADT Use (No vs Yes) | 2.47 | 0.47 | 12.91 |
| EQ5D VAS | |||
| Fractionation (HIMRT vs CIMRT) | 0.78 | 0.14 | 4.46 |
| Year 1 | 5.61 | 1.80 | 17.54 |
| Year 2 | 2.79 | 0.85 | 9.20 |
| Year 3 | 2.18 | 0.65 | 7.38 |
| Year 4 | 1.81 | 0.54 | 6.06 |
| Year 5 | 3.19 | 0.91 | 11.21 |
| Age (Continuous) | 0.98 | 0.88 | 1.10 |
| LN Radiation (Yes vs No) | 1.92 | 0.12 | 32.06 |
| ADT Use (No vs Yes) | 0.63 | 0.05 | 8.35 |
| EQ5D Index | |||
| Fractionation (HIMRT vs CIMRT) | 0.44 | 0.13 | 1.54 |
| Year 1 | 1.51 | 0.50 | 4.56 |
| Year 2 | 3.61 | 1.18 | 11.07 |
| Year 3 | 1.50 | 0.47 | 4.78 |
| Year 4 | 3.06 | 0.94 | 9.92 |
| Year 5 | 0.91 | 0.26 | 3.24 |
| Age (Continuous) | 0.91 | 0.84 | 0.98 |
| LN Radiation (Yes vs No) | 1.44 | 0.22 | 9.48 |
| ADT Use (No vs Yes) | 3.07 | 0.52 | 18.24 |
DISCUSSION
This is one of the first prospective randomized phase III trial presenting long-term QoL outcomes for patients undergoing conventional versus hypofractionated radiation. Due to the multiple treatment options for patients with prostate cancer, assessment of treatment impact on QoL is an important factor in decision-making. Furthermore, due to the growing body of literature demonstrating similar efficacy between fractionation schemes, QoL outcomes are becoming increasingly important to confirm the safety of hypofractionation. In the presented analysis, there was a trend towards worse urinary incontinence scores in the HIMRT group although there were no apparent differences between groups in terms of bowel, sexual, hormonal, or general health status. Furthermore, there was relatively minimal impact of treatment on QoL outcomes over time reflecting the advances in treatment technique in these patients. Our long-term results suggest that hypofractionated radiation can be delivered effectively and safely in a select group of patients with prostate cancer.
One of the concerns associated with hypofractionated radiotherapy is the potential for late treatment related toxicity due to a higher dose delivered per fraction. In our analysis, there was a trend towards worse urinary incontinence scores in the HIMRT group versus the CIMRT group. These findings may reflect an increased risk of late GU toxicity in these patients and should be an important consideration when deciding on treatment options for patients with localized prostate cancer. When examining other QoL domains, there was no association between treatment arm and outcomes. Our results are consistent with those presented in other analyses. Hoffman et al demonstrated similar long term quality of life outcomes in patients receiving conventionally (75.6 Gy in 1.8 Gy fractions) or hypofractionated intensity modulated radiotherapy (72 Gy in 2.4 Gy fractions) with no significant differences in patient reported bowel, urinary, or sexual symptoms. Aluwini et al reported 3 month patient reported outcomes for patients receiving standard radiation consisting of 78 in 2 Gy fractions or 64.6 Gy in 3.4 Gy fractions [18]. In their analysis, patients receiving hypofractionated radiation had higher rates of acute gastrointestinal toxicity versus standard fractionation. Wilkins et al reported 2 year QoL outcomes for patients receiving 74 Gy in 37 fractions, 60 Gy in 20 fractions, or 57 Gy in 19 fractions [19]. In their analysis there was no significant difference in QoL outcomes when comparing standard versus hypofractionated regimens. Similarly, Norkus et al reported no differences in mean EPIC scores between patients receiving conventionally fractionated (76 Gy in 38 fractions) or hypofractionated radiation (63 Gy in 20 fractions) [20]. Overall, clinical outcomes also appear to be similar in patients undergoing conventional versus hypofractionated radiotherapy [5, 6]. There are multiple ongoing phase III trials which will further clarify the role of hypofractionated radiation in the management of prostate cancer [21, 22].
Dose escalation is the standard of care for patients receiving definitive radiation therapy. Multiple randomized trials demonstrated a reduction in biochemical failure rates with long-term follow-up and provided an opportunity to examine QoL metrics as well [23–26]. Talcott et al presented long term QoL outcomes from the Proton Radiation Oncology Group 9509 trial which compared conventional to dose-escalated radiation for prostate cancer. Of 280 (83%) treated patients surveyed, there was no significant difference in patient reported outcomes between the two sub-groups in terms of urinary, bowel, or sexual function [27]. Patients in the standard-dose radiation arm reported more concern regarding their cancer control and regret about treatment choice likely reflecting their inferior treatment outcome. Similarly, Al-Mamgani et al found no significant difference in patient reported outcomes in patients treated on either of the treatment arms on the Dutch CKTO 96-10 dose escalation trial [28]. In addition to clinical outcomes, patient reported outcomes play an important role in selecting the optimal treatment modality in prostate cancer patients.
In order to verify the observations in our dataset, we used multivariable models to account for any potential confounders. There was no association between fractionation scheme and treatment outcomes across any QoL endpoint although there was a trend towards worse urinary incontinence quality of life scores in the HIMRT group. In addition, patients with lymph node irradiation appeared to have worse scores across multiple domains. In a previous analysis, pelvic nodal irradiation also predicted for higher rate of late grade ≥2 GU toxicity although this was not demonstrated in the present dataset [6]. The role of elective pelvic nodal irradiation in prostate cancer remains controversial with three prior randomized trials which showed no improvement in outcomes with pelvic irradiation [29–31]. The role of pelvic nodal irradiation, particularly in patients receiving hypofractionated radiotherapy needs further evaluation.
