Abstract
Purpose of the review
This review highlights recent developments into how intercellular communication through connexin43 facilitates bone modeling and remodeling.
Recent Findings
Connexin43 is required for both skeletal development and maintenance, particularly in cortical bone, where it carries out multiple functions, including preventing osteoclastogenesis, restraining osteoprogenitor proliferation, promoting osteoblast differentiation, coordinating organized collagen matrix deposition, and maintaining osteocyte survival. Emerging data shows that connexin43 regulates both the exchange of small molecules among osteoblast lineage cells and also the docking of signaling proteins to the gap junction, affecting the efficiency of signal transduction.
Summary
Understanding how and what connexin43 communicates to coordinate tissue remodeling has therapeutic implications in bone. Altering the information shared by intercellular communication and/or targeting the recruitment of signaling machinery to the gap junction could be used to impact the skeletal homeostatic set point, either driving osteogenesis or inhibiting resorption.
Keywords: connexin, intercellular communication, signal transduction, osteoblast, osteocyte
I. Bone is a tissue requiring exquisite intercellular coordination
Bone is a dynamically regulated tissue involving cross-talk between cells to coordinate tissue formation, remodeling, and repair. As such, the cells of bone form an extensive interconnected network. Bone embedded osteocytes link up with each other and with surface osteoblasts and osteoprogenitors via long dendritic processes that pass through the bone canalicular system. These osteocytes orchestrate the tightly controlled process of bone remodeling by directing the differentiation and activity of surface osteoblasts and osteoclasts to synthesize, resorb, and repair skeletal tissue (1–3). In the absence of tight coordination of this networks of cells, bone quality can be diminished, leading to skeletal fragility.
II. Gap junctions: conduits on the information superhighway
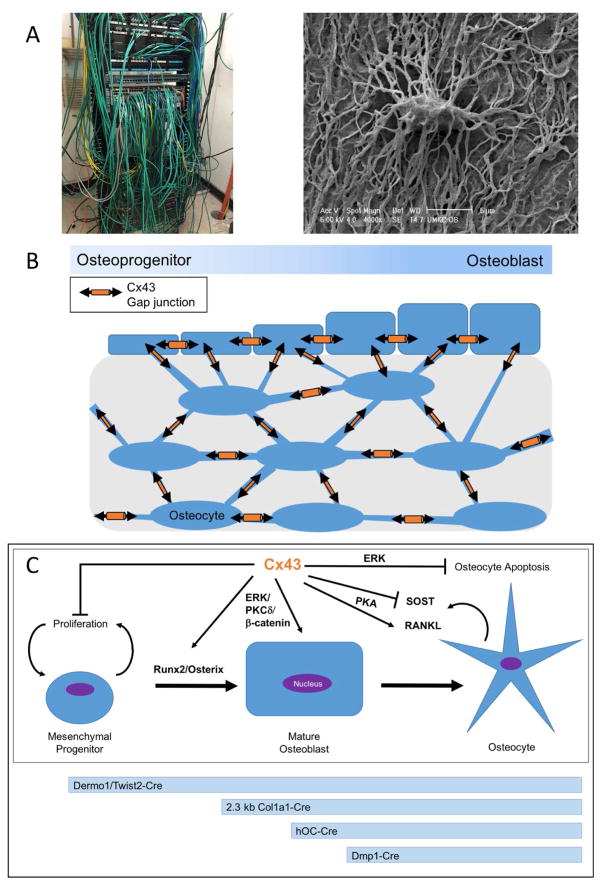
One way bone cells coordinate their actions is by intercellular communication through gap junctions. Gap junctions form aqueous channels that allow the direct exchange of small ions, molecules, and second messengers between cells. This allows for efficient intercellular communication and functional coordination through an interconnected cellular network. A useful analogy is to think of the gap junction-coupled cellular network as a telecommunications network, with gap junctions being the point of data exchange between components that allow efficient communication, comparable perhaps to the modem/router. To extend this analogy in bone, the osteocytic cell processes are comparable to the cables and wires connecting the different cells of the bone communication network (Figure 1A–B). At the intersection of two osteocyte cell processes or where osteocytes and osteoblast lineage cells touch, gap junctions form and facilitate direct cell-to-cell communication. The osteocyte itself would be comparable to the server, sending out information to the workstations (e.g., osteoblasts and their progenitors) on the bone surface. As such, the gap junction plays important roles in permitting, regulating and responding to the shared data in the form of small molecules, ions, and second messengers.
