Abstract
Background
Stress exposure (SE) during adolescence is associated with an increased risk for the development of alcohol use disorders (AUDs). Past research has shown that SE during adolescence increases voluntary alcohol consumption in mice during adulthood; however, little is known about the positive or negative motivational aspects of this relationship.
Methods
High-alcohol preferring (HAP2) and low-alcohol preferring (LAP2) male mice were exposed to stress during adolescence, stress during adulthood, or no stress. After a 30-day interim, subjects were exposed to alcohol-induced place and footshock-induced fear conditioning procedures to measure stress-induced behavioral alterations during adulthood.
Results
SE during adolescence did not increase the magnitude of alcohol-induced conditioned place preference (CPP), as hypothesized, but increased the magnitude of conditioned fear, as measured by fear-potentiated startle (FPS), in HAP2 subjects only. Regardless of stress treatment group, LAP2 subjects showed greater alcohol-induced CPP expression than HAP2 mice. HAP2 mice also showed greater FPS than LAP2 mice, as previously shown.
Conclusions
These results in mice, taken together with past research, suggest that mice exposed to stress during adolescence do not increase alcohol consumption during adulthood because of a greater sensitivity to the rewarding effects of alcohol, as measured via place conditioning. These results in mice also suggest that humans exposed to stress during adolescence may be more susceptible to developing anxiety during adulthood. The findings may be particularly relevant for humans with a familial history of AUDs.
Keywords: Stress, Adolescence, Reward, Alcohol, Genetics
The term alcohol use disorder (AUD) is used to encompass the spectrum of alcohol abuse and alcohol dependence (Boschloo et al., 2012). Approximately 79,000 deaths per year are related to AUDs, costing the nation approximately 220 billion dollars annually (Bouchery et al., 2011). Understanding what makes an individual more susceptible to developing an AUD is vital to the prevention and treatment of AUDs.
One factor that is associated with an increased likelihood of AUD development is stress exposure (SE) (Enoch, 2011). Evidence suggests that SE during adolescence can have enduring effects on behavior because individuals are more sensitive to a variety of stimuli while undergoing maturation and neurological, biological, and neurochemical changes (Witt, 1994). Clinical research suggests that SE during adolescence increases the risk for early-life binge drinking during adolescence (ages 15 to 18; Labouvie, 1986) and throughout the lifetime (Pilowsky et al., 2009), as well as developing a lifetime AUD (Anda et al., 2006). Exposure to adverse life events during adolescence is associated with AUD development directly following the life event (Clark et al., 1997) and later during young adulthood, as well as increased risk of developing anxiety disorders and depression throughout the lifetime (De Bellis, 2002).
Importantly, enduring consequences of stress during adolescence may depend on genetic vulnerability toward the development of AUDs and anxiety disorders. For example, familial AUD history predicts an increased risk for later-life AUD development, as well as some increased risk for anxiety disorders during adulthood (Chassin et al., 1999). In addition, stressful life events and parental anxiety disorders may mediate the relationship between familial AUD history and adolescent alcohol use (Chassin et al., 1991).
Rodent research has demonstrated that repeated SE during adolescence increases voluntary alcohol consumption both directly following SE (Becker et al., 2011; Siegmund et al., 2005) and later during adulthood (Chester et al., 2008). Drug-seeking behaviors are influenced by the perceived rewarding effects of the drug (Stephens et al., 2010); however, increased oral intake of drugs, like alcohol, could be interpreted as increased or decreased sensitivity to the rewarding effects of the drug (Cunningham, 2014). Thus, interpretation of oral alcohol self-administration behavior is somewhat limited in terms of assessing how manipulations may change motivational processes underlying voluntary drinking behaviors, which may include both positive and negative motivational effects.
Paradigms such as place conditioning allow for the assessment of sensitivity to the motivational effects of drugs, either rewarding or aversive effects, in rodents without relying on oral consumption of alcohol. Rodents are tested in a drug-free state for their conditioned response to a stimulus previously paired with the drug, which allows for the assessment of learning and memory mechanisms involved in alcohol’s motivational effects. Classically conditioned drug responses are thought to play a critical role in the maintenance of reward-related behaviors (Cunningham et al., 2000).
Rodents selectively bred for high- or low-alcohol preference provide valuable tools for investigating how sensitivity to stress and its effects on alcohol-related traits may depend on genetic predisposition toward alcohol drinking. In our laboratory, we use the high-alcohol preferring (HAP) and low-alcohol preferring (LAP) selectively bred mouse lines (Grahame et al., 1999). HAP mice show greater sensitivity to stress-induced conditioned fear behavior, as measured by fear-potentiated startle (FPS), than LAP mice (Chester and Barrenha, 2007). The lines also differ in their hypothalamic–pituitary–adrenal (HPA) axis responses to stress (Chester et al., 2013). We have also previously shown that stress during adolescence (but not adulthood) increases voluntary alcohol consumption in adult male HAP2 mice (Chester et al., 2008). Chronic stress during adolescence has been shown to increase alcohol-induced conditioned place preference (CPP) in Kunming outbred mice when they were conditioned during adolescence; acute stress did not have the same effect (Song et al., 2007).