As with most studies presenting QoL data, a limitation is the decreasing number of patients completing patient surveys with each subsequent follow-up. As a result, meaningful long-term data on patients who may have undergone further therapy due to a recurrence is limited. Furthermore, as this study was not powered to detect meaningful differences in QoL outcomes, small differences between subgroups may not be accounted for. One of the strengths of our study is the large, randomized data set and comprehensive prospective QoL data collected for the presented patients. As a result, clinical and treatment characteristics were well balanced in both treatment arms. In addition, all patients were treated with contemporary IMRT using image guidance. Overall, the presented cohort is well translated into the current treatment techniques used by most practitioners.
CONCLUSIONS
Treatment related side effects and long-term toxicity play an important part in decision making due to the long life expectancy of patients diagnosed with localized prostate cancer. Although the body of literature on long-term QoL outcomes in these patients is growing, there is no long term prospective data on QoL outcomes following hypofractionated radiotherapy. In the above analysis, we demonstrate similar long-term QoL outcomes between patients undergoing HIMRT and CIMRT. In an era of health care reform, reducing treatment time and cost while maintaining efficacy is intriguing. Hypofractionated radiotherapy has been shown to be a reasonable treatment option in regard to oncologic outcomes and now appears to result in similar long term QoL outcomes in men with localized prostate adenocarcinoma.
Supplementary Material
Supplementary Figure 1: Mean Quality of Life Scores by Treatment Group. Solid horizontal line represents CIMRT group, dashed horizontal line represents HIMRT. Vertical lines represent standard deviation for each respective interval.
Supplementary Figure 2: Three-year Patient Reported Quality of Life Outcomes According to Baseline Status (no relevant problems, small to moderate problems, severe problems). Three-Year Outcomes Reported As: Gray-“No Problems”, Blue-“Small to Moderate Problems”, Orange-“Severe Problems”
Summary.
There is no long-term randomized prospective data comparing quality of life outcomes of patients treated with conventional versus hypofractionated radiotherapy. In this randomized phase III trial, patients receiving hypofractionated radiotherapy appeared to have similar long-term quality of life outcomes versus patients receiving conventionally fractionated radiation. These long-term results suggest that hypofractionated radiation can be delivered effectively and safely in a select group of patients with prostate cancer.
Acknowledgments
This publication was supported by National Cancer Institute Grants P30 CA006927 and CA101984-01, and a Florida Department of Health Biomed Bankhead Coley Grant 09BW11. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, National Institutes of Health or the Florida Department of Health. This publication was also supported in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Varian Medical Systems, Inc.
Footnotes
Conflicts of Interest: Daniel M. Geynisman – Research Funding from Pfizer, Advisory Board Participation at Prometheus, Pfizer, Novartis; Robert G. Uzzo – Honoraria from Johnson & Johnson, Consulting or Advisory Role at Myriad Genetics, Speakers’ Bureau at Janssen Oncology
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 5.Arcangeli G, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(1):11–8. doi: 10.1016/j.ijrobp.2009.07.1691. [DOI] [PubMed] [Google Scholar]
- 6.Pollack A, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860–8. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanda MG, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 8.Potosky AL, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 9.Pollack A, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64(2):518–26. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 12.Talcott JA, et al. Bringing prostate cancer quality of life research back to the bedside: translating numbers into a format that patients can understand. J Urol. 2006;176(4 Pt 1):1558–63. doi: 10.1016/j.juro.2006.06.067. discussion 1563–4. [DOI] [PubMed] [Google Scholar]
- 13.Barry MJ, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 14.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 16.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 17.Wei JT, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 18.Aluwini S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16(3):274–83. doi: 10.1016/S1470-2045(14)70482-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins A, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605–16. doi: 10.1016/S1470-2045(15)00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norkus D, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol. 2013;8:206. doi: 10.1186/1748-717X-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dearnaley D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13(1):43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 22.Aluwini S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16(3):274–83. doi: 10.1016/S1470-2045(14)70482-6. [DOI] [PubMed] [Google Scholar]
- 23.Zietman AL, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuban DA, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Peeters ST, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–6. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 26.Dearnaley DP, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 27.Talcott JA, et al. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA. 2010;303(11):1046–53. doi: 10.1001/jama.2010.287. [DOI] [PubMed] [Google Scholar]
- 28.Al-Mamgani A, et al. Dose escalation and quality of life in patients with localized prostate cancer treated with radiotherapy: long-term results of the Dutch randomized dose-escalation trial (CKTO 96-10 trial) Int J Radiat Oncol Biol Phys. 2011;79(4):1004–12. doi: 10.1016/j.ijrobp.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Asbell SO, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77-06. Int J Radiat Oncol Biol Phys. 1988;15(6):1307–16. doi: 10.1016/0360-3016(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 30.Roach M, 3rd, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21(10):1904–11. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Pommier P, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25(34):5366–73. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Mean Quality of Life Scores by Treatment Group. Solid horizontal line represents CIMRT group, dashed horizontal line represents HIMRT. Vertical lines represent standard deviation for each respective interval.
Supplementary Figure 2: Three-year Patient Reported Quality of Life Outcomes According to Baseline Status (no relevant problems, small to moderate problems, severe problems). Three-Year Outcomes Reported As: Gray-“No Problems”, Blue-“Small to Moderate Problems”, Orange-“Severe Problems”