Figure 1.
A. Left, Image of IT network cables. Right, back-scatter scanning electron micrograph of an acid-etched resin embedded murine femoral cortical bone sample showing a resin-filled osteocyte lacunae and the many resin filled osteocyte canaliculi (Image provided by Lynda Bonewald, IUPUI). We propose the basic analogy of the osteocyte-osteoblast network to a telecommunication/IT network. B. Model of the interconnected cellular network within bone by gap junctions, containing osteoprogenitors, osteoblasts, and osteocytes. C. Top, osteoblastogenesis model and the influence of connexin43 on various aspects of the differentiation/survival program of these cells. Below, timeline of expression of promoters used in osteoblast-lineage conditional knockout models.
It is becoming increasingly accepted that gap junctions regulate the data they permit to be communicated and acted on in numerous ways, including the monomeric composition of the gap junction channel, the relative abundance of the connexins, and the local recruitment of signaling machinery to the gap junction plaque. Gap junctions form from the assembly of connexin monomers. Each connexin has four trans-membrane domains, 2 extracellular loops, and intracellular N and C terminal domains. Connexin protein hexamers in the plasma membrane form a hemi-channel, also called a connexon. When a hemichannel in one cell aligns with a hemichannel in a neighboring cell, a gap junction pore is formed, creating an aqueous channel between the cytoplasm of the coupled cells. These gap junctions aggregate into larger gap junction plaques, composed of hundreds to thousands of gap junction channels (4, 5). Humans have 21 genes encoding connexin proteins, and the specific connexin composition of a gap junction influences the size and charge selective permeability as well as the open/closed state probability of the channel (6, 7). Further, most tissues, including bone, express multiple connexins and these can interact to form both hexameric (e.g., mixed channels of connexin43 and connexin45) and heterotypic gap junctions (e.g., a connexin43 containing hemichannel docked to a connexin45 hemichannel) (8). Thus, there is great plasticity in the molecules that can be exchanged by gap junctions. Another way that gap junctions can regulate intercellular communication is by regulating the relative abundance of connexins in a plaque, effectively tuning the bandwidth of the communication network with increasing gap junctions enhancing the efficiency of communication (9). Furthermore, emerging data support a model in which gap junctions not only serve as passive conduits for the intercellular exchange of small molecules, but gap junction also assemble large complexes of signaling machinery. These signaling complexes not only regulate the open/closed state probability of the channel (affecting bandwidth), but may also serve as a docking scaffold for recruiting effectors of downstream signaling (10–12). As with any effective communication, there must be a process to receive the communicated information. This pre-assembled complex of signaling machinery at the connexin43 plaque may serve this purpose. In addition, the assembly of specific signaling effectors to the gap junction permits even more specificity and control in regulating the data exchanged through this intercellular network.
III. Gap Junctions in bone: the skeletal information network
Connexin43 is the most abundant (and most studied) connexin present in the osteoblast lineage and allows passages of molecules and second messengers less than ~1.2 kDa, with a preference for negatively charged molecules (13–16). There is growing evidence that connexin43 may be able to pass larger molecules, including siRNA or miRNAs with shapes permissive of traversing the gap junction channel (17, 18). Other connexins are also expressed in osteoblast lineage cells, including connexin45, connexin46 and connexin37 (19–22), but a critical role for these other connexins in the osteoblast lineage has not been established. The gap junctions that form from connexin monomers occur not only between osteoblasts and osteoprogenitors on the bone surface, but also at the long dendritic cell processes of osteocytes that extends through the canalicular network. The result is a functional syncytium of cells throughout bone. It is through this network of cells that information is passed between cells in a gap junction-dependent manner to affect bone formation and resorption to ultimately impact bone quality. While much of what is known about connexin biology in the skeleton has been studied in cells of the osteoblast lineage, osteoclasts also express several gap junction proteins, including connexin37 and connexin43 (22–24). A skeletal phenotype has been reported in osteoclast-lineage knockouts connexin37 in vivo (22). Interestingly, despite overlap in connexin expression, there is little evidence that the osteoblasts and osteoclasts directly communicate via gap junctions. Since the connexin in these bone resorbing osteoclasts do not appear to be directly plugged in to the intercellular communication syncytium of osteoblasts, osteocytes and their progenitors, we will not focus on them here.
