Abstract
Objective
People with schizophrenia are at greater risk for cardiovascular disease and their overallmortality rate is elevated compared to the general population. The metabolic side effects of antipsychotic medications have been widely studied; however, the effect of adding conventional mood stabilizers, such as lithium and valproate, to antipsychotic medication has not been assessed in terms of metabolic risk. The primary purpose of this secondary analysis was to examine whether treatment with lithium or valproate in addition to a second-generation antipsychotic is associated with poorer metabolic outcomes than treatment with a second-generation antipsychotic without lithium or depakote.
Methods
Baseline data from 3 studies, which included measurement of body mass index, waist circumference, fasting glucose, insulin, homeostatic model assessment of insulin resistance, insulin sensitivity index, glucose utilization, and acute insulin response to glucose, were included in the analysis.
Results
No differences were found between those taking lithium or valproate and those who were not in terms of fasting glucose, fasting insulin, and homeostatic model assessment of insulin resistance. Insulin sensitivity was lower among participants taking lithium or valproate. Participants taking lithium or valproate had a higher body mass index than those not taking conventional mood stabilizers, although the difference did not reach statistical significance.
Conclusions
These cross-sectional findings suggest it may be beneficial to monitor insulin sensitivity and body mass index in patients taking lithium or valproate in combination with a second-generation antipsychotic.
Keywords: conventional mood stabilizers, lithium, valproate, second-generation antipsychotics, schizophrenia, insulin sensitivity, body mass index, metabolic side effects
The overall mortality rate for people with schizophrenia is elevated compared to the rate in the general population.1,2 People with schizophrenia and other severe mental illnesses are at greater risk for cardiovascular disease, including myocardial infarction, obesity, hypertension, and dyslipidemia.3–5 Although some patients may present with intrinsic metabolic abnormalities, some second-generation antipsychotics are associated with an increased risk of obesity, impaired glucose tolerance and new-onset diabetes, hyperlipidemia, and cardiovascular disease.6
Although antipsychotic medications are the most common treatment for schizophrenia or schizo-affective disorder, conventional mood stabilizers are widely used in conjunction with a second-generation antipsychotic to assist with the residual mood symptoms that often accompany the positive and negative symptoms of schizophrenia.7 Despite the effectiveness of conventional mood stabilizers in reducing mood symptoms, patients treated with mood-stabilizing drugs may experience a wide range of side effects, including neurological, gastrointestinal, metabolic, thyroid, dermatological, nephrogenic, cognitive, sexual, hematological, hepatogenic, and or teratogenic effects.8 Lithium monotherapy increases the risk for weight gain and hypothyroidism.9–12 Valproate has been found to increase the risk for weight gain, hyperlipidemia, and insulin and glucose abnormalities.13–15 Among these side effects, weight gain is especially concerning, because long-term monotherapy with valproate can lead to several metabolic disturbances that may also play a role in the development of metabolic syndrome.16 In addition to increasing the risk for cardiovascular mortality, weight gain may also have broad negative effects on functioning and quality of life in patients with severe mental illnesses.17
Research has examined the metabolic side effects of combining conventional mood stabilizers, such as lithium or valproate, with second-generation antipsychotics with mixed and conflicting results.18–27 Interpretation of these findings has been limited by small sample sizes and a focus on 1 specific antipsychotic medication in each study. Given the inconsistency of currently available research findings, the metabolic side effects that may occur when combining lithium or valproate with second-generation antipsychotics remain unclear.
The study described here sought to examine the association of metabolic effects and lithium or valproate adjunctive therapy. Specifically, we pooled baseline data from 3 previous studies to create a large sample of patients with schizophrenia and schizoaffective disorder who were treated with a variety of second-generation antipsychotics.28–30 Our goal was to capture the effects of pharmacological combinations in the broader clinical landscape of patients with schizophrenia and schizoaffective disorder by including patients being treated with a variety of antipsychotic medications associated with both high-metabolic and low-metabolic risk (clozapine and olanzapine vs. quetiapine and risperidone, respectively).
