SYNOPSIS
Anal cancer is an increasingly common non-AIDS-defining cancer among HIV-infected individuals. It is associated with human papillomavirus (HPV), the most common sexually transmitted infectious agent. The 14 oncogenic types of HPV are causally associated with 5–10% of all cancers, notably anogenital cancers. HPV16 is the most common genotype detected in about 70% of anal cancers. The HPV types detected in anal cancer are included in the 9-valent vaccine. HPV vaccines have demonstrated efficacy in reducing anal precancerous lesions in HIV-infected individuals. The standard treatment for anal cancer has been fluorouracil (5-FU) and mitomycin (or cisplatin) as chemotherapy agents plus radiation, which can also be effectively used for the HIV-infected patients. Continued studies will be needed to test new treatment strategies in HIV-infected patients with anal cancer to determine which treatment protocols provide the best therapeutic index.
Keywords: HPV, HPV vaccination, anal cancer, HIV, immunosuppression
Introduction
Human papillomavirus (HPV), the most common sexually transmitted infection worldwide, causes approximately 5% of all cancers in men and 10% of all cancers in women [1]. Anal cancer is highly associated with HPV infection. Similar to cervical cancer, anal cancer is typically preceded by precursor lesions. In the general population, anal cancer is a relatively uncommon disease, only accounting for about 3% of all cancers of the gastrointestinal tract [2]. However, the incidence of anal cancer is higher in the human immunodeficiency virus (HIV)-infected population, and it is continuing to increase in the United States [3]. About 80% of all anal cancers arise from the anal canal [4]. In this article, we review the epidemiology of HPV-associated precancerous anal lesions and anal cancer in the HIV-infected population, and highlight some treatment-related issues.
Role of HPV Infection
HPV is responsible for 100% of cervical cancers and 88% of anal cancers, with the majority caused by HPV 16 or 18 [5, 6]. 98% of anal cancer tumor specimens from men who were not exclusively heterosexual were positive for HPV, with 73% harboring HPV-16 [7]. Although the majority of anal cancers associated with HPV are caused by type 16, HPV types 6, 11, and 31 account for 1.4% to 4.1%, whereas HPV 18 accounts for 3.4% to 7% [8, 9]. HPV is a small, non-enveloped, double-stranded DNA virus with over 100 different genotypes identified, of which at least 30 HPV genotypes are sexually transmitted and infect the squamous epithelium of the anogenital tract [10]. HPV is highly prevalent in the young and sexually active population since HPV is transmitted through any sexual activity that involves skin-to-skin or skin-to-mucosa contact, including vaginal, anal, and oral sex [11]. Both symptomatic and asymptomatic individuals can transmit HPV to their sexual partners [12]. Based on conservative assumptions and nationally representative data, more than 50% of sexually active women in the U.S. are estimated to have been infected by one or more genital HPV types at some point in their lifetime [13]. Heterosexual HIV-negative adult men have also been shown to have an overall HPV prevalence of approximately 50% [14]. Concordance of HPV infection between sexual partners is variable and ranges from 40% to 60%, which may be affected by length of sexual relationship, frequency of intercourse, condom use and number of lifetime sexual partners [15, 16]. The overall transmission rate from one heterosexual partner to the other over a 6-month period is estimated to be 3.7 cases per 100 person-months [17]. In a 12-month period, the probability for men to acquire a new genital HPV infection is estimated to be 0.29–0.39, which is similar to previous estimates for women [18, 19].
The transformation of HPV-infected cells to cancer cells is a multi-step process [20]. HPV infects basal cells located in the epithelial transformation zone, a region that extends proximally from the squamocolumnar junction within the rectal columnar mucosa distally to the dentate line. In this area there is active transition from columnar epithelium to squamous epithelium through the process of squamous metaplasia. Upon entry into the anal epithelium, HPV targets actively proliferating basal cells. E6 and E7 oncoproteins act to enhance cellular proliferation, resulting in increased numbers of infected cells and infectious virions [21]. A spectrum of pathologic changes may occur as a result of HPV infection. Currently, it is believed that low-risk HPV types do not cause malignancy due to weaker binding of their E6 and E7 to their target proteins, differences in promoter positioning and regulation, and pattern of mRNA splicing compared with E6 and E7 from the high-risk HPV types [22, 23].
The terminology for HPV-associated squamous lesions of the lower anogenital tract has a long history marked by various diagnostic terms derived from multiple specialties. The Lower Anogenital Squamous Terminology (LAST) project aimed to create a histopathologic nomenclature system that reflects current knowledge of HPV biology. Current data support the 2-tiered system of low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesion (HSIL) [24], which may be further qualified with the appropriate – intraepithelial neoplasia (IN) terminology for specific location. Therefore, LSIL includes condyloma and anal intraepithelial neoplasia (AIN) 1, and are not considered to be precancerous. In contrast, HSIL includes p16-positive AIN 2 and AIN 3. HSIL are considered to be the true cancer precursors [24].