IV. Taking the bone cellular network offline: models of connexin-mutation or deficiency
The fundamental observations that connexins, and connexin43 in particular, play an important role in bone function has come from several loss of function studies performed in animal models. These findings have been confirmed by the observation of a role for connexin43 in human diseases with musculoskeletal involvement, including oculodentodigital dysplasia and craniometaphyseal dysplasia (25, 26). When the bone intercellular communication network is “taken offline” by connexin43 gene deletion in healthy mice, the consequences for the skeleton are reproducibly poor. Connexin43 global knock out mice exhibit delayed mineralization and brittle bones in the axial and appendicular skeleton, craniofacial abnormalities and a cell autonomous osteoblast dysfunction, including a failure to progress through differentiation (27). These mice die at birth due to a cardiac defect (28). This role for connexin43 in bone is evolutionarily conserved in zebrafish, in which gain and loss of function of connexin43 regulate fin ray segment length (29–32).
In order to assess post-natal connexin43 gene deletion, conditional deletion knockout mouse models have been generated using four different promoters to drive Cre recombinase at progressively later stages of osteoblast differentiation (Figure 1C). The Dermo1/Twist2-Cre mouse line was used to conditionally delete connexin43 in cells of the early chondro-osteoblast lineage (cKOTW2) (33). cKOTW2 mice have smaller and hypo-mineralized skulls at birth, reduced chest cavity from shortened ribs, and a 12% reduction in whole body BMD (bone mineral density). cKOTW2 mice have a striking cortical, but no trabecular phenotype, including a more than 40% increase in cross sectional area of the long bones at the mid-diaphysis. This is a result of increased periosteal osteoblast activity, leading to periosteal expansion; cortical thinning, and increased marrow cavity area due to a concomitant increase in endocortical osteoclast activation. This demonstrated that deletion of connexin43 early in the osteoblast lineage not only affects the activity of osteoblasts, which had a cell autonomous differentiation defect, enhanced proliferation and produced a disorganized collagenous matrix, but can also affect osteoclast formation and function. Similar, slightly milder results were observed using the 2.3 kb Col1a1-Cre mice to genetically delete connexin43 at a later stage in the osteoblast-lineage (cKOCol1; Figure 1C). cKOCol1 mice have a 5% reduction whole body BMD, cortical thinning, and a 20% increase in the cross sectional area at the diaphysis (34, 35). These animals had a similar cell autonomous osteoblast differentiation defect and enhanced support of osteoclastogenesis (34). Indeed, much of the cortical phenotype could be restored by administering anti-resorptive bisphosphonates to these animals, indicating that the role of conneixn43 in bone is not only to coordinate osteoblast differentiation and function, but also that communicating networks of bone cells are needed to suppress osteoclast activation (36). Conditional knock outs using the human osteocalcin-Cre mice (cKOhOC) allowed connexin43 knock out to be restricted to mineralizing (late-stage) osteoblasts and osteocytes (Figure 1C). Consistent with the progressively less pronounced skeletal phenotype when connexin43 deletion occurred later in the osteoblast differentiation stage, these cKOhOC mice had only slightly diminished BMD (albeit only ~4%) and only a ~25% increase in cross sectional area at the diaphysis, but without cortical thinning in cKOhOC mice compared to wild type mice (37, 38). The fourth conditional knock out model was generated using the 8 kb DMP1-Cre mice (cKODMP1), which is expressed almost exclusively in mature osteoblasts/osteocytes (Figure 1C) (37, 38). The cKODMP1 mice have no cortical thinning or decrease in whole body BMD along with no trabecular phenotype. cKODMP1 mice do exhibit a 20% increase in diaphysis cross-sectional area resulting from increased osteoclast number. This model also exhibits a disorganized collagenous extracellular matrix with inferior material properties (38, 39). Enhanced osteocyte apoptosis was observed in this model, underscoring an additional role for connexin43 in osteocyte cell survival (37). Notably, mouse models of oculodentodigital dysplasia, a disease caused by loss of function mutations in connexin43, have largely recapitulated the findings of connexin43 conditional deletion models, including osteopenia, an expanded cortical cross sectional area with a widened marrow cavity, and bone with impaired material properties (33, 40–42).
