Abstract
Objective
In clinical practice, patient characteristics predicting resistant hypertension (RH) include higher blood pressure levels, left ventricular hypertrophy, older age, obesity, chronic kidney disease and diabetes. On the contrary little is known about the role of serum uric acid (SUA) as a risk factor for RH in subjects from general population.
Material and methods
580 elderly subjects aged ≥65 years were enrolled in the Risk Of Vascular complications Impact of Genetics in Old people (ROVIGO) study. RH was defined as the failure to maintain blood pressure values below 140 mmHg (systolic) and 90 mmHg (diastolic) despite therapeutic interventions that include appropriate lifestyle measures plus adherence to treatment with full doses of at least three antihypertensive drugs, including a diuretic. RH was confirmed using 24-h ambulatory blood pressure measurement. Hyperuricemic was defined as the subjects having SUA ≥6.8 mg/dl or taking uricosuric drugs. Gender-specific odds ratio (OR) for RH was calculated by logistic regression analysis.
Results
The prevalence of RH was 5.7% in the cohort and was higher in women (8.3%) than in men (3.0%, p < 0.05). Independent of chronic kidney disease (OR 3.89, 95% confidence interval 1.49–10.1), hyperuricemia predicted resistant hypertension in women (odds ratio 3.11, 95% confidence intervals 1.06–9.1, p = 0.03) but not in men.
Conclusions
In elderly women from the general population, an SUA value of ≥6.8 mg/dl triples the risk of RH. SUA assessment should be recommended to better define the pattern of risk associated with RH.
Keywords: Cardiovascular risk, Epidemiology, Resistant hypertension, Uric acid
1. Introduction
In the 2013 Guidelines of the European Societies of Hypertension and Cardiology, resistant hypertension (RH) is defined as blood pressure (BP) remaining above the goal (140/90 mmHg) despite a therapeutic strategy taking into consideration appropriate lifestyle measures plus the adherence to treatment with full doses of at least 3 antihypertensive drugs including a diuretic [1]. In clinical practice, patient characteristics predicting RH include higher BP (particularly systolic), older age, left ventricular hypertrophy, diabetes, obesity, chronic kidney disease and African-American race [2]. On the contrary, little is known about the role of serum uric acid (SUA) as a risk factor for RH, particularly in elderly subjects from general population.
Hyperuricemia is commonly associated with hypertension [3] and is present in 25% of untreated patients with hypertension, in 50% of patients taking diuretics and in >75% of patients with malignant hypertension [4]. In addition hypertensive patients with hyperuricemia have a 3-to-5-fold increased risk of coronary or cerebrovascular disease compared to hypertensive patients with normal SUA [5].
The aim of this study was to evaluate the role of SUA as a risk factor for RH in elderly people from general population.
2. Material and methods
2.1. Study population
The Risk Of Vascular Complications Impact of Genetics in Old people (ROVIGO) study, was conceived as a longitudinal population-based study aimed at evaluating in the long term whether cardiovascular disease depends on a particular genetic profile and/or environmental risk factors. The study cohort consisted of 580 unselected subjects (272 men and 308 women) aged 65 years or over, representative of the elderly of the general population of Rovigo in the Italian Veneto region. The results of the initial cross-sectional survey are described herein [6]. Data from the longitudinal analysis, including morbidity, mortality and incidence of new cases of hypertension, has not yet been published. A complete genetic analysis is on going. The local Ethics Committee approved the study and all the procedures were carried out in accordance with the Helsinki declaration and with institutional guidelines. Informed consent was obtained for each participant.
2.2. Data collection and disease criteria
The protocol of the study was previously published [6] and included gathering of demographic information, medical and social questionnaires, fasting blood tests, anthropometrics, electrocardiogram, clinic BP and 24-h ambulatory BP measurements. Procedures for taking and preparing blood specimens and laboratory analysis were standardized.