Infection by multiple oncogenic types of HPV has been associated with a greater likelihood of anal HSIL in HIV-negative MSM [25]. HIV-infected individuals, regardless of HIV risk factor, have a high prevalence of HPV infection and are at higher risk for anal squamous intraepithelial lesions (ASIL) despite good virologic suppression of their HIV [26, 27]. In cross-sectional studies, anal HPV infection is almost universal among HIV-infected men who have sex with men (MSM), with reported prevalence estimates between 87 and 98% [28–30]. A prospective cohort study to assess the natural history of anal HPV infection in HIV-infected MSM in the highly active antiretroviral therapy (HAART) era showed that the incidence of any anal HPV infection and oncogenic anal HPV infection was 21.3/100 person-years and 13.3/100 person-years, respectively [31]. 20% of these men with an incident HPV infection also had more than one new HPV type detected during follow-up [31]. Low CD4 counts are a risk factor for HIV-positive individuals developing ASIL. Palefsky et al showed that, for HIV-positive men, having CD4 cell counts below 200/mm3 was associated with more than 3-fold increased incidence of progression (based on cytology and/or biopsy) of normal or atypical epithelium to ASIL, or from anal LSIL to a higher grade lesion [32].
Role of HIV/AIDS
It is estimated that there were approximately 37 million people worldwide living with HIV/AIDS at the end of 2014[33], including about 1.2 million HIV-infected individuals in the United States.[34] Approximately 1% of women and 28% of men with anal cancer also have HIV infection [35]. Cancer is estimated to be responsible for over one-third of all deaths in HIV-infected individuals [36]. The immunosuppression associated with HIV infection reduces the ability to control oncogenic viral processes, which could explain the greater risk of infection-related cancer. This hypothesis is supported by a meta-analysis by Grulich and colleagues who compared cancer incidences in population-based cohort studies of persons with HIV infection and organ transplant recipients [37].
Prior to the availability of highly active antiretroviral therapy (HAART), the estimated incidence of anal cancer among human immunodeficiency virus (HIV)-infected MSM was nearly 60-fold higher than men in the general population [38]. Since the advent of HAART, the incidence of malignancies associated with Epstein-Barr virus and Kaposi sarcoma herpesvirus has decreased in HIV-infected MSM. However, the incidence of HPV-associated anal cancer has increased (see Figure 1). A recent report from the French 2010 survey of deaths in 82,000 HIV-infected patients evaluated the underlying causes of over 700 deaths from 2000 to 2010. Non-AIDS defining cancers (NADCs) were the cause of death in 26% of the patients in the most recent period, doubling from 2000. Of the 193 NADC deaths, the commonest were bronchopulmonary malignancies (32%), hepatocellular carcinoma (17%), head and neck cancers (8%), and anal cancer (8%) [39]. In a study of 34,189 HIV-infected individuals and 114,260 HIV-uninfected individuals from 13 North American cohorts with follow-up between 1996 and 2007, the unadjusted anal cancer incidence rates per 100,000 person-years were 30 for HIV-infected women, 0 for HIV-uninfected women, 131/100,000 for HIV-infected MSM, 46/100,000 for other HIV-infected men, and 2/100,000 for HIV-uninfected men. Therefore, the incidence of anal cancer in HIV-infected MSM is now estimated to be 80 times higher than men in the general population [40]. This increase in incidence of anal cancer has been shown to be associated with the HIV epidemic in men [35].
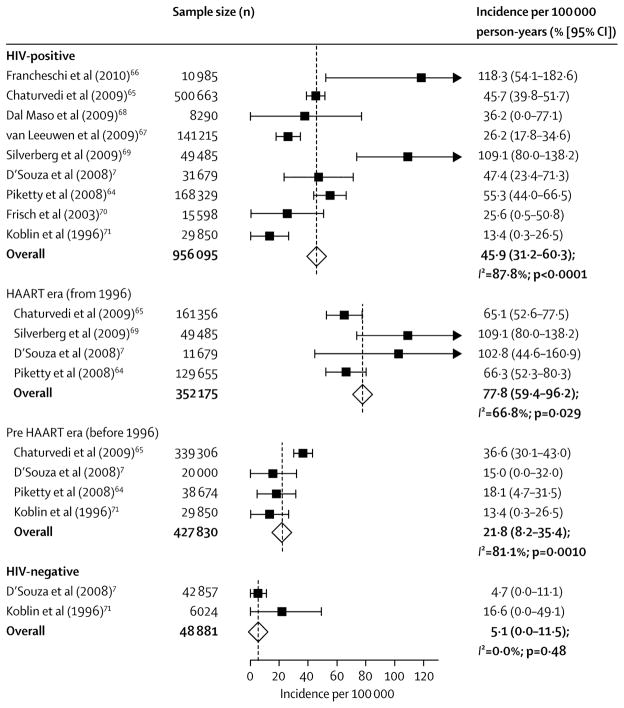
Figure 1.