A common theme in these models of connexin43 deficiency in the osteoblastic lineage is reduced bone mass, cortical widening, and reduced cortical thickness. Another consistent finding is an increase in bone resorption, caused by alterations in the production of osteoclastogenic factors by osteoblasts and osteocytes. These findings confirm connexin43 as an important regulator of osteoblastogenesis and the production of osteoclastogenic factors by osteoblasts and osteocytes, especially in cortical bone. It is also notable that aspects of the phenotype are more pronounced the earlier in differentiation that connexin43 is deleted. The demonstration of the altered proliferative capacity of osteo-progenitors in the cKOTW2 model is unique among the osteoblast-lineage conditional knockout models and implies that connexin43 plays a role in restraining proliferation in early osteoprogenitor populations (Figure 1C) (33). A cell autonomous defect in osteoblast differentiation was exclusive to the models of connexin43 deletion early in the lineage (i.e., cKOCol1 and cKOTW2) (Figure 1C) (33, 34). In the cKODMP1 model, connexin43 expression is obligated for osteocyte cell survival, as osteocyte apoptosis was substantially increased in connexin43 deleted cells (Figure 1C) (37). A similar observation of connexin43-dependent support of osteocyte survival was made in cKOhOC mice (43). In addition, loss of connexin43 in this model promoted osteocytic osteolysis in this model (44). Interestingly, aspects of the skeletal phenotypes in these mice are similar to osteocyte ablation studies (e.g., fragile bones with increased cortical porosity, decreased cortical BMD, and osteoblast dysfunction) (45–48). A computer simulation of the intercellular communication between osteocytes and bone cells on the bone surface predicted that even a modest 5–9% level of osteocyte apoptosis would have a 25–37% reduction in the signaling received at the cell surface (49). This suggests that at least some signals communicated through this cellular network travel through osteocytes to target the effector cells (i.e., osteoblasts, osteoclasts and their progenitors). Not only that, the osteoblast-stage specific progressive skeletal phenotype supports a role for connexin43 throughout the lineage, including osteo-progenitors, osteoblasts, and osteocytes, underscoring bone as a coordinated remodeling unit. The effects of defective intercellular communication caused by loss of connexin43 in bone become more complex in aging, loading and disuse (reviewed (50–52)). Thus, it is clear that data exchanged through this intercellular communication network is dynamic. The signals passed in a healthy animals likely fundamentally differ from those in an aged animal, under mechanical load stimuli, or in disuse. Understanding the code of communicating anabolic and catabolic data signals will allow us to hack the systems to modulate bone responses.