Fasting SUA and lipids was determined by enzymatic method. For the purpose of this study, we analysed only those subjects having asymptomatic hyperuricemia, defined as SUA levels ≥6.8 mg/dl without symptoms or signs of urate crystal deposition disease or clinical history of uric acid renal disease, as well as subjects taking hypouricemic drugs. The cut-off of «abnormal» SUA is still disputed; however we used a pathophysiological approach considering the super-saturation of SUA concentration (i.e. 6.8 mg/dl) observed at 37 °C of temperature [7]. Subjects with total cholesterol ≥200 mg/dl or low-density-lipoprotein cholesterol >130 mg/dl [8] were labelled as hypercholesterolemic, those with serum triglycerides ≥150 mg/dl as hypertriglyceridemic [9]. Subjects were classified as diabetic if their fasting serum glucose was repeatedly ≥126 mg/dl, or if they had a history of diabetes and/or treatment with anti-diabetic drugs. Serum creatinine (mg/dl) was measured using by an enzymatic method (Hitachi Modular P, Roche diagnostic, USA). Albuminuria was measured using the turbidimetric method (Cobas Mira Plus, Roche, Montclair, NJ) in 24-h urine. Subjects having albuminuria ≥300 mg/day were classified as proteinuric.
Creatinine clearance (ml/min) was calculated from serum creatinine (mg/dl) using the formula of Cockroft and Gault [10]. Subjects having a creatinine clearance <60 ml/min were classified as having chronic renal disease.
Subjects currently smoking ≥1 cigarette daily were classified as smokers. Alcohol intake was recorded by means of a questionnaire and considered as ethanol consumption (ml/week), mainly from wine. Anthropometrics were measured in patients wearing light underwear without shoes. Subjects were considered obese when body mass index was ≥30 kg/m2.
Following a 20-min rest, trained physicians measured BP using a mercury sphygmomanometer. The measurements were obtained from subjects while sitting in the supine posture position. The measurements were performed in triplicate at 10-min intervals avoiding any terminal digit preference. To minimise the “white-coat effects”, if any, the average of the last two measurements was taken into consideration as a measure of blood pressure for data analysis. Heart rate was also measured at the same time and averaged. Arterial hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg or current treatment with antihypertensive drugs. According to the 2013 guidelines [2], hypertension was classified as resistant to treatment after a therapeutic strategy including appropriate lifestyle measures + diuretic + two other antihypertensive drugs belonging to different classes at adequate doses failed to lower systolic BP <140 and diastolic BP <90 mmHg.
An expert who did not know the aim and design of the study evaluated 12-lead standard electrocardiogram. Subjects satisfying the Sokolow-Lyon criterion [11] were classified as having left ventricular hypertrophy.
2.3. Statistical analysis
SPSS/PASW Statistics 18.0 for Windows (SPSS, Chicago, USA) was used for statistical analysis. Continuous variables were expressed as mean and standard deviation. Analysis of variance was used to compare grouped continuous variables, and the Pearson’s χ2 test to compare the prevalence of categorical variables. Gender-specific odds ratio (OR) and 95% confidence intervals for RH were calculated for independent variables by logistic regression analysis. The null hypothesis was rejected when p was <0.05.
3. Results
The general characteristics of the population, also showing stratification in subjects with and without RH, are summarised in Table 1. Using office BP values, the prevalence of RH was 9.8% in the mixed gender cohort. No statistically significant gender differences were observed in men and women (9.2 vs. 10.6%, respectively). In contrast, using the 24-h ambulatory measurement, the prevalence of RH was 5.7% in the whole cohort and higher in women (8.3%) than in men (3.0%), p < 0.05. Other parameters, including SUA, clinic systolic BP, 24-h systolic and daytime systolic BP, serum triglycerides, number of antihypertensive drugs, prevalence hyperuricemia and of left ventricular hypertrophy were higher in subjects with RH. Diuretic treatment was also more prevalent in subjects with RH. In contrast, eGFR and HDLC values as well as the prevalence of uricosuric drugs were higher in the latter than in the former (Table 1).
Table 1.
General characteristics of the 580 unselected men and women stratified according to the presence of resistant hypertension (RH). BMI: body mass index. SUA: serum uric acid; SBP, DBP: systolic and diastolic blood pressure; HR: heart rate; TC, HDLC, LDL: total, high-density- and low-density-lipoprotein serum cholesterol; TG: serum triglycerides; ATH: anti-hypertensive; ABPM: ambulatory blood pressure monitoring. NS: non-significant difference.