METHODS
Source of Data
This study involved a secondary analysis of data from 3 studies conducted between 1999 and 2008 at an urban outpatient schizophrenia treatment clinic at the Erich Lindemann Mental Health Center in Boston, MA. The sample consisted of baseline data from 112 male and female participants enrolled in at least 1 of these 3 medication trials. The 3 studies were an insulin secretion study,28 a rosiglitazone study,29 and an aripiprazole study,30 each of which was approved by the Massachusetts General Hospital Institutional Review Board. The insulin secretion study examined the relationship between anthropometric measures such as waist circumference and insulin resistance in patients taking clozapine or olanzapine.28 The rosiglitazone study was a controlled trial that examined the effects of rosiglitazone on glucose utilization and insulin sensitivity in patients treated with clozapine.29 The aripiprazole study examined the effect of aripiprazole as adjunctive therapy to clozapine on metabolic factors such as weight, lipid levels, and glucose metabolism. Readers are referred to the original publications describing these studies for the specific procedures that were used.28–30
The aggregate data for this analysis included results of the intravenous glucose tolerance test and fasting laboratory measures taken at the baseline visit of each of the 3 studies before any study medication was dispensed. Diagnoses were confirmed by a research psychiatrist using the Structured Clinical Interview for DSM-IV.31 Medications being taken and demographic data were determined by interview and a review of medical records. Fasting glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), insulin sensitivity index, glucose utilization, 32 and acute insulin response to glucose were measured using standard blood analysis. Intravenous glucose tolerance test data were collected at the Mallinckrodt General Clinical Research Center at Massachusetts General Hospital in Boston, MA.
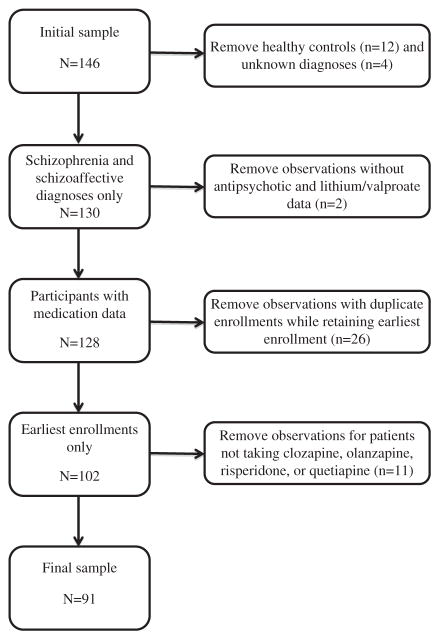
Many of the 112 participants were enrolled in >1 study, resulting in 146 observations. For this analysis, we included only patients with a diagnosis of schizophrenia or schizoaffective disorder and only those who were taking clozapine, olanzapine, risperidone, or quetiapine. For participants who had been enrolled in >1 study, we also included only the earliest study enrollment for each participant. Thus, the analysis involved data from 91 patients (81.3% of the 112 participants, Fig. 1). After controlling for these factors, we included 1 participant from the insulin secretion study taking a mood stabilizer and 52 participants not taking a mood stabilizer (Table 1). The aripiprazole study included 12 participants taking a mood stabilizer and 12 participants not taking a mood stabilizer. The rosiglitazone study included 6 participants taking a mood stabilizer and 8 participants not taking a mood stabilizer.
FIGURE 1.
Flow chart of participant inclusion/exclusion.
TABLE 1.