V. Connexin43’s mechanisms of action: identifying data packets and downstream effectors
The need for this elaborate communication network in bone is most evident in the coordinated bone formation process, in which hundreds of osteoblasts in a local area become activated to deposit and mineralize the extracellular matrix. One of the key roles of connexin43 in this process is likely the ability to detect, amplify and coordinately respond to subtle signals relative to background noise. This concept has been beautifully demonstrated in mammary epithelial cells, where gap junctional communication among cells was shown to enable detection of shallow concentration gradients of epidermal growth factor that could not be sensed by a single cell by improving the efficiency of the signaling relay among cells (53). In this context, the exchanged data was in the form of Ca2+ signaling. Indeed, bone cell networks of osteocytes and osteoblasts have been shown to exchange Ca2+-dependent signals in response to hormonal, growth factor and mechanical cues (54–57). But, Ca2+ is not the only data that this network exchanges. Connexin43 has been shown to be involved directly or indirectly in the regulation of signaling cascades involving low molecular weight second messengers, like cAMP, inositol polyphosphates and ATP release (58–61). Interestingly, ATP release and the release of prostaglandin E2 have been shown to occur through unpaired hemichannels of connexin43 (60, 62, 63). In a transgenic model of mutant connexin43 overexpression in osteocytes, a dominant negative connexin43 construct (connexin43 Δ130–136) that blocked both gap junctions and hemichannels recapitulated the increase in cortical cross-sectional area, endocortical resorption and osteocyte apoptosis that is observed in models of connexin43 deficiency (64). These phenotypes were not observed when a dominant negative construct (connexin43 R76W) that only blocked gap junctional communication, not hemichannels was expressed in osteocytes. These data strongly indicate that connexin43 hemichannel activity is a driving force behind osteocytic signaling in this context. This interpretation is not without controversy as closely related pannexin channels have also been implicated in ATP release from these cells (65).
The downstream effectors of connexin43 in bone include various signaling pathways, including ERK, PKCδ, β-catenin and protein kinase A (9, 58, 66–70) (Figure 1C). The connexin43-dependent effect on ERK and PKCδ regulate the transcriptional activity of Runx2, a master regulator of osteoblast differentiation, with increased connexin43 levels promoting Runx2 activity driving the expression of several osteoblast genes and vice versa (Figure 1C) (9, 59, 66, 67). Similarly, the connexin43-dependent effect on ERK impact the recruitment and activity of the transcriptional activator Sp1, the required osteoblast transcription factor, osterix/Sp7, and osteocyte survival (Figure 1C) (68, 71–74). Loss of osteocyte connexin43 has been shown to increase β-catenin, which has been speculated to prime endocortical bone formation in response to mechanical load (70). Communication of cAMP through connexin43 gap junctions activates protein kinase A-dependent cascades to increase expression of the pro-osteoclastogenic signal, RANKL, and suppress sclerostin, an inhibitor of osteogenesis through the Wnt/ β-catenin pathway (Figure 1C) (58). Consistent with this finding, loss of connexin43 in vivo has been shown to attenuate the bone anabolic actions of parathyroid hormone, which acts in part via the cAMP/ protein kinase pathway (34). The role of connexin43 in this effect appears to be more in the osteoblasts than osteocytes, as this effect is observed in connexin43 deficiency in osteoblasts and osteocytes (cKOCol1), but not in cKODMP1 mice lacking connexin43 only in the mature osteoblast/osteocyte compartment (34, 39).
Undoubtedly, these second messengers and signaling pathways are not comprehensive, nor is it likely they are all operant under all conditions. Different stimuli are likely to elicit very different second messengers and signaling cascades. Indeed, such diversity in signals likely explains the differential roles for connexin43 in load, aging, disuse, hormonal signal, fracture healing, and even differences in function on the periosteal versus endocortical surfaces of bone. Regardless, in healthy adult animals, the role of connexin43 in the osteoblast lineage seems to be to inhibit osteoclastogenesis, suppress osteoblast proliferation, support early osteogenic differentiation, and promote osteocyte survival (Figure 1C). In the absence of connexin43, there is an expansion of the progenitor pool and a bottleneck as these cells fail to differentiate effectively, this combined with the deposition of a disorganized collagenous extracellular matrix leads to poor bone quality.