Incidence of anal cancer in men who have sex with men, by HIV status. The incidence of anal cancer was higher in HIV-positive men than it was in HIV-negative men. In HIV-positive men, the incidence of anal cancer was higher from 1996 onwards (after introduction HAART) than it was before 1996. From Machalek, D.A., et al., Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol, 2012. 13(5): p. 487–500, with permission.
Immunosuppression plays a pivotal role in the pathogenesis of anal cancer. In a large French HIV cohort study, the risk of anal cancer increased with the time during which the CD4 count was < 200 cells/mm3 and viral load was > 100,000 copies/mL [41]. A recent analysis from the HIV/AIDS Cancer Match Study, a linkage of population-based state HIV and cancer registries, showed that anal cancer is the 3rd most common cancer occurring in excess in the HIV-positive population. 83% of excess cases of anal cancer occurred among men who have sex with men, and 71% among those living five or more years since AIDS onset [42].
In the general population, the risk of most cancers increases with age, including cancers frequently diagnosed in HIV-infected individuals [43]. As effective antiretroviral treatment has greatly prolonged life expectancy, the proportion of the HIV population in older age groups has increased and will likely continue increasing in the future. Yanik et al used a linkage between data from cancer registries in the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and Medicare claims (SEER-Medicare) to estimate absolute cancer risk among people age 65 years or alder with an HIV diagnosis and evaluate the association between HIV and cancer in this age group [44]. HIV was found to be associated with elevated incidence of anal cancer (adjusted harazard ratio = 34.2) among those aged 65 or older [45]. This highlights a clear need for cancer prevention in this age group and the importance of screening.
Treatment outcomes of HIV+ anal cancers
In the general population, concurrent chemoradiotherapy (CRT) with 5-fluorouracil (5-FU) infusion and mitomycin (or cisplatin) has been established as the standard-of-care regimen for non-metastatic anal cancer [46–51]. Intensity-modulated radiotherapy (IMRT) has also been shown to reduce acute toxicities compared with conventional three-dimensional radiotherapy [52, 53]. For information on treatment of anal cancer in HIV-negative patients see Craig A. Messick and Miguel Rodriguez-Bigas’s article, “Anal Dysplasia,” in this issue.
Outcomes for HIV-infected patients with anal cancer are not as well described as for HIV-negative individuals. Patients with HIV infection had been excluded from the 3 large randomized phase III trials on anal cancer (ACT I, RTOG 98-11, and ACCORD 03) because of the uncertainties regarding toxicity, compliance, and clinical outcome. When CRT was first applied to HIV-infected patients in the pre-HAART era, reduced doses of radiotherapy and/or chemotherapy were administered as a precaution against the compromised immunologic status and the presumed increased hematologic and mucosal toxicity [54, 55]. However, when therapy was applied in standard doses, increased toxicity, requiring treatment breaks or dose reductions, and poorer clinical outcome were reported [56, 57]. In five studies that included 53 patients, the incidence of grade 3–4 skin toxicity was 50–78% [54, 56–59]. Pre-treatment CD4 count less than 200 was identified as a factor associated with poorer anal cancer control and increased treatment morbidity in a small retrospective cohort [58].
In the HAART era, reports on clinical outcomes of HIV-infected patients with anal cancer have been conflicting. Immune restoration with effective suppression of HIV viral load and elevation in CD4 count could be achieved in most HIV-infected patients, with a positive impact on treatment-related side effects and compliance. Recent studies with most HIV-infected patients receiving concomitant effective HAART and CRT found no statistically significant correlation between toxicity and CD4 cell count. Blazy et al. reported that high-dose CRT with radiotherapy doses of 60 to 70 Gy with concurrent 5-FU and cisplatin is feasible [60]. Some studies show that HIV-infected patients had comparable disease control and survival to HIV-negative patients [61–65], whereas others suggested that HIV-positive patients may do worse in terms of enhanced treatment-related toxicity and/or an increased risk for local relapse [66–70]. There are no clear explanations for the differences, or lack of differences, in the outcomes of anal cancer in the HIV-infected versus the HIV-negative population. Almost all of these reports are limited by small patient numbers and the retrospective nature of the data. Wexler et al reported the local failure rate was only 16% in their cohort, but 44% of patients had T1N0 disease [65], which could reflect the fact that many of the referring providers are experienced in caring for HIV-infected individuals and more likely to examine patients for HSIL and anal cancer. In contrast, one of the largest series of anal cancer patients (total = 107, HIV-infected, HIV-negative) showed that HIV-infected patients had significantly worse overall survival and colostomy free-survival compared with a similar cohort of HIV-negative patients, despite having similar treatment approach, patient adherence, and cancer stage [70]. There were also no differences in radiation-related acute toxicity based on HIV status.