In this study, we examined the effects of chronic stress during adolescence on sensitivity to alcohol-induced place conditioning and fear conditioning procedures in adulthood. We exposed adolescent and adult male HAP2 and LAP2 mice to same stress procedures used in Chester and colleagues (2008), in which stress during adolescence increased subsequent alcohol drinking in adult male HAP2 mice. It was hypothesized that HAP2 mice exposed to chronic stress during adolescence would show greater alcohol-induced CPP compared to HAP2 mice exposure to chronic stress during adulthood (Chester et al., 2008). We also hypothesized that chronic SE during adolescence would increase FPS to a greater extent in HAP2 than LAP2 mice, based on greater sensitivity to stress-related anxiety in HAP2 than LAP2 mice (Chester et al., 2013) and the previously discussed literature. We also examined the effects of re-exposure to stress, as some research indicates that stress re-exposure might further enhance alcohol-induced CPP expression (Adell et al., 1988; Chester et al., 2006; Matsuzawa et al., 1998).
MATERIALS AND METHODS
Subjects
Alcohol-naïve male HAP2 and LAP2 mice were used (Grahame et al., 1999). HAP and LAP mice from both replicates 1 and 2 were originally selected from an outbred HS/Ibg stock (Grahame et al., 1999). All subjects in this study were generated at Purdue University from HAP2 and LAP2 breeders (44th generation [Study 1] and 47th generation [Study 2]) obtained from the Indianapolis Alcohol Research Center. Subjects were counterbalanced across 25 (Study 1) and 15 (Study 2) breeding pairs. Weanings took place between postnatal days (PD) 21 to 23 (birth of pups = PD 1) and all subjects were group-housed with siblings (2 to 4 mice per cage). Siblings from each litter were assigned to each experimental subgroup in a balanced fashion to the best extent possible. Mice within a cage were assigned to the same treatment group to minimize disruption to the cage. See Table 1 for ages of mice in each experimental group.
Table 1.
Age Ranges, Age Means, and Number of Subjects in Each Group at the Start of Each Behavioral Paradigm for Studies 1 and 2
| Study 1 | SE day 1
|
CPP pretest
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adolescent stress
|
Control
|
Adult stress
|
Adolescent stress
|
Control
|
Adult stress
|
|||||||
| HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | |
| Age (PD) range; M: | 22 to 34; 28 | 23 to 33; 28 | 22 to 35; 28 | 23 to 34; 28 | 63 to 162; 97 | 64 to 157; 92 | 62 to 76; 70 | 62 to 76; 70 | 62 to 76; 70 | 62 to 75; 69 | 104 to 201; 139 | 105 to 202; 134 |
| n: | 70 | 68 | 71 | 68 | 69 | 62 | 70 | 68 | 71 | 68 | 69 | 62 |
|
| ||||||||||||
| Study 2 | SE day 1
|
FPS conditioning
|
||||||||||
| Adolescent stress
|
Control
|
Adult stress
|
Adolescent stress
|
Control
|
Adult stress
|
|||||||
| HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | HAP2 | LAP2 | |
|
| ||||||||||||
| Age (PD) range; M: | 26 to 29; 28 | 27 to 29; 27 | 26 to 29; 27 | 27; 27 | 83 to 85; 84 | 83 to 84; 84 | 66 to 69; 68 | 67 to 69; 67 | 66 to 69; 67 | 67; 67 | 123 to 125; 124 | 123 to 124; 124 |
| n: | 16 | 7 | 12 | 4 | 12 | 12 | 16 | 7 | 12 | 4 | 12 | 12 |
SE, stress exposure; CPP, conditioned place preference; FPS, fear-potentiated startle; PD, postnatal day; HAP2, high-alcohol preferring mice (replicate line 2); LAP2, low-alcohol preferring mice (replicate line 2).
Control mice were age-matched to the adolescent stress group. The adult stress group was older (M = 134 to 139) than the adolescent stress and control groups (M = 69 to 70) when the CPP and FPS procedures began due to the 30-day interim period and the counterbalanced assignment across littermates.
Subjects were housed in clear polycarbonate cages (11.5 × 7.5 × 5 in) with ad libitum access to food and water. All behavioral experiments were conducted during the light portion of the light/dark cycle.
Stress Exposure
Footshock Stress Apparatus
Footshock was delivered using an Animal Acoustic Startle System (Coulbourn Instruments, Allentown, PA), which consisted of 2 sound-sensitive compartment chambers containing 4 weight-sensitive platforms. Four open-air bins (8 × 8 × 16 cm) held individual animals and were placed on top of the platforms. Footshocks were administered through metal rod floors (rod diameter 0.19 cm separated by 0.99 cm) within the bins. Individual platforms measured the grams of force (g/F) exerted by each subject during each footshock.
SE Procedures
Groups received chronic SE during adolescence (adolescent stress) or adulthood (adult stress). The control group was age-matched to the adolescent stress group but received no SE; control subjects were placed in the footshock apparatus for the same amount of time as the adolescent stress and adult stress subjects.
For 10 consecutive days, adolescent stress and adult stress subjects received 15 footshocks (0.2 mA) during a 30-minute time period (Chester et al., 2008). Each shock platform was cleaned with Alconox Powdered Precision Cleaner (Alconox, Inc., White Plains, NY between each squad to minimize odors. Subjects were weighed on days 1 to 10 of SE. All subjects were handled normally during routine animal husbandry.
