The allostatic model posits that chronic drug use induces hedonic homeostatic dysregulation, in which motivation to obtain natural rewards (e.g., eating, copulation, affiliation) is re-organized around seeking drug-induced reward to alleviate dysphoria [1]. The downward shift in salience of natural reward relative to drug reward may represent a crucial tipping point leading to the loss of control over drug use that is characteristic of addiction. Heightened responsiveness to drug reward coupled with decreased responsiveness to natural reward has been observed opioid-dependent individuals [2,3], predicts opioid consumption [4], and may drive prescription opioid misuse and addiction [5]. Therapies that restructure reward responsiveness from valuation of drug reward to valuation of natural reward may be an effective means of treating opioid misuse.
We conducted a RCT of a Mindfulness-Oriented Recovery Enhancement (MORE) intervention for opioid misuse among chronic pain patients, which integrates skills to amplify natural reward processing with mindfulness and reappraisal techniques. In this RCT, relative to a support group (SG) control, MORE reduced opioid misuse and craving while decreasing pain symptoms [6]. Given its focus on orienting attention away from drug-related cues and towards healthful and socially affiliative objects and events, it is possible that MORE may regulate attention to hedonically-relevant stimuli to shift the relative salience of drug and natural rewards, and thereby ameliorate opioid misuse.
To test this exploratory hypothesis, we examined unpublished psychophysiological data from this trial (ClinicalTrials.gov identifier NCT01505101) [6]. Individuals with complete data (17 men and 34 women, mean age = 45.7, SD = 13.7, MORE n = 20; SG n = 31) from an affective picture viewing paradigm conducted one week before and after the study treatments were selected for the present analysis. Chronic pain patients were recruited from primary and specialty care clinics, and met inclusion criteria if they took opioids nearly every day for >90 days. Most (84.3%) reported opioid misuse as defined by a validated cut-point on the Current Opioid Misuse Measure (COMM >12; [7]). We used heart rate (HR) to measure cue-responsiveness, and high-frequency heart rate variability (HRV), to index parasympathetic regulation of hedonic responses, including attention to emotional information [8]. Participants were paid $200 for completing the IRB-approved study; all procedures complied with the Helsinki Declaration.
The manualized 8-session MORE treatment involved group training in mindfulness to disengage attention from drug cues and intentionally re-orient attention to visual, auditory, olfactory, gustatory, or tactile features of a pleasant experience (e.g., the warmth of the sun on one’s skin) while cultivating metacognitive awareness of positive emotions and cognitions arising in response to the pleasant event [9]. The two-hour sessions were led by a social worker, supervised weekly by the developer of MORE. Participants engaged in daily 15-minute mindfulness sessions at home guided by a CD. The manualized SG control condition consisted of 8 weekly, 2-hour support group sessions, in which a social worker facilitated emotional expression and discussion of chronic pain-related topics. MORE and SG session recordings were reviewed to maintain treatment fidelity.
Participants performed a randomized, event-related affective picture viewing task [10] in which they passively viewed neutral (furniture, neutral faces), pain-related (injuries, medical procedures), natural reward (social attachment, nature scenes), and opioid-related cues (pills, pill bottles). After a 500 ms fixation cross, pictures were presented for 6 seconds while HR was recorded, after which stimulus valence and arousal was rated on 9-point Likert scales. HR was recorded at 1000 Hz on a Biopac MP150. Kubios 2.0 computed time domain HRV analyses, yielding the root mean square of successive differences (RMSSD) to estimate parasympathetically-mediated HRV. Cue-elicited HRV responsivity scores were generated by covarying HRV during a 5-minute baseline from HRV during affective picture viewing. Cue-elicited HR was averaged for each cue type.
RM-ANOVA revealed a significant group (MORE vs SG) X time (pre vs post-treatment) effect on HRV responsivity, F(1,49) = 4.42, P = .04, η2partial = .08, indicating that compared to the SG, the MORE group experienced significantly greater increases in HRV responsivity during affective picture viewing. RM-ANOVA was conducted on cue-elicited HR with group, time, and cue type (opioid, natural reward, pain, and neutral) as factors. We identified a significant group X time X condition effect, F(3,47) = 4.54, P = .007, η2partial = .09, indicating that compared to the SG, the MORE group experienced significantly greater decreases in cue-elicited HR – this effect was most pronounced for drug cue-elicited HR relative to natural reward cue-elicited HR, F(1,49) = 7.59, P = .004, η2partial = .13.