The HPV-associated E5 protein amplifies the mitogenic signals mediated by the epidermal growth factor receptor (EGFR) [71], which is broadly expressed in epithelial cancers, including SCC of the anogenital tract and oropharynx [72] [73], There is rationale, therefore, for therapeutically exploiting the association between HPV infection and anal cancer. Cetuximab is a chimeric IgG1 monoclonal antibody that binds EGFR with high specificity and with greater affinity than its ligands, thus blocking ligand-induced activation of EGFR [74]. Cetuximab prolongs survival when used in combination with radiation therapy (RT) in patients with locally advanced squamous cell carcinoma of the oropharynx [75] [76], another cancer that is typically associated with HPV infection [77–79] but not other head and neck cancers not associated with HPV [80]. Cetuximab also enhances the effectiveness of cisplatin in advanced head and neck carcinoma [81]. Based on these observations, investigators from the AIDS Malignancy Consortium (AMC) and Eastern Cooperative Oncology Group (ECOG) designed two trials that were concurrently conducted to determine the effectiveness of cetuximab plus chemoradiation (CRT) in patients with HIV infection (AMC045) and without HIV infection (E3205) [82]. CRT included cisplatin (75 mg/m2) and 5-FU (1000 mg/m2/day × 5 days) × 2 cycles plus RT (45–54 Gy), plus 2 cycles of neoadjuvant cisplatin/5-FU in the first 28 patients in E3205 prior to a study amendment. Cetuximab (400 mg/m2 IV, then 250 mg/m2 IV weekly × 8 weeks) began 1 week prior to CRT. When the two trials are considered together, a noteworthy finding is that patients with HIV infection had similar clinical outcomes as those who did not have HIV infection, with about 70% being alive and recurrence-free at 3 years. Treatment tolerance and the overall side effect profile were also similar in the two populations. These findings are consistent with population-based data indicating that although cancer specific mortality is increased in HIV-infected subjects compared with the general population for some cancers (eg, colorectal, pancreas, larynx, lung, melanoma, and breast cancer), this is not true for anal cancer [83]. These findings therefore provide additional data indicating that anal cancer in HIV-infected individuals should be treated with curative intent similar to immunocompetent individuals.
Tumor-infiltrating lymphocytes (TIL) are found in a variety of solid cancers and have been considered to be a manifestation of a host immune response directed against cancer cells. Virus-encoded antigens expressed in the neoplastic cells may represent neoantigens targeted by the immune system. In anal cancer, TIL have been demonstrated to predict overall survival and recurrence-free survival [84]. Some have hypothesized that impaired immune response in HIV-infected patients may allow anal cancer to escape surveillance and results in poorer outcomes. Clearly, the biological basis for poor cancer outcomes in HIV-infected patients requires further study.
Management issues in HIV+ patients
As HAART allows HIV-infected cohorts to live longer, with a concomitant increased incidence of non-HIV associated cancers, including anal cancer, concurrent treatment with HAART and anticancer therapy is increasingly common [85]. Extrapolating from treatment studies of HIV-associated lymphomas, concomitant use of HAART and chemotherapy is tolerable in most cases and is not associated with life-threatening toxic effects, similar to those observed in patients with cancer without HIV infection [86–88]. In HIV-infected patients receiving chemotherapy for cancer, most HAART regimens can be safely implemented to suppress viral replication. Typically, the preferred HAART regimens for HIV-infected patients are 2 nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI) (preferably boosted with ritonavir), or an integrase strand transfer inhibitor (INSTI). Recent guidelines state that INSTI-based regimens may be preferred in cancer patients receiving anticancer treatment because of their favorable drug interaction profile [89]. Zidovudine is often avoided because it commonly causes nausea, anemia, and myelosuppression, which can be potentiated by chemotherapy [90]. Tenofovir may lead to renal dysfunction, particularly in patients receiving other nephrotoxic drugs such as cisplatin. For PIs and NNRTIs, the potential for drug-drug interactions is high because these agents are extensively metabolized by and induce or inhibit the CYP450 system, which mediates the metabolism of more than half of all drugs that undergo hepatic metabolism [91]. PIs also may act as radiosensitizers by inhibiting proteasome function and causing apoptosis [92], thereby potentially increasing both tumor control and toxicity.
In HIV-infected cancer patients, as with other HIV-infected patients, CD4 count, HIV-1 RNA level, and HAART adherence should be monitored [89]. Because CD4 counts can be affected by malignancies or their treatment, CD4 count should be interpreted with caution as an indicator of immunologic response to HAART. For anal cancer patients who receive pelvic radiation, myelosuppression may be severe since the major source of bone marrow is also radiated. Specifically, the CD4+ T cell count may fall even more severely and may not readily recover to pre-treatment values. In a single institution study of 60 HIV-infected patients with anal cancer, those who received CRT with effective HAART had higher pre-treatment CD4 compared those who received CRT without HAART. However, median CD4 at 3 months after anal cancer diagnosis was more than 50% lower than their pre-treatment value, and their median CD4 at 12 months after diagnosis was only 200 cells/mm3 [61]. Scatter of radiation may also affect the gut, which is also an important compartment for CD4+ T cells [93, 94]. Another group reported that 4 patients (11%) developed opportunistic illnesses such as candida esophagitis during long-term follow-up of their anal cancer [95]. Therefore, antibiotic prophylaxis should be implemented to further reduce infectious complications during the treatment of HIV/AIDS-associated anal cancers based on careful assessment of risk.