Study 1
Study 1 Procedure
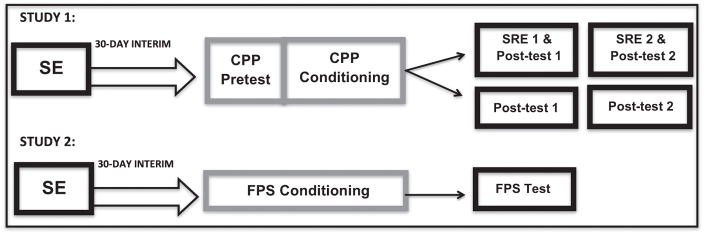
Following SE, there was a 30-day interim period for all subjects to allow the adolescent stress and control subjects to mature into adulthood after which they received place conditioning procedures. Half the subjects in each stress treatment group were re-exposed to the stressor (SRE) immediately before both place preference tests (termed “post test” herein; see Fig. 1). In Study 1, there were 138 subjects in the adolescent stress group (HAP2: 70, LAP2: 68), 139 subjects in the adult stress group (HAP2: 71, LAP2: 68), and 131 subjects in the control group (HAP2: 69, LAP2: 62; see Table 1).
Fig. 1.
Methodological flowchart for behavioral paradigms in studies 1 and 2.
Conditioned Place Preference
Apparatus
The place conditioning apparatus consisted of 8 open-top plexiglass boxes housed within separate light- and sound-sensitive front-open boxes, as previously described (Chester and Coon, 2010). Subjects’ locations and locomotor activity levels were monitored using the Hamilton-Kinder MotorMonitor program (Model HMM100; San Diego, CA).
Procedure
The CPP paradigm for Study 1 included 3 phases: pretest (60 minutes), conditioning trials (5 minutes), and 2 consecutive post tests (60 minutes), and has been explained previously (Powers et al., 2010). During the pretest, subjects were place on a half GRID/half HOLE floor to measure baseline floor preference. Twenty-four hours later, conditioning trials began. Each of the 4 conditioning trials consisted of 1 alcohol (+) and 1 saline (−) floor pairing. On alternating conditioning days, subjects in the G+ subgroup received an intraperitoneal injection of alcohol (2.0 g/kg; Powers et al., 2010) and were placed on a GRID floor for 5 minutes. Conversely, the G− subgroup was injected with saline and placed on the GRID floor for 5 minutes. During the intervening days, subjects received the opposite floor/treatment pairing. Apparatus enclosure, floor assignment, +/− pairing order, and floor placement order during the preference tests (left/right) were counterbalanced across groups. During each post test (24 hours apart), subjects had free access to both the GRID and HOLE floors for 60 minutes.
Stress Re-Exposure
Apparatus
The apparatus used for SE was also used for SRE in Study 1.
Procedure
SRE subjects received 1 SE session immediately before the posttests. The remaining noSRE subjects were placed in the chambers, but no footshocks were given.
Blood Collection and Corticosterone Analyses
During Study 1, blood samples were obtained following SE on days 1 and 10 and following posttests 1 and 2 using the submandibular collection technique, as previously described (Chester et al., 2013; Golde et al., 2005). Plasma samples were kept frozen in a −80°C freezer until corticosterone (CORT) analyses were performed.
CORT levels were determined using a competitive enzyme (sheep polyclonal antibody to CORT) immunoassay kit from Enzo Life Sciences (Farmingdale, NY). CORT densities were read by a microplate reader at a 405-nm wavelength and interpolated from standard curves using a multiple parameter curve-fitting program (Assay Blaster; Enzo Life Sciences). All samples were run in duplicate.
Study 2
Study 2 Procedure
Following SE, there was a 30-day interim period for all subjects to allow the adolescent stress and control subjects to mature into adulthood after which they received fear conditioning procedures (see Fig. 1). In Study 2, there were 23 subjects in the adolescent stress group (HAP2: 16, LAP2: 7), 16 subjects in the adult stress group (HAP2: 12, LAP2: 4), and 24 subjects in the control group (HAP2: 12, LAP2: 12; see Table 1).
Fear-Potentiated Startle
Apparatus
A separate apparatus from that used for SE was used during Study 2 to avoid the possibility of latent inhibition during fear conditioning with footshock. The fear-conditioning apparatus consisted of 8 sound- and light-attenuated startle boxes (Hamilton-Kinder Startle Monitor System, San Diego, CA), each containing a single weight-sensitive platform located 25 cm from the back of the box, 25.8 cm from the speaker, and 26.5 cm from the light. A plexiglass mouse restraint holder (4 × 8.5 × 15 cm) was located on top of the platform. Metal rod floors (rod diameter 0.32 cm separated by 0.47 cm) were used for conditioning days. Peak forces of startle responses were recorded in newtons.
Procedure
The FPS paradigm for Study 2 consisted of 1 conditioning day and 1 testing day, as previously described (Chester et al., 2013). Conditioning stimuli consisted of a light stimulus (30 seconds, 6 W 18 V DC candelabra base) paired with a footshock (0.5 seconds, 0.8 mA) during the last 0.5 seconds, separated by 120-second intertrial intervals (ITIs). The test session consisted of 36 trials (randomized ITIs 10 to 120 seconds: blank [12 trials], noise alone [12 trials, 40 ms, 100 dB], or light + noise [12 trials, 30 seconds, 100 dB noise]).