Demographic and Clinical Characteristics of the Sample by Lithium/Valproate Status
| n (%) | t/χ2 | P | ||
|---|---|---|---|---|
|
| ||||
| No Lithium/Valproate (N=72) | Lithium/Valproate (N=19) | |||
| Age (Mean±SD) | 43.39±10.60 | 42.39±8.49 | 0.37 | 0.713 |
| Sex: male | 55 (76.4) | 13 (68.4) | 0.51 | 0.477 |
| Race: white | 56 (77.8) | 15 (79.0) | 0.01 | 0.913 |
| Diagnosis: schizophrenia | 58 (80.6) | 11 (57.9) | 4.21 | 0.040 |
| Study | ||||
| Insulin secretion | 52 (72.2) | 1 (5.3) | 27.71 | 0.000 |
| Aripiprazole | 12 (16.7) | 12 (63.2) | 16.73 | 0.000 |
| Rosiglitazone | 8 (11.1) | 6 (31.6) | 4.84 | 0.028 |
| Medication* | ||||
| Olanzapine, clozapine | 52 (72.2) | 19 (100.0) | 6.76 | 0.009 |
| Risperidone, quetiapine | 23 (31.9) | 4 (21.1) | 0.85 | 0.355 |
Seven participants were prescribed >1 type of antipsychotic, so that the proportion does not sum to 100%.
Statistical Analysis
We divided the sample into 2 groups: participants who were taking lithium and/or valproate in addition to an antipsychotic compared with those who were taking only 1 or more antipsychotics. We compared baseline demographic and clinical characteristics between these 2 groups using independent samples t tests and χ2 analyses. We then stratified the entire sample by study (insulin secretion, aripiprazole, or rosiglitazone) and compared these groups in terms of the same demographic and clinical variables using analyses of variance and χ2 tests to see if there were any systematic differences between the study samples. Post hoc logistic regression models were constructed to further characterize significant between-group differences for dichotomous outcomes.
To test the primary hypotheses, we fit linear mixed-effects regression models using Proc Mixed that evaluated the association of adjuvant lithium or valproate use with the following metabolic variables: fasting glucose, fasting insulin, HOMA-IR, glucose effectiveness, insulin sensitivity, acute insulin response to glucose, waist circumference, and body mass index. Most dependent variables were positively skewed and thus required a log transformation (fasting insulin, HOMA-IR, insulin sensitivity), a negative reciprocal transformation (fasting glucose), or a square root transformation (acute insulin response to glucose). Glucose effectiveness, waist circumference, and body mass index were normally distributed and did not necessitate any transformations. To account for within-study correlations, we fit a random intercept and slope for each study. We also controlled for the originating study as a covariate in the models with the insulin secretion study as the reference group. The insulin secretion study was chosen as the reference group because it had the largest sample size of the 3 studies. On the basis of previous research documenting the effects of antipsychotics on metabolic outcomes,33–38 we created a dichotomous variable for antipsychotic metabolic profile risk in terms of metabolic side effects, which indicated whether participants were prescribed a high-metabolic risk antipsychotic (clozapine, olanzapine) and controlled for this in the models. The coefficient of interest in these mixed-effects models was the one comparing metabolic outcomes for patients receiving adjuvant lithium or valproate with those who did not receive either medication; this coefficient represents a within-study difference in means. All analyses were conducted using SAS Version 9.2, SAS Institute Inc., Cary, NC.
RESULTS
There were no demographic differences (in age, sex, race) between the groups taking lithium/valproate and those not taking a conventional mood stabilizer at baseline (Table 1). Participants who were not taking lithium or valproate were more likely to have a diagnosis of schizophrenia (80.6%) compared with those who were taking lithium or valproate (57.9%; χ2=4.21, P=0.040). With regard to differences among the 3 studies, participants enrolled in the aripiprazole and rosiglitazone studies were more likely to be taking lithium/valproate (P<0.05). In contrast, participants enrolled in the insulin secretion study were less likely to be taking lithium or valproate (P=0.000). Participants taking lithium or valproate were also more likely to be prescribed a high-metabolic risk antipsychotic (clozapine, olanzapine; χ2=6.76, P=0.009). No significant differences between groups were found in the proportion of the sample that was prescribed a lower metabolic risk antipsychotic (risperidone or quetiapine).