The guidelines for prophylaxis against opportunistic infections in patients with HIV take into account risk and history of exposure, as well as the status of the immune system, particularly as reflected by the CD4 count, the receipt of and duration of HAART, and the response to HAART [96]. The guidelines for preventing of infections in patients with cancer are centered on the degree and duration of neutropenia, a key risk factor for infection [97]. CRT also potentiates the neutropenia associated with HIV/AIDS. Granulocyte colony-stimulating factors (GCSF) can reduce the effects of chemotherapy-induced neutropenia, and is often liberally used by oncologists when treating cancer in HIV-infected patients. The caveat is that GCSF should not be given concurrently with CRT due to concern for worsening hematologic toxicity [98]. The immunological deterioration following CRT may have an impact on the clinical course of the HIV disease and may be associated with an increased risk of opportunistic infections and diseases. Both the HIV-related and cancer-related guidelines need to be considered to prevent opportunistic infections in HIV-infected patients with anal cancer.
Prevention of Anal Cancer in HIV-Infected Patients
Anal cancer shares biological similarities with cervical cancer, including detectable precancerous lesions and oncogenic HPV infection. Administration of the prophylactic HPV vaccine prior to the onset of sexual activity is primary prevention tactic for cervical cancer prevention; this strategy can be mirrored for primary prevention of anal cancer. In one double-blinded trial, 602 sexually active MSM, age 16 to 26, were randomized to receive 3 doses of the quadrivalent HPV (qHPV) vaccine or placebo and evaluated every 6 months by HRA and HPV testing over 3 years. There was significant reduction of anal HSIL associated with any type of HPV (not only those associated with HPV 6, 11, 16 and 18) in those who received the qHPV compared to those who received the placebo [99]. Wilkin et al evaluated 112 HIV+ men (ages 27 or older with no evidence of anal HSIL) with the 3-dose course of qHPV vaccine and found that all of these HIV+ men seroconverted [100]. Therefore, qHPV vaccine has been demonstrated to be both immunogenic and safe in HIV-infected men. The efficacy of primary prevention of anal HSIL in HIV-infected MSM is being evaluated in an ongoing trial [101].
Deshmukh et al found that qHPV vaccination of HIV-negative MSM age 27 or older treated for anal HSIL reduced the lifetime risk of anal cancer by 60.77% at an incremental increase of cost effectiveness ratios (ICER) of US $87,240 per quality-adjusted life-year [102]. Their modeling suggests that qHPV vaccination for MSM may decreases their lifetime risk of anal cancer and is a cost-saving strategy because it decreases lifetime costs and increases quality-adjusted life expectancy. In 2015, the 9-valent (9v) HPV vaccine became available. Joura et al evaluated the safety and efficacy of the 9v HPV vaccine through a double-blind international multicenter trial of 14,215 young women randomized to 9v HPV vaccine or qHPV vaccine. The investigators found that the 9v HPV vaccine prevented infection and disease related to HPV 31, 33, 45, 52, and 58 in a susceptible population and generated an antibody response to HPV 6, 11, 16, and 18 that was non-inferior to that generated by the qHPV vaccine [103]. From these data, it is assumed that the 9v HPV vaccine will provide the same degree of protection from persistent HPV infections and development of anal HSIL (and possibly progression to anal cancer) in patients without evidence of prior vaccine-type HPV infection.
Similar to the cervical cancer screening, secondary prevention of anal cancer consists of detection and treatment of HSIL. Anal HSIL can be detected by anal cytology, digital anorectal examination, high-resolution anoscopy (HRA) and/or biopsy. Sensitivity of anal cytology is in the range of 50%–80%, with sensitivity being higher in the HIV-infected population [104]. Individuals with abnormal anal screening cytology are referred for HRA in which the anal canal is examined with a colposcope after the application of 5% acetic acid and/or Lugol’s solution and detected lesions are biopsied for histological diagnosis. Patients with histologic results of anal HSIL are recommended for treatment to prevent progression from anal HSIL to invasive cancer. However, unlike the treatment of cervical HSIL where the entire squamocolumnar junction (SCJ) of the cervix is either ablated or excised, the entire SCJ of the anal canal cannot be surgically treated for concerns of stricture or other complications.
Currently, the most commonly used treatment is HRA-directed ablation of apparent anal HSIL lesions. Unfortunately, recurrence rates are very high and frequently additional treatments are needed [105]. The Anal Cancer/HSIL Outcomes Research (ANCHOR) Study is an ongoing phase III, randomized, multi-institutional trial to determine whether treating anal HSIL is effective in reducing the incidence of anal cancer in HIV-infected men and women [106]. Although definitive efficacy data for an anal cancer screening program are lacking, the potential benefits are quite significant [107–109].