Statistics
Data were analyzed using analysis of variance (ANOVA) in the Statistical Package for Social Sciences (SPSS; IBM Corp., Armonk, NY). The significance level was set at p < 0.05. Bonferroni-corrected t-tests, Tukey’s post hoc assessments, and Pearson r correlations were used where appropriate. Between group factors were stress treatment (adolescent stress, adult stress, control), line (HAP2, LAP2), SRE subgroup (SRE, noSRE), and conditioning subgroup (G+, G−) and within-subject factors were minute, day, and conditioning trial type, where applicable.
Place conditioning was assessed using time on the GRID floor and the GRID difference score (time spent on the GRID floor during the posttest minus time spent on the GRID floor during the pretest). Significant differences between time spent on the GRID floor between the conditioning subgroups (G+, G−) indicated place conditioning, and any interactions with conditioning subgroup indicated differences in place conditioning magnitude across groups.
For CORT (ng/ml) analyses, duplicate CORT values were subjected to the Dixon’s Extreme Score Test (Dixon, 1950) in reference to data from the same experimental group. If only 1 of 2 values passed the outlier test, the other value was used. If both values passed, the subject was removed from all analyses. All CORT data from 2 subjects (1 male HAP2 adolescent stress noSRE, 1 male LAP2 adolescent stress SRE) were dropped because both values from at least 1 time point qualified as outliers.
Percent FPS was used as the dependent variable for FPS analyses, which is the proportional change score in startle response that adjusts for individual and group differences in startle reactivity (Walker and Davis, 2002). The % FPS values were calculated with the following formula: [((startle amplitude on light + noise trials − startle amplitude on noise-alone trials)/startle amplitude on noise-alone trials) × 100].
RESULTS
Study 1
Tactile Startle Responses During SE
Control subjects were not included in the SE analyses as they had no g/F per kg data. Grams of force per kg data analyzed by a 2 (Stress Treatment) × 2 (Line) × 10 (Day) ANOVA yielded interactions of Day × Stress Treatment, F(9, 2,385) = 2.0, p < 0.05, and Stress Treatment × Line, F(1, 265) = 21.1, p < 0.001 (Fig. 2).
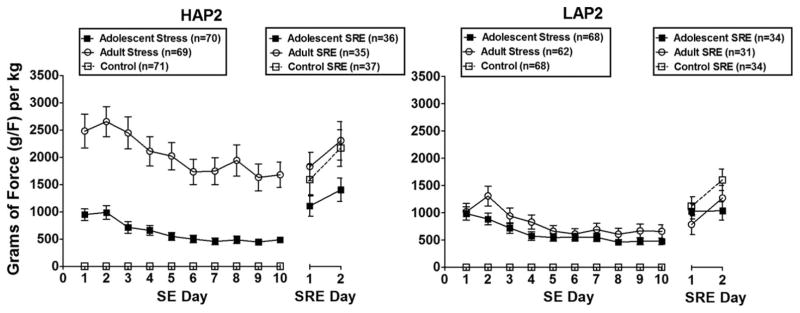
Fig. 2.
Mean (±SEM) grams of force (g/F) per kg in response to footshock stressor exposure (SE; days 1 to 10) and stressor re-exposure (SRE; days 1 to 2) in high-alcohol preferring (HAP2) and low-alcohol preferring (LAP2) mice exposed to the footshock stressor during adolescence and adulthood. Control mice are shown at 0 g/F.
Follow-up analyses of Day within each Stress Treatment group indicated main effects in both the adolescent stress, F(9, 1,233) = 23.9, p < 0.001, and adult stress, F(9, 1,170) = 12.3, p < 0.001, subjects due to decreased g/F per kg over days in both groups, with a more drastic decrease in the adult stress subjects. Follow-up analyses of Stress Treatment within each Day yielded main effects on each of the 10 days of SE (Fs > 30.9, ps < 0.001; Bonferroni-corrected; adult stress > adolescent stress).
Follow-up analyses of Line within each Stress Treatment group yielded a main effect of Line only in the adult stress subjects, F(1, 129) = 22.8, p < 0.001; HAP2 > LAP2. Follow-up analyses of Stress Treatment within each Line yielded a main effect of Stress Treatment in HAP2 subjects only, F (1, 137) = 36.3, p < 0.001; adult stress > adolescent stress.
CORT Levels Following SE
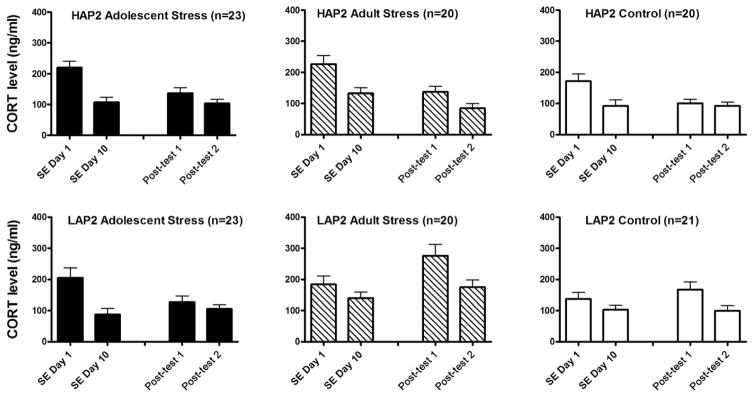
A 3 (Stress Treatment) × 2 (Line) × 2 (Day) ANOVA on CORT levels for SE days 1 and 10 yielded an interaction of Day × Stress Treatment, F (2, 121) = 3.1, p < 0.05. Follow-up analyses of Day within each Stress Treatment group yielded main effects in each Stress Treatment group (Fs > 11.0, ps < 0.01); the interaction appeared to be due to a greater decrease in CORT levels from day 1 to day 10 in the adolescent stress subjects (Fig. 3). Follow-up analyses of Stress Treatment on days 1 and 10 showed main effects on both days (Fs > 3.0, ps < 0.05). Tukey’s post hoc tests indicated greater CORT in adolescent stress subjects than control subjects on day 1 only (p < 0.05).