Similar to the findings shown in Table 1, no significant differences in demographic characteristics were found among participants in the 3 studies. With respect to clinical differences, the prevalence of schizophrenia was significantly higher in the insulin secretion study (84.9%) compared with the aripiprazole study (58.3%; χ2=6.54, P=0.038). In addition, significantly more patients were prescribed high-metabolic risk antipsychotics in the aripiprazole and rosiglitazone studies (100%) than in the insulin secretion study (62.3%; χ2=18.38, P=0.000). There were no significant differences among the 3 studies in the proportion of the samples prescribed a lower metabolic risk antipsychotic (risperidone, quetiapine) (P>0.05). Seven participants (7.7%) were prescribed both a low-risk and a high-risk antipsychotic; therefore, the medication proportions shown in Table 1 do not sum to 100%. In these cases, antipsychotic usage was coded based on the higher metabolic risk.
Table 2 displays the mixed-effects model regression results for the association of lithium or valproate use with metabolic laboratory outcomes, controlling for study enrollment and antipsychotic metabolic risk profile. Fasting glucose, fasting insulin, and HOMA-IR were not related to lithium or valproate use, antipsychotic metabolic risk profile, or the specific study in any of these models. However, the participants taking lithium or valproate had 47% lower insulin sensitivity compared with participants who were not taking lithium or valproate (t=−2.02, P=0.047). Participants taking lithium or valproate also had higher body mass index levels of approximately 3.28 units compared with those not taking lithium or valproate; however, this relationship did not reach statistical significance (t=1.85, P=0.068).
TABLE 2.
The Relationship Between Use of Valproate/Lithium and Clinical Metabolic Variables
| β | SE | t | P | |
|---|---|---|---|---|
| Fasting glucose† | ||||
| Aripiprazole | 1.359 | 1.649 | 0.82 | 0.412 |
| Rosiglitazone | 0.220 | 1.658 | 0.13 | 0.895 |
| Antipsychotics§ | 0.437 | 0.433 | 1.01 | 0.315 |
| Lithium/valproate | −0.744 | 0.515 | −1.44 | 0.153 |
| Fasting insulin* | ||||
| Aripiprazole | 0.293 | 0.898 | 0.33 | 0.745 |
| Rosiglitazone | −0.331 | 0.903 | −0.37 | 0.715 |
| Antipsychotics§ | 0.390 | 0.236 | 1.65 | 0.102 |
| Lithium/valproate | 0.257 | 0.280 | 0.92 | 0.363 |
| HOMA-IR* | ||||
| Aripiprazole | 0.431 | 0.313 | 1.38 | 0.171 |
| Rosiglitazone | −0.308 | 0.329 | 0.94 | 0.353 |
| Antipsychotics§ | 0.409 | 0.263 | 1.55 | 0.124 |
| Lithium/valproate | 0.165 | 0.308 | 0.54 | 0.594 |
| Insulin sensitivity* | ||||
| Aripiprazole | 0.177 | 0.244 | 0.73 | 0.471 |
| Rosiglitazone | −0.117 | 0.239 | −0.49 | 0.627 |
| Antipsychotics§ | −0.582 | 0.187 | −3.12 | 0.003 |
| Lithium/valproate | −0.474 | 0.235 | −2.02 | 0.047 |
| Glucose effectiveness | ||||
| Aripiprazole | −0.001 | 0.002 | −0.44 | 0.662 |
| Rosiglitazone | 0.001 | 0.002 | 0.44 | 0.661 |
| Antipsychotics§ | −0.005 | 0.002 | −2.68 | 0.009 |
| Lithium/valproate | −0.002 | 0.002 | −1.02 | 0.310 |
| Acute insulin response to glucose‡ | ||||
| Aripiprazole | −10.445 | 3.043 | −3.43 | 0.001 |
| Rosiglitazone | −8.006 | 3.118 | −2.57 | 0.012 |
| Antipsychotics§ | 4.407 | 2.294 | 1.92 | 0.059 |
| Lithium/valproate | −1.126 | 3.013 | −0.37 | 0.710 |
| Waist circumference | ||||
| Aripiprazole | 14.285 | 4.302 | 3.32 | 0.001 |
| Rosiglitazone | 6.509 | 4.716 | 1.38 | 0.171 |
| Antipsychotics§ | 5.272 | 3.544 | 1.49 | 0.141 |
| Lithium/valproate | 6.137 | 4.287 | 1.43 | 0.156 |
| Body mass index | ||||
| Aripiprazole | 5.079 | 1.815 | 2.80 | 0.006 |
| Rosiglitazone | 3.640 | 1.902 | 1.91 | 0.059 |
| Antipsychotics§ | 2.008 | 1.508 | 1.33 | 0.187 |
| Lithium/valproate | 3.280 | 1.773 | 1.85 | 0.068 |
Values shown in bold were statistically signifucant.