Conclusion
Given the epidemiologic relationship between HIV, HPV and anal cancer, patients with anal cancer should be screened for HIV infection. Early detection and treatment of anal HPV-related disorders in patients with HIV is a research priority and an unmet need. Clinicians treating HIV-infected patients for anal cancer need to monitor for possible and unexpected interactions between CRT and HIV disease as well as between CRT and HAART. HIV-infected anal cancer patients should be included in clinical trials of both cancer drugs and ART. In the era of HAART, anal cancer can be successfully treated in HIV-positive patients with standard CRT, with clinical outcomes similar to their HIV-negative counterparts. Careful monitoring and management of toxicities are paramount to achieving long-term survival.
KEY POINTS.
Anal cancer is preceded by precursor lesions (anal high-grade intraepithelial lesions or anal HSIL); it is also almost always associated with human papillomavirus (HPV) infection.
The degree to which highly active antiretroviral therapy (HAART) prevents the development of anal HSIL or progression of anal HSIL to anal cancer is not known.
Both of these events clearly occur in individuals well controlled on HAART and overall the incidence of anal cancer is higher in the HAART era than the pre-HAART era.
HIV-infected individuals with anal cancer can receive similar treatment as HIV-negative individuals and achieve similar outcomes, but they may require more careful monitoring for toxicities.
The use of a screening anal cytology in high-risk patients, i.e. HIV-infected MSM, may lead to an earlier diagnosis of anal HSIL, and treatment of HSIL may decrease the risk for the development of anal cancer.
Footnotes
DISCLOSURE STATEMENT
Dr. Palefsky is a member of a Merck scientific advisory board, and receives travel support form Merck. He receives grant support from Merck and Hologic. He is a consultant to Ubiome, VaxGen, Agenovir and Antiva Biosciences. Drs. Wang and Sparano have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chia-ching J. Wang, Clinical instructor, Division of Hematology/Oncology, Department of Medicine, Zuckerberg San Francisco General Hospital, San Francisco, CA
Joseph Sparano, Professor of Medicine & Women’s Health, Albert Einstein College of Medicine, Montefiore Medical Center, Department of Oncology, Bronx, NY
Joel M. Palefsky, Professor, Division of Infectious Diseases, Department of Medicine, University of California at San Francisco, San Francisco, CA
References
- 1.Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol. 2013;10(7):400–10. doi: 10.1038/nrclinonc.2013.84. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Kauh J, et al. Management of anal cancer in the HIV-positive population. Oncology (Williston Park) 2005;19(12):1634–8. discussion 1638–40, 1645 passim. [PubMed] [Google Scholar]
- 5.Arbyn M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131(9):1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 7.Daling JR, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 8.Steinau M, et al. Human papillomavirus prevalence in invasive anal cancers in the United States before vaccine introduction. J Low Genit Tract Dis. 2013;17(4):397–403. doi: 10.1097/LGT.0b013e31827ed372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zandberg DP, et al. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63(1):57–81. doi: 10.3322/caac.21167. [DOI] [PubMed] [Google Scholar]
- 10.Humans IWGotEoCRt. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 11.Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS. 2013;24(11):843–51. doi: 10.1177/0956462413481527. [DOI] [PubMed] [Google Scholar]
- 12.Barr E, Sings HL. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26(49):6244–57. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Satterwhite CL, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AR, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchell AN, et al. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010;21(1):31–7. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyitray AG, et al. Genital human papillomavirus (HPV) concordance in heterosexual couples. J Infect Dis. 2012;206(2):202–11. doi: 10.1093/infdis/jis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burchell AN, et al. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis. 2011;204(11):1723–9. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliano AR, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124(6):1251–7. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano AR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377(9769):932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egawa N, et al. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015;7(7):3863–90. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid NA, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci. 2009;66(10):1700–17. doi: 10.1007/s00018-009-8631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doorbar J, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 23.Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology. 2012;424(2):77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darragh TM, et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32(1):76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 25.Chin-Hong PV, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study. J Natl Cancer Inst. 2005;97(12):896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 26.Piketty C, et al. High prevalence of anal squamous intraepithelial lesions in HIV-positive men despite the use of highly active antiretroviral therapy. Sex Transm Dis. 2004;31(2):96–9. doi: 10.1097/01.OLQ.0000109515.75864.2B. [DOI] [PubMed] [Google Scholar]
- 27.Wilkin TJ, et al. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004;190(9):1685–91. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 28.Darwich L, et al. Prevalence, clearance, and incidence of human papillomavirus type-specific infection at the anal and penile site of HIV-infected men. Sex Transm Dis. 2013;40(8):611–8. doi: 10.1097/01.OLQ.0000430798.61475.08. [DOI] [PubMed] [Google Scholar]
- 29.de Pokomandy A, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J Infect Dis. 2009;199(7):965–73. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 30.Vajdic CM, et al. Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sex Transm Infect. 2009;85(5):330–5. doi: 10.1136/sti.2008.034744. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez AL, et al. Incidence of and risk factors for type-specific anal human papillomavirus infection among HIV-positive MSM. AIDS. 2014;28(9):1341–9. doi: 10.1097/QAD.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palefsky JM, et al. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(4):314–9. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 33.UNAIDS. Fact Sheet 2015. 2015 http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- 34.Control, C.f.D. HIIV in the United States: At A Glance. 2015 http://www.cdc.gov/hiv/statistics/overview/ataglance.html.