Fig. 3.
Mean (±SEM) CORT levels (ng/ml) on stressor exposure (SE) days 1 and 10 and following posttests 1 and 2 in mice exposed to adolescent stress (adolescent stress), adult stress (adult stress), or no stress (control) during Study 1. HAP2, high-alcohol preferring; LAP2, low-alcohol preferring; CORT, corticosterone.
Grams of force per kg data was significantly and positively correlated with CORT values on SE day 1 (r = 0.3, p < 0.05), but not on SE day 10.
Baseline Floor Preferences During the Pretest
Mice spent more time on the GRID floor versus the HOLE floor during the pretest (40.6 ± 0.5), particularly the LAP2, F(1, 407) = 131.0, p < 0.001; LAP2 (45.0 ± 0.7) > HAP2 (36.4 ± 0.5), and adult stress subjects, F(2, 407) = 28.6, p < 0.001; adult stress (44.3 ± 0.9) > adolescent stress (40.0 ± 0.7) and control (37.6 ± 0.7); ps < 0.05; Tukey’s post hoc. Importantly, no effects of Conditioning Subgroup were observed in a 1-way ANOVA, indicating similar baseline preference between the conditioning subgroups prior to conditioning.
Tactile Startle Responses During SRE
NoSRE subjects were not included in the g/F analyses as they had no data. A 3 (Stress Treatment) × 2 (Line) × 2 (Day) ANOVA on the g/F per kg responses on SRE days 1 and 2 yielded a main effect of Day, F(1, 201) = 27.4, p < 0.001, due to increased g/F per kg responses on SRE day 2 versus. 1, as well as a main effect of Line, F(1, 201) = 10.8, p < 0.01; HAP2 > LAP2 (Fig. 2).
Posttest Preference
First, we analyzed the raw time on the GRID floor with a 3 (Stress Treatment) × 2 (Line) × 2 (SRE) × 2 (Conditioning Subgroup) for minutes 1 to 60 of posttest 1, which yielded a significant 3-way interaction of Minute × Line × Conditioning Subgroup, F(59, 22,656) = 2.30, p < 0.001. Due to the interaction with the minute factor, we decided to separately analyze the preference test behavior in three 20-minute segments, as temporal parameters are an important factor to consider when analyzing behavior during a place preference test (Cunningham et al., 2006). Furthermore, to account for the initial GRID floor bias during the pretest, all posttest data were analyzed using the GRID difference score (posttest–pretest). Analyses on the first 20-minute segment are reported and shown in Fig. 4; analyses on the second and third 20-minute segment of posttest 1 and all 20-minute segments of posttest 2 yielded only interactions of Line × Conditioning Subgroup (ps < 0.01), due to greater CPP in LAP2 than HAP2 subjects (data not shown).
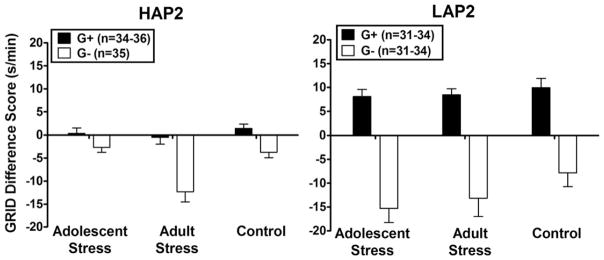
Fig. 4.
Average (±SEM) GRID difference score (time on the GRID floor during posttest 1 minus time on the GRID floor during the pretest) for the first 20 minutes of posttest 1 for G+ and G− conditioning subgroups in each stress treatment and stress re-exposure subgroup of high-alcohol preferring (HAP2) and low-alcohol preferring (LAP2) mice in Study 1.
The overall 4-way ANOVA (3 [Stress Treatment] × 2 [Line] × 2 [SRE] × 2 [Conditioning Subgroup]) on the GRID difference score during the first 20 minutes of the preference test yielded an interaction between Line × Conditioning Subgroup, F(1, 407) = 36.70, p < 0.001, and a near-significant Line × Stress Treatment interaction, F(2, 407) = 2.7, p = 0.07.
Follow-up analyses of the Line × Conditioning Subgroup interaction (Conditioning Subgroup ANOVAs within each Line and Line ANOVAs within each Conditioning Subgroup) yielded main effects of Conditioning Subgroup in both HAP2, F(1, 209) = 30.7, p < 0.001, and LAP2 subjects, F(1, 197) = 100.4, p < 0.001. Follow-ups also yielded main effects of Line in both G+, F(1, 203) = 54.17, p < 0.001; LAP2 > HAP2, and G− subgroups, F(1, 203) = 7.75, p < 0.01; HAP2 > LAP2. Thus, this interaction was due to greater CPP in LAP2 subjects compared to HAP2 subjects.