Log transformations: fasting insulin, HOMA-IR, insulin sensitivity.
Negative reciprocal transformation: fasting glucose.
Square root transformation: acute insulin response to glucose.
High severity metabolic risk antipsychotics: clozapine and olanzapine.
HOMA-IR indicates homeostatic model assessment of insulin resistance.
The metabolic risk profile of the antipsychotic agents the participants were taking was also related to metabolic health outcomes. In this sample, participants on high-risk metabolic profile antipsychotics (clozapine, olanzapine) had 58% lower insulin sensitivity (t=−3.12, P=0.003) and reduced glucose effectiveness (t=−2.68, P=0.009). Although not reaching statistical significance, it appeared that participants on higher metabolic risk antipsychotics also had greater acute insulin response to glucose compared with individuals on antipsychotics with a lower metabolic risk (t=1.92, P=0.059).
Some metabolic outcomes also differed depending on the study in which the individual participated, which may be explained by the inclusion/exclusion criteria specific to the study. Compared with participants in the insulin secretion study, participants in the aripiprazole and rosiglitazone studies had significantly lower acute insulin response to glucose (P<0.05). Participants in the aripiprazole study on average also had a significantly greater waist circumference and body mass index compared with participants in the insulin secretion study (P<0.05).
DISCUSSION
This cross-sectional study explored the impact of adjunctive lithium or valproate therapy on metabolic risk factors in individuals with schizophrenia. Key findings were that treatment with lithium or valproate plus a second-generation antipsychotic compared with treatment with such an antipsychotic without lithium or valproate was associated with (1) lower insulin sensitivity and (2) higher body mass index. Patients taking lithium or valproate had 47% lower insulin sensitivity than those not taking a conventional mood stabilizer irrespective of the metabolic risk profile associated with the antipsychotic they were receiving. Although the glucose effectiveness variable was significantly decreased in subjects taking high-metabolic risk antipsychotics (clozapine and olanzapine), we did not find any statistical difference in glucose effectiveness in subjects taking lithium or valproate as adjunctive therapy to second-generation antipsychotics. HOMA-IR, which is a computation based on fasting insulin and glucose values, represents the most common surrogate measure of insulin resistance.39 It has a moderate correlation with insulin sensitivity, which is likely why it was not statistically significant in our study. We also found that participants taking adjunctive lithium or valproate had higher average body mass index of approximately 3.28 units (kg/m2) compared with those not taking lithium or valproate, although this relationship did not reach statistical significance. Increased appetite secondary to improved mood, lower metabolic rate, hypothyroidism, and ingestion of high sugar drinks secondary to polydipsia are all related to weight gain.40 Although we did adjust our results on the basis of whether participants were receiving antipsychotics associated with a higher metabolic risk, other potential confounding factors (eg, lifestyle and behavioral differences) were not measured. It is also possible that the observed metabolic changes were influenced by the metabolic side effects of the primary antipsychotic medications.