VI. Connexin43 as a docking platform: a firewall for regulating which communicated data is acted on
While gap junctions are generally thought of as passive conduits for small molecules to be shared between cells, growing evidence indicates that connexins actively contribute to downstream signaling. Numerous protein complexes, including kinases associated with signal transduction cascades, assemble on the connexin43 C-terminus (10–12). This led to the intriguing notion that, in order for efficient signaling to occur following the exchange of small molecules through the gap junction pore, the signal machinery must be spatially recruited to the gap junction plaque to be available to initiate a response. This also implies an additional level of control where data can be communicated, but not responded to if the appropriate signaling machinery is not present at the gap junction plaque. In support of this model, several of the signaling molecules involved in osteoblasts and osteocyte signaling have been shown to bind to the connexin43 C-terminus. In MC3T3 osteoblasts, PKCδ transiently binds to the connexin43 C-terminus and upon stimulation translocates to the nucleus where it can regulate Runx2 transcriptional activity (59). In OB-6 cells, interactions between connexin43 C-terminus and β-arrestin allow survival from apoptosis from PTH, by sequestering β-arrestin and allowing cAMP-dependent cascades to be activated (69). Lastly, in MLO-Y4 osteocyte like cells, the connexin43 C-terminus interacts with α5β1 integrin, which controls opening and closing of the hemi channels by causing a conformational change in the extracellular domain of the integrin in response to mechanical load, leading to activation of AKT signaling (75).
Further validating this model of the local recruitment of signaling machinery to the connexin43 C-terminus for efficient signal transduction, the connexin43 C-terminus is required (necessary but not sufficient) for the efficient activation of the ERK and PKCδ signaling cascades, activation of Runx2 transcriptional activity, and the expression of osteoblast genes (9). An in vivo model of connexin43 C-terminal truncation (connexin43 K258Stop) had reduced trabecular bone, but surprisingly no changes in cortical cross sectional area or marrow area in female mice (76). Careful inspection did reveal a cortical phenotype in these mice that included decreased crystallinity and fracture toughness (77).
VII. Hacking the network: modulating communicated molecules to impact bone quality
Bone forms an elaborate communication network with osteoblast, osteocytes and osteoprogenitors sharing data. The communication of this data throughout the network plays a key role in how bone modeling and remodeling occurs. It has become clear that taking the cellular network offline by loss of connexin43 function has negative consequences for the skeleton, at least in healthy mice. But the mechanisms leading to these effects are less clear. We have only begun to scratch the surface of the actual data being communicated through these cells. Cracking the code of the data passing through this network will have obvious implications into bone therapeutic targets. For example, if we can hack the communication network to communicate data informing the skeleton that it is under load, even during disuse, we can have a profound impact on skeletal fragility and the maintenance of bone mass. The emerging data that this communicated data is diverse, with multiple targets, acting differently in distinct skeletal compartments, and in response to a broad array of extracellular cues only enhances the need to understand this fundamental process in bone homeostasis.
Acknowledgments
This work was supported by a grant, R01-AR063631 (JPS) from the National Institutes of Health/National Institute for Arthritis, Musculoskeletal and Skin Diseases. We thank Lynda Bonewald (Indiana University-Purdue University Indianapolis) for providing the EM image of the osteocyte lacunae-canalicular network.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Joseph Stains and Megan Moorer declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
• • Of major importance
- 1.Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol. 2016;12(10):593–605. doi: 10.1038/nrendo.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prideaux M, Findlay DM, Atkins GJ. Osteocytes: The master cells in bone remodelling. Curr Opin Pharmacol. 2016;28:24–30. doi: 10.1016/j.coph.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNutt NS, Weinstein RS. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970;47(3):666–88. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296(5567):503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 6.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62(2):228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588(8):1423–9. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koval M, Molina SA, Burt JM. Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014;588(8):1193–204. doi: 10.1016/j.febslet.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Hebert C, Stains JP. An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells. J Cell Biochem. 