Activity levels during the posttest were not significantly correlated with the GRID difference scores.
CORT Levels Following Posttests
A 3 (Stress Treatment) × 2 (Line) × 2 (SRE) × 2 (Conditioning Subgroup) repeated-measures ANOVA on CORT levels during posttests 1 and 2 (Day) yielded interactions of Day × Stress Treatment, F(2, 103) = 4.6, p < 0.05, and Day × Line, F(1, 103) = 5.2, p < 0.05 (Fig. 3). Follow-up analyses of Day within each Line showed main effects in HAP2 subjects, F(1, 62) = 16.7, p < 0.001, and LAP2 subjects, F(1, 63) = 24.5, p < 0.001, where CORT decreased between posttests 1 and 2. Follow-up ANOVAs of Line within each Day showed greater CORT levels in LAP2 than HAP2 mice on posttest 1 and posttest 2 (Fs > 5.5, ps < 0.05).
Follow-up analyses of Day within each Stress Treatment group yielded main effects in all Stress Treatment groups (Fs > 7.9, ps < 0.01), where CORT decreased between posttests 1 and 2. Follow-up ANOVAs of stress treatment within each Day yielded a main effect only on posttest 1, F(2, 126) = 5.7, p < 0.01, where adult stress subjects showed greater CORT levels than adolescent stress (p < 0.01) and control subjects (p < 0.05; Tukey’s post hoc).
CORT levels after posttest 1 or posttest 2 were not significantly correlated with the corresponding GRID difference scores.
Study 2
Tactile Startle Responses During SE
Control subjects were not included in SE analyses as they did not have g/F per kg data. A 2 (Stress Treatment) × 2 (Line) × 10 (Day) ANOVA on the g/F per kg yielded an interaction of Day × Stress Treatment, F(9, 387) = 2.2, p < 0.05. Follow-up analyses of Day within each Stress Treatment group yielded main effects of Day in both adolescent stress and adult stress groups (Fs > 1.9, ps < 0.05), where g/F per kg decreased across days. Follow-up analyses of Stress Treatment within each of the 10 days yielded main effects of Stress Treatment on day 5 only, F(1, 46) = 18.8, p < 0.001; adult stress > adolescent stress; Bonferroni-corrected; data not shown. In general, this data pattern is similar to that observed in Study 1.
Fear-Potentiated Startle
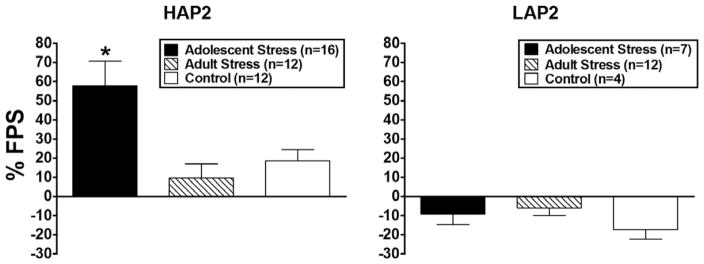
A 3 (Stress Treatment) × 2 (Line) ANOVA on % FPS yielded a Stress Treatment × Line interaction, F(2, 62) = 3.7, p < 0.05 (see Fig. 5). Follow-up analyses of Stress Treatment within each Line showed a main effect of Stress Treatment in HAP2 subjects only, F(2, 39) = 6.7, p < 0.01, and Tukey’s post hoc confirmed that adolescent stress subjects showed greater % FPS than adult stress and control subjects (ps < 0.05). Follow-up analyses of Line within each Stress Treatment group yielded main effects of Line in the adolescent stress, F(1, 22) = 11.2, p < 0.01; HAP2 > LAP2, control subjects, F(1, 15) = 10.8, p < 0.01; HAP2 > LAP2, and a trend toward a main effect in the adult stress subjects, F(1, 23) = 3.6, p = 0.07; HAP2 > LAP2.
Fig. 5.
Mean (±SEM) % fear-potentiated startle (FPS) during the FPS test for high-alcohol preferring (HAP2) and low-alcohol preferring (LAP2) subjects exposed to stress during adolescence (adolescent stress), stress during adulthood (adult stress), and no stress (control) in Study 2. *p < 0.01 HAP2 adolescent stress > HAP2 adult stress and HAP2 control.
DISCUSSION
The results did not support our first hypothesis that SE during adolescence would increase sensitivity to the rewarding effects of alcohol during adulthood, as measured by place conditioning. If anything, SE during adolescence reduced alcohol-induced CPP, although we were unable to statistically show support for this effect (see Fig. 4; Adolescent vs. Adult Group). Re-exposure to stress prior to the preference tests did not alter the expression of alcohol-induced CPP in any groups. However, our second hypothesis was supported: SE during adolescence increased FPS in HAP2 but not LAP2 mice. These findings suggest that the HAP2 mice may be more sensitive to the effects of SE during adolescence than LAP2 mice and are consistent with past literature showing that HAP2 mice are more sensitive to stress-related anxiety than LAP2 mice (Chester et al., 2013).