- 35.Shiels MS, et al. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104(20):1591–8. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet F, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009;48(5):633–9. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 37.Grulich AE, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 38.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 39.Morlat P, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014;28(8):1181–91. doi: 10.1097/QAD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 40.Silverberg MJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guiguet M, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 42.Robbins HA, et al. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howlader N, et al. SEER Cancer Statistics Review, 1975–2012. Based on November 2014 SEER data submission. National Cancer Institute; Bethesda, MD: [Google Scholar]
- 44.Engels EA, et al. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol. 2011;174(7):860–70. doi: 10.1093/aje/kwr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanik EL, Katki HA, Engels EA. Cancer Risk among the HIV-Infected Elderly in the United States. AIDS. 2016 doi: 10.1097/QAD.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348(9034):1049–54. [PubMed] [Google Scholar]
- 47.Ajani JA, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299(16):1914–21. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 48.Flam M, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 49.Gunderson LL, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30(35):4344–51. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James RD, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14(6):516–24. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 51.Northover J, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102(7):1123–8. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuong MD, et al. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res. 2013;6(2):39–45. [PMC free article] [PubMed] [Google Scholar]
- 53.Kachnic LA, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chadha M, et al. Squamous-cell carcinoma of the anus in HIV-positive patients. Dis Colon Rectum. 1994;37(9):861–5. doi: 10.1007/BF02052589. [DOI] [PubMed] [Google Scholar]
- 55.Peddada AV, et al. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1997;37(5):1101–5. doi: 10.1016/s0360-3016(96)00596-2. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum. 2001;44(10):1496–502. doi: 10.1007/BF02234605. [DOI] [PubMed] [Google Scholar]
- 57.Place RJ, et al. Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum. 2001;44(4):506–12. doi: 10.1007/BF02234322. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman R, et al. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys. 1999;44(1):127–31. doi: 10.1016/s0360-3016(98)00528-8. [DOI] [PubMed] [Google Scholar]
- 59.Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology. 1994;193(1):251–4. doi: 10.1148/radiology.193.1.8090901. [DOI] [PubMed] [Google Scholar]
- 60.Blazy A, et al. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48(6):1176–81. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 61.Alfa-Wali M, et al. Chemoradiotherapy for anal cancer in HIV patients causes prolonged CD4 cell count suppression. Ann Oncol. 2012;23(1):141–7. doi: 10.1093/annonc/mdr050. [DOI] [PubMed] [Google Scholar]
- 62.Chiao EY, et al. Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26(3):474–9. doi: 10.1200/JCO.2007.14.2810. [DOI] [PubMed] [Google Scholar]
- 63.Fraunholz I, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for anal carcinoma: are there differences between HIV-positive and HIV-negative patients in the era of highly active antiretroviral therapy? Radiother Oncol. 2011;98(1):99–104. doi: 10.1016/j.radonc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Seo Y, et al. Outcomes of chemoradiotherapy with 5-Fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75(1):143–9. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 65.Wexler A, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51(1):73–81. doi: 10.1007/s10350-007-9154-7. [DOI] [PubMed] [Google Scholar]
- 66.Hogg ME, et al. HIV and anal cancer outcomes: a single institution’s experience. Dis Colon Rectum. 2009;52(5):891–7. doi: 10.1007/DCR.0b013e31819eefa6. [DOI] [PubMed] [Google Scholar]
- 67.Munoz-Bongrand N, et al. Anal carcinoma in HIV-infected patients in the era of antiretroviral therapy: a comparative study. Dis Colon Rectum. 2011;54(6):729–35. doi: 10.1007/DCR.0b013e3182137de9. [DOI] [PubMed] [Google Scholar]
- 68.Oehler-Janne C, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26(15):2550–7. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 69.Meyer JE, et al. HIV positivity but not HPV/p16 status is associated with higher recurrence rate in anal cancer. J Gastrointest Cancer. 2013;44(4):450–5. doi: 10.1007/s12029-013-9543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grew D, et al. HIV Infection Is Associated With Poor Outcomes for Patients With Anal Cancer in the Highly Active Antiretroviral Therapy Era. Dis Colon Rectum. 2015;58(12):1130–6. doi: 10.1097/DCR.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 71.Tsai TC, Chen SL. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch Virol. 2003;148(8):1445–53. doi: 10.1007/s00705-003-0111-z. [DOI] [PubMed] [Google Scholar]
- 72.Le LH, Chetty R, Moore MJ. Epidermal growth factor receptor expression in anal canal carcinoma. Am J Clin Pathol. 2005;124(1):20–3. doi: 10.1309/X4UADHVN317V2XMW. [DOI] [PubMed] [Google Scholar]