| Items | All subjects (n = 580) |
Without RH (n = 547) |
With RH (n = 33) |
p-value |
|---|---|---|---|---|
| Age (yrs) | 73.2 ± 4.8 | 73.1 ± 4.8 | 73.7 ± 5.2 | NS |
| Female gender (%) | 53.1 | 51. 6 | 78.8 | <0.05 |
| BMI (kg/m2) | 26.7 ± 3.7 | 26.7 ± 3.8 | 27.0 ± 3.0 | NS |
| Obesity (%) | 49.5 | 46.2 | 3.3 | NS |
| SUA (mg/dl) | 5.29 ± 1.4 | 5.26 ± 1.4 | 5.80 ± 1.6 | <0.05 |
| SUA >6.8 mg/dl (%) | 20.3 | 17. 7 | 63.6 | <0.05 |
| Clinic BP values | ||||
| SBP (mmHg) | 143.3 ± 18.4 | 141.3 ± 16.6 | 158.2 ± 4.7 | <0.05 |
| DBP (mmHg | 79.6 ± 11.6 | 79.8 ± 11.6 | 77.1 ± 0.3 | NS |
| HR (bpm) | 71.8 ± 9.6 | 71.9 ± 9.5 | 71.2 ± 11.7 | NS |
| 24-h ABPM values | ||||
| SBP (mmHg) | 137.9 ± 13.6 | 137.4 ± 13.7 | 146.6 ± 9.2 | <0.05 |
| DBP (mmHg) | 76.8 ± 6.7 | 76.9 ± 6.7 | 75.7 ± 6.5 | NS |
| HR (bpm) | 71.2 ± 8.0 | 71.6 ± 8.2 | 68.1 ± 6.8 | NS |
| Day-time SBP (mmHg) | 141.1 ± 13.8 | 140.4 ± 13.8 | 151.6 ± 8.7 | <0.05 |
| Day-time DBP (mmHg) | 78.8 ± 7.0 | 78.9 ± 7.2 | 90.2 ± 4.6 | NS |
| Day-time HR (bpm) | 73.2 ± 8.2 | 73.4 ± 8.1 | 70.0 ± 7.7 | NS |
| Night-time SBP (mmHg) | 126.5 ± 16.4 | 126.5 ± 16.6 | 128.7 ± 11.1 | NS |
| Night-time DBP (mmHg) | 70.0 ± 8.4 | 70.1 ± 8.5 | 67.9 ± 7.5 | NS |
| Night-time HR (bpm) | 64.5 ± 8.6 | 64.4 ± 8.6 | 64.0 ± 8.1 | NS |
| Diuretic treatment (%) | 23.8% | 19.2% | 10 0 | <0.05 |
| Number of ATH-drugs | 1.0 ± 1.24 | 0.8 ± 0.9 | 4.2 ± 0.5 | <0.05 |
| Serum glucose (mg/dl) | 102.1 ± 25.1 | 102.6 ± 25.4 | 104.4 ± 18.6 | NS |
| Diabetes (%) | 18.8 | 18.5 | 24.2 | NS |
| Serum creatinine (mg/dl) | 0.92 ± 0.32 | 0.92 ± 0.34 | 0.99 ± 0.31 | NS |
| eGFR (ml/min) | 68.3 ± 16.8 | 62.2 ± 16.7 | 60.1 ± 16.9 | <0.05 |
| Uricosuric drugs (%) | 9.4 | 13.4 | 4.2 | <0.05 |
| Proteinruia (%) | 14.3 | 11. 6 | 17. 8 | NS |
| LVH (%) | 45 | 38.7 | 52.6 | <0.05 |
| TC (mg/dl) | 206.4 ± 70.6 | 200.6 ± 72.1 | 210.8 ± 41.2 | NS |
| HDLC (mg/dl) | 61.2 ± 16.0 | 61.3 ± 16.2 | 60.1 ± 14.3 | NS |
| TG (mg/dl) | 114.4 ± 63.6 | 113.3 ± 64.4 | 133.2 ± 45.4 | <0.05 |
| LDCL (mg/dl) | 120.1 ± 33.7 | 120.5 ± 33.6 | 121.5 ± 34.5 | NS |
| Smoking (%) | 6.7 | 6.9 | 3.0 | NS |
| Ethanol (g/week) | 28.4 ± 21.9 | 27.44 ± 20.0 | 29.2 ± 23.5 | NS |
As shown in Table 2, only chronic kidney disease and hyperuricemia predicted RH (OR 4.4, 95% confidence intervals 1.24–15.7, p = 0.02) in women but not in men (OR 1.46, 95% confidence intervals 0.51–4.21, p = 0.5).
Table 2.