Past literature suggests that repeated footshock can elicit changes in behavior more effectively than other stress paradigms, such as a forced swim test (Siegmund et al., 2005). In the current study, we used the same footshock procedure that we used previously to demonstrate increased voluntary alcohol consumption in adult HAP2 male mice previously exposed to footshock stress during adolescence (Chester et al., 2008). The finding that adolescent mice exposed to this footshock procedure subsequently showed increased FPS in Study 2 suggests that this footshock procedure was effective in eliciting long-term changes in behavior.
Previous research using pharmacological and genetic manipulations suggests an overlapping association between voluntary alcohol consumption and alcohol-induced CPP in mice (Green and Grahame, 2008). However, there is also evidence that they are dissociated (Powers et al., 2010). In our past work, we showed that SE during adolescence increased subsequent alcohol intake in adult male HAP2 mice (Chester et al., 2008). In the current study, we used the place conditioning procedure to further explore how adolescent stress may change alcohol’s motivational effects, as increased alcohol drinking could reflect either increased or decreased sensitivity to alcohol’s rewarding effects.
Contrary to our hypothesis, our results suggest that SE during adolescence did not increase sensitivity to alcohol’s rewarding effects. Results of a study by Mathews and colleagues (2008) are consistent with this idea. Adolescent rats that were exposed to a social stress paradigm and then tested for amphetamine-induced CPP during adulthood were found to be less sensitive to amphetamine-induced CPP based on a left-shifted dose–response curve. The current study used a single dose of alcohol known to produce robust alcohol-induced CPP in adult HAP and LAP mice (2.0 g/kg). However, future studies should include a dose-dependent analysis to provide information about line differences in sensitivity to alcohol and stress and interactions with developmental age.
These data also show for the first time in replicate line 2 an inverse relationship between propensity toward alcohol consumption and alcohol-induced CPP (2.0 g/kg). Past research has shown that LAP1 mice show greater alcohol-induced CPP than HAP1 mice at a 4.0 g/kg dose, but not at a 1.5 or 3.0 g/kg dose (Grahame et al., 2001). This inverse genetic relationship between sensitivity to alcohol-induced CPP and voluntary alcohol drinking behavior is consistent with a broader literature in many different mouse strains (see review by Cunningham, 2014). HAP1/LAP1 and HAP2/LAP2 lines have not been shown to differ in alcohol metabolic rate (Grahame et al., 1999).
As previously mentioned, there was a trend toward reduced alcohol-induced CPP in HAP2 mice exposed to stress during adolescence (see Fig. 4), although the required interaction term needed to support this difference (Stress Treatment × Line × Conditioning Subgroup) was not significant. It is possible that this trend could be due to differential effects of SE on the adolescent versus adult brain that could have affected a general learning mechanism. As well, chronological age differences between the adolescent and control groups versus the adult group, which was about 60 to 70 days older (see Table 1) at the time of CPP testing, could be related to this trend. Although chronological age differences might influence general learning ability, recall that all groups were categorically adults at the time of place conditioning (see Table 1). In Study 2, these groups had the same differences in chronological age ranges and yet HAP2 mice exposed to stress during adolescence showed enhanced FPS (a classically conditioned behavior like place conditioning) compared to the other groups. Taken together, these data do not support a possible learning ability difference between groups. However, it is certainly possible that stress differentially affects learning about a reward-related stimulus versus an anxiety-related stimulus.
The current study design did not include a separate drug-free control group to assess a possible change in unconditioned preference for the floor types, which could affect interpretation of the CPP data. However, past research suggests that GRID and HOLE floor preference do not change following repeated drug-free exposure to the floor types in LAP1 (Chester and Coon, 2010; Grahame et al., 2001) or HAP1 (Grahame et al., 2001) mice. Thus, it seems likely that any group differences in CPP can be attributed to differences in sensitivity to alcohol’s rewarding effects. Furthermore, the greater CPP present in LAP2 subjects does not appear to be due to differential unconditioned changes in floor preference between the lines as LAP2 subjects initially preferred the GRID floor during the pretest, yet still showed greater CPP magnitude during the posttests.
Results of our study differ from those of Song and colleagues (2007), where chronic SE enhanced alcohol-induced CPP. However, in the Song and colleagues (2007) study place conditioning was conducted immediately after each SE during adolescence. Also, different alcohol doses were used in this study to induce place conditioning in the adolescent versus adult Kunming mice. Past research suggests that SE may differ in its immediate and long-term effects on drug-related behaviors (Becker et al., 2011).
No effects of re-exposure to stress on place conditioning were seen during the study. However, the current study did not simultaneously condition the footshock stimulus with alcohol exposure, as was carried out in a prior study (Matsuzawa et al., 1998). Additionally, the interim period between the original stressor and re-exposure to the stressor was 24 hours in the Matsuzawa and colleagues (1998) study and 30 days in the current study.
Overall, HAP2 adult subjects showed greater g/F per kg responses than LAP2 adult subjects. The g/F measurement provides information about sensitivity to noxious, anxiety-provoking stimuli while accounting for differences in body weight (Barrenha and Chester, 2007). There were no differences between the adolescent HAP2 and LAP2 subjects’ tactile startle responses. These results support the notion that SE during adolescence may not be immediately evident but manifest into altered behaviors in adulthood, possibly due to potentiation and incubation effects (Lupien et al., 2009).