Differences in lithium/valproate use and metabolic outcomes were found between the 3 studies that were examined. This supports our decision to avoid introducing confounds by focusing on within-study associations, although this decision may have sacrificed statistical power for improved internal validity. Although pooling results across studies does improve statistical precision, the non-significant results reported here should be interpreted cautiously as the effective sample size was still quite small and larger studies are needed to confirm these findings.
Some metabolic outcomes also differed on the basis of the study in which the individual participated, which may be explained by differences in the inclusion/exclusion criteria used in the different studies. Compared with the insulin secretion study, participants in the aripiprazole and rosiglitazone studies had significantly lower acute insulin response to glucose (P<0.05). Participants in the aripiprazole study also had significantly greater waist circumference and body mass index on an average compared with participants in the insulin secretion study (P<0.05). These findings may reflect the higher proportion of patients in those studies who were taking high-metabolic profile risk antipsychotics (all patients in the aripiprazole and rosiglitazone studies were taking clozapine). In addition, the insulin secretion study included fewer participants taking lithium or valproate compared with the other 2 studies, thereby providing only a small sample of participants who were taking lithium or valproate in addition to an antipsychotic with a low-metabolic risk profile. This small number of patients in the lithium/valproate group who were taking antipsychotics with a low-metabolic risk profile may have contributed to the observed differences. Our findings are consistent with previous research that has found that clozapine is associated with a variety of adverse metabolic effects, including weight gain.38
Side effects are well known to be among the most frequent causes of increased rates of nonadherence to treatment by preventing patients from taking their medications regularly. Our findings suggest that clinicians should carefully evaluate the need for lithium or valproate in patients who are already being treated with an antipsychotic medication. They also suggest the importance of regular monitoring of glucose metabolism and body mass index in patients taking lithium or valproate in combination with a second-generation antipsychotic. Switching to another conventional mood stabilizer such as carbamazepine or lamotrigine might also be considered if clinically appropriate. It may also be beneficial to provide dietary counseling and encourage patients to start an exercise program before initiating treatment with a conventional mood stabilizer, especially for patients who are already overweight or have metabolic abnormalities. A recent meta-analysis of studies that included a nutritional element, physical activity, and/or a psychological intervention aimed at weight loss or preventing weight gain found that lifestyle interventions can be effective in treating and preventing obesity and reducing cardiometabolic risk factors in patients with psychotic disorders,41 so that such interventions are recommended for all at-risk individuals. Clinicians should also consider the use of pharmacological interventions (metformin, top-iramate) for weight gain related to treatment with antipsychotics or mood stabilizers as non-pharmacologic interventions may be too challenging and difficult to implement and also because combining the 2 strategies may offer additive benefits.42 In addition, choice of the most appropriate conventional mood stabilizer and use of the lowest possible dosage for such patients should be carefully considered.
Future research in this area should involve larger samples, longitudinal designs, follow-up beginning at the initiation of lithium/valproate adjuvant therapy, collection of data concerning measures of lifestyle and health behaviors related to metabolic outcomes and adjustment of results to take into account such measures, and exploration of whether any potential effect of lithium/valproate therapy is influenced by the type of antipsychotic therapy received.
CONCLUSIONS
The results of this study suggest that the combination of lithium or valproate with antipsychotic medications may negatively affect insulin sensitivity and body weight. This risk should be taken into account in deciding on the best treatment for patients with both psychotic and mood symptoms. The results of this study also suggest that it may be beneficial to monitor for changes in insulin sensitivity, glucose tolerance, and body mass index in patients taking lithium or valproate adjunctive to second-generation antipsychotics, particularly clozapine and olanzapine.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
BRENDA VINCENZI, Schizophrenia Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, Boston, MA.
CLAIRE M. GREENE, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
MELISSA ULLOA, Schizophrenia Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, Boston, MA.