Multivariate logistic regression analysis for resistant hypertension by gender. CKD: chronic kidney disease. Other abbreviations as in Table 1.
| Items | Wald | Coefficient (SE) |
p value |
Odds ratio | 95% confidence intervals |
|---|---|---|---|---|---|
| Women (n = 308) | |||||
| CKD (yes/no) | 7. 71 | 1.35 (0.48) | 0.005 | 3.89 | 1.49–10.1 |
| SUA >6.8 mg/dl (yes, no) | 4.32 | 1.13 (0.54) | 0.038 | 3.11 | 1.06–9.10 |
| Diabetes (yes/no) | 0.76 | 0.44 (0.54) | 0.383 | 1.61 | 0.55–4.72 |
| LVH (yes, no) | 0.65 | 0.35 (0.43) | 0.423 | 1.41 | 0.61–3.2 |
| Age (years) | 0.43 | 0.09 (0.34) | 0.509 | 1.13 | 0.89–1.36 |
| Obesity (yes, no) | 0.14 | −0.20 (0.55) | 0.707 | 0.81 | 0.27–2.42 |
| Diuretics (yes, no) | 0.12 | 1.01 (0.32) | 0.246 | 1.24 | 0.83–2.89 |
| Men (n = 272) | |||||
| CKD (yes/no) | 2.71 | 1.27 ±0.772 | 0.099 | 3.59 | 0.78–16.2 |
| SUA >6.8 mg/dl (yes, no) | 0.49 | 0.51 ±0.722 | 0.481 | 1.66 | 1.06–9.1 |
| Diabetes (yes/no) | 0.41 | 0.36 ±0.761 | 0.629 | 1.44 | 0.32–6.4 |
| LVH (yes, no) | 0.36 | −0.42 ± 0.69 | 0.574 | 0.65 | 0.16–2.52 |
| Diuretics (yes, no) | 0.23 | 0.93 ± 0.46 | 0.416 | 1.02 | 0.76–3.10 |
| Age (years) | 0.03 | 0.09 ± 0.34 | 0.509 | 1.00 | 0.69–1.16 |
| Obesity (yes, no) | 0.04 | −0.01 ± 0.74 | 0.954 | 1.19 | 0.27–6.6 |
4. Discussion
Five to 15% of hypertensive patients do not achieve an adequate BP control despite three or more antihypertensive agents [1]. Subjects with RH represent one of the most important clinical challenges in the management of hypertension. Resistant hypertensives are characterized by several factors: longer history of hypertension, obesity and other accompanying factors, such as diabetes, left ventricular hypertrophy, albuminuria and renal dysfunction [12]. In addition to other diagnostic and therapeutic manoeuvres (excluding secondary hypertension, ensuring treatment adherence and optimizing therapeutic schemes), 24-h ambulatory BP monitoring (24h-ABPM) is crucial in the clinical evaluation of these patients. 24h-ABPM discriminates between those with out-of-office BP elevation (true resistant hypertensives) and those with white-coat RH. The latter represent about one-third of subjects with RH [13]. In agreement with published studies [12,13], these studies confirm that 24h-ABPM leads to a better characterization of true RH, that is commonly overestimated when diagnosed using office BP values [1]. In addition, although patients with RH may have elevations in both systolic and diastolic BP, in our study isolated systolic hypertension was the more common form of RH diagnosed in the elderly.
A large number of observational and epidemiological studies have revealed an increase risk of hypertension with increasing levels of SUA, an association that remains independent of the traditional cardiovascular risk factors [14–16]. From a pathophysiological point of view, oxidative stress due to xanthine oxidase activity appears to be involved in the association between hyperuricemia and hypertension [15]. In detail, this mechanism has been clearly investigated in animal models [17,18], where SUA increase leads a rise in BP that can be prevented or reversed with uricosuric drugs or xanthine oxidase inhibitors [19]. However, studies performed in humans and specifically in the elderly led controversial results regarding the relationship between SUA and BP [20,21].
The association between SUA and BP is not as strongly supported in the elderly population, and SUA-lowering agents have different effects on controlling BP in the elderly and in the young adults with hyper-uricemia [22,23]. However, large controlled studies are needed to formally establish the effect of SUA lowering on BP control and on hard cardiovascular and renal endpoints. The ongoing Febuxostat for Cerebral and caRdiorenovascular Events prEvEntion stuDy (FREED; ClinicalTrials.gov Identifier: NCT01984749) is currently investigating the effect of xanthine oxidase inhibition on cardiovascular and renal endpoints in elderly hyperuricemic patients. The results are expected in late 2017.