The footshock procedure did increase CORT levels in both adolescent and adult mice to a similar degree on SE day 1. Adolescent stress subjects showed a more dramatic decrease in CORT levels from SE day 1 to day 10 and showed CORT levels comparable to the control group by day 10. These results are contradictory to some of the literature, which has shown that chronic SE often leads to a potentiated CORT response in adolescents (Laviola et al., 2002; Romeo et al., 2006). On the other hand, other research suggests that chronic SE may enhance negative feedback inhibition of CORT release in adolescent subjects (Schmidt et al., 2007).
There were no line differences in CORT levels in any group at 30 minutes following SE. However, LAP2 mice showed higher CORT than HAP2 mice after the posttests. These results are very interesting in light of our prior findings where LAP2 mice showed higher CORT levels than HAP2 mice after fear conditioning and testing (Chester et al., 2013). In the current study where we saw no line difference in CORT after SE, blood was sampled at 30 minutes after exposure to 15 footshocks of 0.2 mA intensity. In our prior study, the footshock intensity was 0.8 mA and blood was sampled after a 2-hour footshock conditioning session or after a 1-hour FPS test session. The current and prior data taken together suggest that the line differences in CORT (LAP2 > HAP2) after stress or behavioral testing reflects greater negative feedback inhibition and consequently lower CORT levels in HAP2 mice when blood is sampled at least 1 hour after the stressor. Greater negative feedback inhibition in HAP2 versus LAP2 mice is a hypothesis we are pursuing in our ongoing studies. Future follow-up studies that explore possible mechanisms in the HAP2-specific enhancement of fear conditioning following adolescent SE will be valuable. There are a variety of ways in which these possible mechanisms could be explored, such as with pharmacological manipulations of the HPA axis at various time points after SE and at different developmental ages.
Overall, the results of the current studies suggest that chronic SE during adolescence does not increase alcohol-induced CPP but does increase FPS in HAP2 mice. These data do not support the previous hypothesis that individuals with a genetic propensity toward high-alcohol preference and exposed to stress during adolescence may increase alcohol consumption during adulthood because they are more sensitive to the rewarding effects of alcohol. In addition, these data are the first to show that SE during adolescence increases FPS in HAP2 mice but not LAP2 mice. These data in mice suggest that individuals with familial history of high-drinking behavior or AUDs may be more susceptible to the development of anxiety following stressful life events during adolescence, a finding reported in the human literature (Chassin et al., 1999). Future research should continue to characterize line differences in the enduring effects of SE during adolescence on alcohol-related neurochemical and behavioral traits.
Acknowledgments
Supported by an American Psychological Association of Graduate Students Basic Psychological Sciences Research Grant and American Psychological Association Dissertation Research Award to KRB. We are grateful to Dr. Nicholas J. Grahame for providing the breeders for the HAP2 and LAP2 mice and to Molly Craig for breeding and maintaining the experimental animals. We are also grateful to Aaron M. Kirchhoff for his assistance with blood samples. The experiments described herein comply with the current laws of the United States of America. The authors do not have any financial conflicts of interest to report.
References
- Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;50:1678–1681. doi: 10.1111/j.1471-4159.1988.tb02462.x. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res. 2007;31:1081–1088. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Becker H, Lopez M, Doremus-Fitzwater T. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Van Den Brink W, Penninx BW, Wall MM, Hasin DS. Alcohol-use disorder severity predicts first-incidence of depressive disorders. Psychol Med. 2012;42:695–703. doi: 10.1017/S0033291711001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Delucia C, Todd M. A Longitudinal Study of Children of Alcoholics: Predicting Young Adult Substance Use Disorders, Anxiety, and Depression. American Psychological Association; Washington, DC: 1999. [DOI] [PubMed] [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. J Abnorm Psychol. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Coon LE. Pentylenetetrazol produces a state-dependent conditioned place aversion to alcohol withdrawal in mice. Pharmacol Biochem Behav. 2010;95:258–265. doi: 10.1016/j.pbb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, De Paula Barrenha G, Demaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Chester JA, Kirchhoff AM, Barrenha GD. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addict Biol. 2013;19:663–675. doi: 10.1111/adb.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Lesnick L, Hegedus AM. Traumas and other adverse life events in adolescents with alcohol abuse and dependence. J Am Acad Child Psychiatry. 1997;36:1744–1751. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci. 2014;128:430–445. doi: 10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- Dixon W. Analysis of extreme values. Ann Math Stat. 1950;21:488–506. [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Chester JA, Rodd-Henricks K, Li TK, Lumeng L. Alcohol place preference conditioning in high- and low-alcohol preferring selected lines of mice. Pharmacol Biochem Behav. 2001;68:805–814. doi: 10.1016/s0091-3057(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouvie E. Alcohol and marijuana use in relation to adolescent stress. Subst Use Misuse. 1986;21:333–345. doi: 10.3109/10826088609074838. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova M. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mathews I, Mills R, McCormick C. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–459. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol. 1998;341:127–130. doi: 10.1016/s0014-2999(97)01456-8. [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Keyes KM, Hasin DS. Adverse childhood events and lifetime alcohol dependence. Am J Public Health. 2009;99:258–263. doi: 10.2105/AJPH.2008.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MS, Barrenha GD, Mlinac NS, Barker EL, Chester JA. Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference. Psychopharmacology. 2010;212:571–583. doi: 10.1007/s00213-010-1997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, Greetfeld M, Uhr M, Holsboer F, Müller MB. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer M, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31:2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC. Reward sensitivity: issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology. 2002;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]