LINDSEY PARNAROUSKIS, Schizophrenia Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, Boston, MA.
JOHN W. JACKSON, Schizophrenia Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, and Department of Epidemiology, Harvard School of Public Health, Boston, MA.
DAVID C. HENDERSON, Schizophrenia Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, and Harvard Medical School, Boston, MA.
References
- 1.Joukamaa M, Heliövaara M, Knekt P, et al. Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry. 2006;188:122–127. doi: 10.1192/bjp.188.2.122. [DOI] [PubMed] [Google Scholar]
- 2.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–194. doi: 10.4088/jcp.v66n0205. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Hägg S, Lindblom Y, Mjörndal T, et al. High prevalence of the metabolic syndrome among a Swedish cohort of patients with schizophrenia. Int Clin Psychopharmacol. 2006;21:93–98. doi: 10.1097/01.yic.0000188215.84784.17. [DOI] [PubMed] [Google Scholar]
- 6.Henderson D, Vincenzi B, Andrea NV, et al. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–464. doi: 10.1016/S2215-0366(15)00115-7. [DOI] [PubMed] [Google Scholar]
- 7.Centorrino F, Sani G, Fogarty KV, et al. Combinations of mood-stabilizers with antipsychotics as treatment strategies in hospitalized psychiatric patients. Clin Neuropsychiatry. 2006;3:322–326. [Google Scholar]
- 8.Dols A, Sienaert P, Van Gerven H, et al. The prevalence and management of side effects of lithium and anticonvulsants as mood stabilizers in bipolar disorder from a clinical perspective: a review. Int Clin Psychopharmacol. 2013;28:287–296. doi: 10.1097/YIC.0b013e32836435e2. [DOI] [PubMed] [Google Scholar]
- 9.Haden ST, Stoll AL, McCormick S, et al. Alterations in parathyroid dynamics in lithium-treated subjects. J Clin Endocrinol Metab. 1997;82:2844–2848. doi: 10.1210/jcem.82.9.4218. [DOI] [PubMed] [Google Scholar]
- 10.Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27:104–107. [PMC free article] [PubMed] [Google Scholar]
- 11.Peselow ED, Dunner DL, Fieve RR, et al. Lithium carbonate and weight gain. J Affect Disord. 1980;2:303–310. doi: 10.1016/0165-0327(80)90031-2. [DOI] [PubMed] [Google Scholar]
- 12.Vendsborg PB, Bech P, Rafaelsen OJ. Lithium treatment and weight gain. Acta Psychiatr Scand. 1976;53:139–147. doi: 10.1111/j.1600-0447.1976.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang HH, Yang YK, Gean PW, et al. The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord. 2010;124:319–323. doi: 10.1016/j.jad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.El-Khatib F, Rauchenzauner M, Lechleitner M, et al. Valproate, weight gain and carbohydrate craving: a gender study. Seizure. 2007;16:226–232. doi: 10.1016/j.seizure.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Chen S, Tong N, et al. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure. 2012;21:578–582. doi: 10.1016/j.seizure.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Belcastro V, D’Egidio C, Striano P, et al. Metabolic and endocrine effects of valproic acid chronic treatment. Epilepsy Res. 2013;107:1–8. doi: 10.1016/j.eplepsyres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Cameron AJ, Magliano DJ, Dunstan DW, et al. A bi-directional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes (Lond) 2012;36:295–303. doi: 10.1038/ijo.2011.103. [DOI] [PubMed] [Google Scholar]
- 18.Bender S, Linka T, Wolstein J, et al. Safety and efficacy of combined clozapine-lithium pharmacotherapy. Int J Neuropsychopharmacol. 2004;7:59–63. doi: 10.1017/S1461145703003870. [DOI] [PubMed] [Google Scholar]
- 19.Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34:1330–1338. doi: 10.1038/npp.2008.209. [DOI] [PubMed] [Google Scholar]
- 20.Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28:182–192. doi: 10.1038/sj.npp.1300023. [DOI] [PubMed] [Google Scholar]