In our study, performed in elderly subjects from the general population, the risk of RH independently tripled in women but not in men when SUA was ≥6.8 mg/dl. Age was an independent predictor for the elevation of SUA, and it is well known that the relationship between age and the occurrence of hyperuricemia differs between men and women [23]. Hyperuricemia correlates negatively with age in men but positively in women [24,25], possibly due to sex hormonal interactions [26]. Elevated SUA levels and the incidence of hypertension is exceedingly rare in women during the fertility phase, but rises with menopause, potentially explained by an effect of oestrogen on SUA renal handling [27].
In our study, chronic kidney disease (CKD) was the highest risk factor for RH. CKD is very frequently associated with RH as a risk factor or comorbidity, and suggests an impaired prognosis in hypertensive subjects with RH [28]. In patients with CKD, RH is a common condition due to a combination of factors including sodium retention and volume expansion, increased activity of the renin-angiotensin system, and enhanced activity of the sympathetic nervous system [29]. On the other hand chronic hyperuricemia seems to be associated with a greater risk of arterial stiffness in women but not in men [30]. We postulate that when it coexists with CKD is likely to promote a vascular remodelling leading RH (Fig. 1).
Fig. 1.
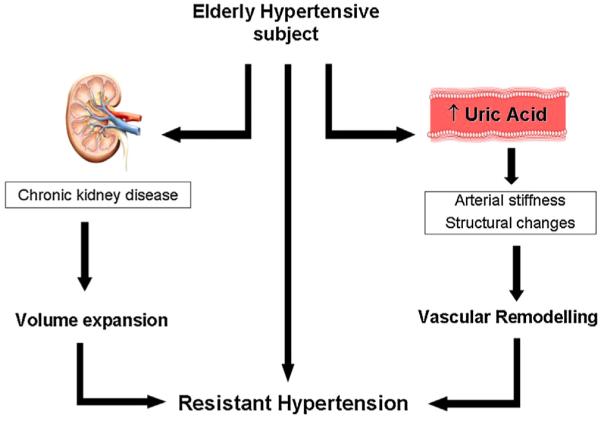
The proposed mechanisms supporting resistant hypertension development in the hypertensive elderly subjects are shown. Chronic kidney disease and hyperuricemia (i.e. serum uric acid ≥6.8 mg/dl) independently increase the risk of resistant hypertension by volume expansion (left side) and vascular remodelling respectively (right side).
Finally, among a small proportion of subjects the prevalence of uricosuric treatment was higher in those without than with hyperuricemia, which explains in part the higher level of SUA detected in the latter. We speculate that uricosuric treatment improves BP and, as a consequence, reduces the prevalence of RH. In support of our hypothesis, the literature shows a trend toward improvement associated with administration of urate-lowering drugs, in particular for the xanthine oxidase inhibitors [31,32]. The demonstrated efficacy of urate-lowering therapy on outcomes other than gout flares leads to the consideration that treatment may be beneficial even in the absence of overt gout when hyperuricemia accompanies other clinical conditions, such as CKD or cardiovascular risk factors like RH. However to confirm this hypothesis, randomized clinical trials aimed at evaluating the effect of treatment of hyper-uricemia in reducing RH prevalence are mandatory.
Acknowledgements
DMT is supported by the National Institute of General Medical Sciences (P20-GM103542-02) and the South Carolina Centers of Excellence program.
Footnotes
Conflict of interest statement
None.