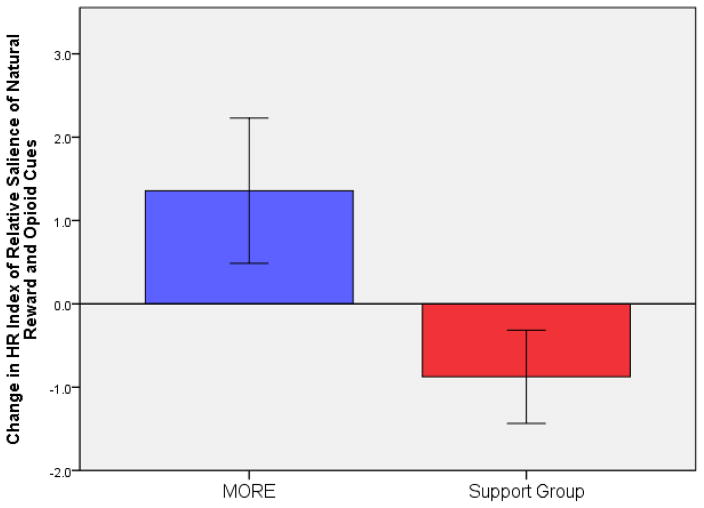
We computed a measure of relative responsiveness to natural reward cues compared to drug cues by extracting residuals generated from regression analyses in which drug cue-elicited HR was regressed on natural reward cue-elicited HR. RM-ANOVA revealed a significant group X time effect on this relative responsiveness measure, F(1,49) = 7.61, P = .008, η2partial = .13, indicating that compared to the SG, the MORE group experienced significantly greater increases in responsiveness to natural reward cues relative to drug cues from pre- to post-treatment (see Figure 1). Controlling for pre-treatment opioid misuse severity, residualized change in relative responsiveness significantly predicted COMM opioid misuse scores at 3-month follow-up, B = −.32, P <.05 (model R2=.32). Compared to the SG, MORE was associated with significantly higher post-treatment arousal ratings of natural reward cues, F(1,49) = 9.30, P = .004, η2partial = .21.
Figure 1.
Changes in relative responsiveness to natural reward and opioid cues from pre- to post-intervention (N=51). Positive scores indicate increased natural reward cue-elicited HR relative to opioid cue elicited HR. Negative scores indicate decreased natural reward cue-elicited HR relative to opioid cue-elicited HR. The Group X Time effect was significant, F(1,49) = 7.61, P = .008, η2partial = .13.
These preliminary findings tentatively suggest that MORE may enhance autonomic regulation of perturbations by hedonic stimuli, and in so doing, modulate the relative salience of natural and drug-related cues to reduce opioid misuse. MORE is a sequenced treatment designed to modify associative learning mechanisms by strengthening top-down cognitive control to restructure bottom-up reward learning from valuation of drug reward to valuation of natural reward. It is possible that this restructuring of reward may arise from restoration of prefrontal-striatal feedback [9], but more well-controlled mechanistic research is needed to test this neural hypothesis. Insofar as addiction involves a downward shift in salience of natural reward relative to drug reward, interventions that reverse this allostatic process by restructuring reward learning may prove to be highly efficacious.
Acknowledgments
This work was supported by grant numbers R03DA032517 and R01DA042033 from the National Institutes of Health awarded to E.L.G.; and a grant from the Fahs Beck Fund for Research and Experimentation, also awarded to E.L.G. The conclusions in this article are those of the authors and do not necessarily represent the official position of the NIH. The authors have no conflict of interest to report with regard to the contents of this manuscript. E.L.G. has conducted MORE trainings for which he received monetary incentives.
Contributor Information
Eric L. Garland, University of Utah
Matthew O. Howard, University of North Carolina at Chapel Hill
Jon-Kar Zubieta, University of Utah.
Brett Froeliger, Medical University of South Carolina.
References
- 1.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 2.Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. J Psychopharmacol. 2008;22:836–42. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- 3.Garland EL, Froeliger B, Howard MO. Allostatic dysregulation of natural reward processing in prescription opioid misuse: Autonomic and attentional evidence. Biol Psychol. 2015 Feb;105:124–129. doi: 10.1016/j.biopsycho.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–12. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- 5.Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013;37:2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448–459. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Garland EL. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.13034. [cited 2016 Apr 14];Available from: http://onlinelibrary.wiley.com/doi/10.1111/nyas.13034/full. [DOI] [PMC free article] [PubMed]
- 10.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]