- 21.Citrome L, Shope CB, Nolan KA, et al. Risperidone alone versus risperidone plus valproate in the treatment of patients with schizophrenia and hostility. Int Clin Psychopharmacol. 2007;22:356–362. doi: 10.1097/YIC.0b013e3281c61baf. [DOI] [PubMed] [Google Scholar]
- 22.Kelly DL, Conley RR, Feldman S, et al. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatr Q. 2006;77:81–95. doi: 10.1007/s11126-006-7963-9. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JM. A retrospective comparison of weight, lipid and glucose changes between risperidone and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63:425–433. doi: 10.4088/jcp.v63n0509. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura M, Nagamine T. Metabolic effects of sodium valproate on atypical antipsychotics in Japanese psychotic patients. Clin Neuropsychopharmacol Ther. 2013;4:13–19. [Google Scholar]
- 25.Peterson GA, Byrd SL. Diabetic ketoacidosis from clozapine and lithium cotreatment. Am J Psychiatry. 1996;153:737–738. doi: 10.1176/ajp.153.5.737b. [DOI] [PubMed] [Google Scholar]
- 26.Small JG, Klapper MH, Malloy FW, et al. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. 2003;23:223–228. doi: 10.1097/01.jcp.0000084026.22282.5f. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Uchida H, Takeuchi H, et al. Augmentation of atypical antipsychotics with valproic acid: an open-label study for most difficult patients with schizophrenia. Hum Psychopharmacol. 2009;24:628–638. doi: 10.1002/hup.1073. [DOI] [PubMed] [Google Scholar]
- 28.Henderson DC, Fan X, Sharma B, et al. Waist circumference is the best anthropometric predictor for insulin resistance in nondiabetic patients with schizophrenia treated with clozapine but not olanzapine. J Psychiatr Pract. 2009;15:251–261. doi: 10.1097/01.pra.0000358312.99233.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson D, Fan X, Sharma B, et al. A double-blind, placebo-controlled trial of rosiglitazone for clozapine-induced glucose metabolism impairment in patients with schizophrenia. Acta Psychiatr Scand. 2009;119:457–465. doi: 10.1111/j.1600-0447.2008.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan X, Borba C, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127:217–226. doi: 10.1111/acps.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Arlington, VA: American Psychiatric Publishing; 2012. [Google Scholar]
- 32.Kenna HA, Jiang B, Rasgon NL. Reproductive and metabolic abnormalities associated with bipolar disorder and its treatment. Harv Rev Psychiatry. 2009;17:138–146. doi: 10.1080/10673220902899722. [DOI] [PubMed] [Google Scholar]
- 33.Newcomer JW, Ratner RE, Eriksson JW, et al. A 24-week, multicenter, open-label, randomized study to compare changes in glucose metabolism in patients with schizophrenia receiving treatment with olanzapine, quetiapine, or risperidone. J Clin Psychiatry. 2009;70:487–499. doi: 10.4088/jcp.08m04132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 35.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson DC, Cagliero E, Copeland PM, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Henderson D, Kunkel L, Nguyen D, et al. An exploratory open-label trial of aripiprazole as an adjuvant to clozapine therapy in chronic schizophrenia. Acta Psychiatr Scand. 2006;113:142–147. doi: 10.1111/j.1600-0447.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 38.Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- 39.Venkataraman K, Khoo CM, Leow MK, et al. New measure of insulin sensitivity predicts cardiovascular disease better than HOMA estimated insulin resistance. PLoS One. 2013;8:e74410. doi: 10.1371/journal.pone.0074410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torrent C, Amann B, Sánchez-Moreno J, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. doi: 10.1111/j.1600-0447.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 41.Bruins J, Jorg F, Bruggeman R, et al. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLoS One. 2014;9:e112276. doi: 10.1371/journal.pone.0112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]