References
- [1].Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J. Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- [2].De la Sierra A, Banegas JR, Oliveras A, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J. Hypertens. 2012;30:1211–1216. doi: 10.1097/HJH.0b013e328353634e. [DOI] [PubMed] [Google Scholar]
- [3].Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N. Engl. J. Med. 1966;275:457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- [4].Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, Takishita S. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa. Japan Hypertens. Res. 2004;27:835–841. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- [5].Verdecchia P, Schillaci PG, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–1078. doi: 10.1161/01.hyp.36.6.1072. [DOI] [PubMed] [Google Scholar]
- [6].Mazza A, Zamboni S, Ramazzina E, Schiavon L, Rempelou P, Zorzan S, et al. The ROVIGO study (risk of vascular complications: impact of genetics in old people): protocol, study design, and preliminary results of the initial survey: cardiovascular epidemiology in the elderly. High Blood Press Cardiovasc. Prev. 2015;22:73–78. doi: 10.1007/s40292-014-0072-1. [DOI] [PubMed] [Google Scholar]
- [7].Borghi C, Agabiti Rosei E, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- [8].Tikhonoff V, Casiglia E, Mazza A, Scarpa R, Thijs L, Pessina AC, Staessen JA. Low-density-lipoproteins cholesterol and mortality in the elderly. J. Am. Geriatr. Soc. 2005;53:2159–2164. doi: 10.1111/j.1532-5415.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- [9].Mazza A, Tikhonoff V, Schiavon VL, Casiglia E. Triglycerides + HDL-cholesterol dyslipidemia: a coronary risk factor in the elderly women: the Cardiovascular study in the elderly. Int. J. Med. 2005;35:604–610. doi: 10.1111/j.1445-5994.2005.00940.x. [DOI] [PubMed] [Google Scholar]
- [10].Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- [11].Sokolow JA, Lyon TP. The ventricular hypertrophy as obtained by unipolar or precordial and limbs lead. Am. Heart J. 1949;37:161–185. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- [12].Acelajado MC, Pisoni R, Dudenbostel T, Dell'Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S, Calhoun DA. Refractory hypertension: definition, prevalence, and patient characteristics. J. Clin. Hypertens. 2012;14:7–12. doi: 10.1111/j.1751-7176.2011.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J. Hum. Hypertens. 2014;28:213–217. doi: 10.1038/jhh.2013.77. [DOI] [PubMed] [Google Scholar]
- [14].Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann. Intern. Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- [15].Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I Epidemiologic Follow-up Study, 1971–1992. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- [16].Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- [17].Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- [18].Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal. Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- [19].Sanchez-Lozada LG, Tapia E, Soto V, Avila-Casado C, Franco M, Zhao L, Johnson RJ. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol. Dial. Transplant. 2008;23:1179–1185. doi: 10.1093/ndt/gfm783. [DOI] [PubMed] [Google Scholar]
- [20].Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, Applegate WB. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP) J. Hypertens. 2000;18:1149–1154. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- [21].Mazza A, Zamboni S, Rizzato E, Pessina AC, Tikhonoff V, Schiavon L, Casiglia E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The Cardiovascular Study in the Elderly (CASTEL) Acta Diabetol. 2007;44:99–105. doi: 10.1007/s00592-007-0249-3. [DOI] [PubMed] [Google Scholar]
- [22].Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee JJ, Ahn J, Hwang J, Han SW, Lee KN, Kim JB, Lee S, Na JO, Lim HE, Kim JW, Rha SW, Park CG, Seo HS, Oh DJ, Kim EJ. Relationship between uric acid and blood pressure in different age groups. Clin. Hypertens. 2015;21:14. doi: 10.1186/s40885-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nakanishi N, Tatara K, Nakamura K, Suzuki K. Risk factors for the incidence of hyperuricemia: a 6-year longitudinal study of middle-aged Japanese men. Int. J. Epidemiol. 1999;28:888–893. doi: 10.1093/ije/28.5.888. [DOI] [PubMed] [Google Scholar]
- [25].Kawamoto R, Tabara Y, Kohara K, Kusunoki T, Abe M, Miki T. Serum uric acid is more strongly associated with impaired fasting glucose in women than in men from a community-dwelling population. PLoS One. 2013;8:e65886. doi: 10.1371/journal.pone.0065886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Levine W, Dyer AR, Shekelle RB, Schoen berger JA, Stamler J. Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago Heart Association Detection Project in Industry. J. Clin. Epidemiol. 1989;42:257–267. doi: 10.1016/0895-4356(89)90061-9. [DOI] [PubMed] [Google Scholar]
- [27].Antón FM, García Puig L, Ramos T, González P, Ordás J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35(4):343–348. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- [28].Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015;386(10003):1588–1598. doi: 10.1016/S0140-6736(15)00418-3. [DOI] [PubMed] [Google Scholar]
- [29].Borrelli S, De Nicola L, Stanzione G, Conte G, Minatolo R. Resistant hypertension in nondialysis chronic kidney disease. Intern. J. Hypertens. 2013;2013:929183. doi: 10.1155/2013/929183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fang JI, Wu JS, Yang YC, Wang RH, Lu FH, Chang CJ. High uric acid level associated with increased arterial stiffness in apparently healthy women. Atherosclerosis. 2014;236:389–393. doi: 10.1016/j.atherosclerosis.2014.07.024. [DOI] [PubMed] [Google Scholar]
- [31].Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J. Rheumatol. 2014;41:955–962. doi: 10.3899/jrheum.131159. [DOI] [PubMed] [Google Scholar]
- [32].Hosoya T, Kimura K, Itoh S, Inaba M, Uchida S, Tomino Y, et al. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidneyx disease stage 3: study protocol for a multicenter randomized controlled study. Trials. 2014;15:26. doi: 10.1186/1745-6215-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]