2013;114(11):2542–50. doi: 10.1002/jcb.24603. Demonstrates the necessary, but insufficient role for the connexin43 C-terminus in regulating the downstream effects of ERK1/2 and PKCδ In vitro, setting the stage for the notion of Cx43 as a docking platform for signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herve JC, Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell and tissue research. 2013;352(1):21–31. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- 11.Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818(8):1844–65. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2012;1818(8):1831–43. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91(5):1888–96. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schirrmacher K, Schmitz I, Winterhager E, Traub O, Brummer F, Jones D, et al. Characterization of gap junctions between osteoblast-like cells in culture. Calcif Tissue Int. 1992;51(4):285–90. doi: 10.1007/BF00334489. [DOI] [PubMed] [Google Scholar]
- 15.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129(3):805–17. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SJ, Gray C, Sakamaki H, Arora M, Boyde A, Gourdie R, et al. The incidence and size of gap junctions between the bone cells in rat calvaria. Anatomy and embryology. 1993;187(4):343–52. doi: 10.1007/BF00185892. [DOI] [PubMed] [Google Scholar]
- 17.Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver? Biochim Biophys Acta. 2012;1818(8):2076–81. doi: 10.1016/j.bbamem.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Lemcke H, Steinhoff G, David R. Gap junctional shuttling of miRNA--A novel pathway of intercellular gene regulation and its prospects in clinical application. Cell Signal. 2015;27(12):2506–14. doi: 10.1016/j.cellsig.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, et al. Defective vascular development in connexin 45-deficient mice. Development. 2000;127(19):4179–93. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994;13(4):744–50. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137(4):847–57. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, et al. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J Biol Chem. 2014;289(12):8508–20. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schilling AF, Filke S, Lange T, Gebauer M, Brink S, Baranowsky A, et al. Gap junctional communication in human osteoclasts in vitro and in vivo. J Cell Mol Med. 2008;12(6A):2497–504. doi: 10.1111/j.1582-4934.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilvesaro J, Vaananen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Miner Res. 2000;15(5):919–26. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 25.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72(2):408–18. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Chen IP, de Almeida S, Tiziani V, Do Amaral CM, Gowrishankar K, et al. A novel autosomal recessive GJA1 missense mutation linked to Craniometaphyseal dysplasia. PLoS One. 2013;8(8):e73576. doi: 10.1371/journal.pone.0073576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151(4):931–44. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 29.Misu A, Yamanaka H, Aramaki T, Kondo S, Skerrett IM, Iovine MK, et al. Two Different Functions of Connexin43 Confer Two Different Bone Phenotypes in Zebrafish. J Biol Chem. 2016;291(24):12601–11. doi: 10.1074/jbc.M116.720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoptak-Solga AD, Klein KA, DeRosa AM, White TW, Iovine MK. Zebrafish short fin mutations in connexin43 lead to aberrant gap junctional intercellular communication. FEBS Lett. 2007;581(17):3297–302. doi: 10.1016/j.febslet.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iovine MK, Higgins EP, Hindes A, Coblitz B, Johnson SL. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev Biol. 2005;278(1):208–19. doi: 10.1016/j.ydbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Sims K, Jr, Eble DM, Iovine MK. Connexin43 regulates joint location in zebrafish fins. Dev Biol. 2009;327(2):410–8. doi: 10.1016/j.ydbio.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22(8):1240–51. doi: 10.1091/mbc.E10-07-0571. Using osteoblast-lineage conditional knockout models, this paper established the major role of connexin43 as a controller of osteoblast progenitor proliferation, osteoblast differentiation, and as a regulator of osteoclastogenesis, impacting cortical bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119(Pt 20):4187–98. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 35.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23(6):879–86. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH, et al. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice. Bone. 2012;51(4):787–94. doi: 10.1016/j.bone.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27(2):374–89. doi: 10.1002/jbmr.548. Established the fundamental importance of connexin43 in the survival of osteocytes in vivo and the contribution to cortical bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int. 2012;91(3):215–24. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacheco-Costa R, Davis HM, Atkinson EG, Katchburian E, Plotkin LI, Reginato RD. Osteocytic connexin 43 is not required for the increase in bone mass induced by intermittent PTH administration in male mice. Journal of musculoskeletal & neuronal interactions. 2016;16(1):45–57. [PMC free article] [PubMed] [Google Scholar]