- 73.Paliga A, et al. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors? Br J Cancer. 2012;107(11):1864–8. doi: 10.1038/bjc.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67(17):2585–607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 75.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 76.Bonner JA, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 77.Mork J, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 78.Herrero R, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 79.D’Souza G, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 80.Termine N, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007) Ann Oncol. 2008;19(10):1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 81.Burtness B, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 82.Garg M, Lee JY, Kachnic LA, Catalano PJ, Henry DH, Cooley TP, Ratner L, Wachsman W, Aboulafia DM, Benson AB, Palefsky J, Whittington R, Mitsuyasu RT, Sparano JA. Phase II trials of cetuximab (CX) plus cisplatin (CDDP), 5fluorouracil (5FU) and radiation (RT) in immunocompetent (ECOG 3205) and HIV positive (AMC045) patients with squamous cell carcinoma of the anal canal (SCAC): Safety and preliminary efficacy results. J Clin Oncol (suppl; abstr 4030) 2012:30. [Google Scholar]
- 83.Coghill AE, et al. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–83. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grabenbauer GG, et al. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12(11 Pt 1):3355–60. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 85.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011;12(9):905–12. doi: 10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montoto S, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30(33):4111–6. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ratner L, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol. 2001;19(8):2171–8. doi: 10.1200/JCO.2001.19.8.2171. [DOI] [PubMed] [Google Scholar]
- 88.Vaccher E, et al. Concomitant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy plus highly active antiretroviral therapy in patients with human immunodeficiency virus-related, non-Hodgkin lymphoma. Cancer. 2001;91(1):155–63. doi: 10.1002/1097-0142(20010101)91:1<155::aid-cncr20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 89.Gunthard HF, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312(4):410–25. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 90.Margolis AM, et al. A review of the toxicity of HIV medications. J Med Toxicol. 2014;10(1):26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudek MA, et al. A phase 1/pharmacokinetic study of sunitinib in combination with highly active antiretroviral therapy in human immunodeficiency virus-positive patients with cancer: AIDS Malignancy Consortium trial AMC 061. Cancer. 2014;120(8):1194–202. doi: 10.1002/cncr.28554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pajonk F, et al. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62(18):5230–5. [PubMed] [Google Scholar]
- 93.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 94.Chun TW, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5(6):651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 95.Fraunholz IB, et al. Long-term effects of chemoradiotherapy for anal cancer in patients with HIV infection: oncological outcomes, immunological status, and the clinical course of the HIV disease. Dis Colon Rectum. 2014;57(4):423–31. doi: 10.1097/DCR.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 96.Kaplan JE, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1–4. [PubMed] [Google Scholar]
- 97.Segal BH, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6(2):122–74. doi: 10.6004/jnccn.2008.0013. [DOI] [PubMed] [Google Scholar]
- 98.Bunn PA, Jr, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol. 1995;13(7):1632–41. doi: 10.1200/JCO.1995.13.7.1632. [DOI] [PubMed] [Google Scholar]
- 99.Palefsky JM, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 100.Wilkin T, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.AIDS Malignancy Consortium. Vaccine Therapy in Preventing Human Papillomavirus Infection in Young HIV-Positive Male Patients Who Have Sex With Males. clinicaltrials.gov [Internet] [cited 2016 March 18]; Available from: https://clinicaltrials.gov/ct2/show/NCT01209325.
- 102.Deshmukh AA, et al. Long-Term Outcomes of Adding HPV Vaccine to the Anal Intraepithelial Neoplasia Treatment Regimen in HIV-Positive Men Who Have Sex With Men. Clin Infect Dis. 2015;61(10):1527–35. doi: 10.1093/cid/civ628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joura EA, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 104.Uronis HE, Bendell JC. Anal cancer: an overview. Oncologist. 2007;12(5):524–34. doi: 10.1634/theoncologist.12-5-524. [DOI] [PubMed] [Google Scholar]
- 105.Stier EA, Chigurupati NL, Fung L. Prophylactic HPV vaccination and anal cancer. Hum Vaccin Immunother. 2016:0. doi: 10.1080/21645515.2016.1149274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.AIDS Malignancy Consortium. Topical or Ablative Treatment in Preventing Anal Cancer in Patients With HIV and Anal High-Grade Squamous Intraepithelial Lesions. clinicaltrials.gov [Internet] [cited 2016 March 18]; Available from: https://clinicaltrials.gov/show/NCT02135419.
- 107.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Dis Colon Rectum. 2014;57(3):316–23. doi: 10.1097/DCR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 108.Park IU, Palefsky JM. Evaluation and Management of Anal Intraepithelial Neoplasia in HIV-Negative and HIV-Positive Men Who Have Sex with Men. Curr Infect Dis Rep. 2010;12(2):126–33. doi: 10.1007/s11908-010-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scholefield JH, Harris D, Radcliffe A. Guidelines for management of anal intraepithelial neoplasia. Colorectal Dis. 2011;13(Suppl 1):3–10. doi: 10.1111/j.1463-1318.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- 110.Machalek DA, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]