- 40.Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132(19):4375–86. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- 41.Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, et al. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem. 2005;280(12):11458–66. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- 42.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17(4):539–54. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57(1):76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis. BMC musculoskeletal disorders. 2014;15:122. doi: 10.1186/1471-2474-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komori T. Mouse models for the evaluation of osteocyte functions. J Bone Metab. 2014;21(1):55–60. doi: 10.11005/jbm.2014.21.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Hasegawa T, Hogo H, Tatsumi S, Liu Z, Guo Y, et al. Histological examination on osteoblastic activities in the alveolar bone of transgenic mice with induced ablation of osteocytes. Histol Histopathol. 2013;28(3):327–35. doi: 10.14670/HH-28.327. [DOI] [PubMed] [Google Scholar]
- 48.Moriishi T, Maruyama Z, Fukuyama R, Ito M, Miyazaki T, Kitaura H, et al. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PLoS One. 2011;6(11):e27487. doi: 10.1371/journal.pone.0027487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahani M, Genever PG, Patton RJ, Ahwal F, Fagan MJ. The effect of osteocyte apoptosis on signalling in the osteocyte and bone lining cell network: a computer simulation. J Biomech. 2012;45(16):2876–83. doi: 10.1016/j.jbiomech.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Stains JP, Civitelli R. Connexins in the skeleton. Semin Cell Dev Biol. 2016;50:31–9. doi: 10.1016/j.semcdb.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimston SK, Watkins MP, Stains JP, Civitelli R. Connexin43 modulates post-natal cortical bone modeling and mechano-responsiveness. BoneKEy reports. 2013;2:446. doi: 10.1038/bonekey.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Shifting paradigms on the role of connexin43 in the skeletal response to mechanical load. J Bone Miner Res. 2014;29(2):275–86. doi: 10.1002/jbmr.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellison D, Mugler A, Brennan MD, Lee SH, Huebner RJ, Shamir ER, et al. Cell-cell communication enhances the capacity of cell ensembles to sense shallow gradients during morphogenesis. Proc Natl Acad Sci U S A. 2016;113(6):E679–88. doi: 10.1073/pnas.1516503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henriksen Z, Hiken JF, Steinberg TH, Jorgensen NR. The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium. 2006;39(5):435–44. doi: 10.1016/j.ceca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Geneau G, Defamie N, Mesnil M, Cronier L. Endothelin1-induced Ca(2+) mobilization is altered in calvarial osteoblastic cells of Cx43(+/−) mice. J Membr Biol. 2007;217(1–3):71–81. doi: 10.1007/s00232-007-9024-1. [DOI] [PubMed] [Google Scholar]
- 56.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139(2):497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishihara Y, Kamioka H, Honjo T, Ueda H, Takano-Yamamoto T, Yamashiro T. Hormonal, pH, and calcium regulation of connexin 43-mediated dye transfer in osteocytes in chick calvaria. J Bone Miner Res. 2008;23(3):350–60. doi: 10.1359/jbmr.071102. [DOI] [PubMed] [Google Scholar]
- 58•.Gupta A, Anderson H, Buo AM, Moorer MC, Ren M, Stains JP. Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell Signal. 2016;28(8):1048–57. doi: 10.1016/j.cellsig.2016.04.014. Demonstrates that cAMP is a biologically relevant second messenger passed by connexin43 channels with a biological consequence on osteoblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Niger C, Luciotti MA, Buo AM, Hebert C, Ma V, Stains JP. The regulation of runt-related transcription factor 2 by fibroblast growth factor-2 and connexin43 requires the inositol polyphosphate/protein kinase Cdelta cascade. J Bone Miner Res. 2013;28(6):1468–77. doi: 10.1002/jbmr.1867. Shows that inostiol polyphosphates might be novel second messengers communicated by gap junctions in bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–14. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romanello M, Pani B, Bicego M, D’Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289(5):1275–81. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- 62.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–6. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283(39):26374–82. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res. 2015;30(3):436–48. doi: 10.1002/jbmr.2374. Using transgenic mice expressing mutant connexin43, this paper addressed the role of Cx43 gap junctions versus connexin43 hemichannels in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thi MM, Islam S, Suadicani SO, Spray DC. Connexin43 and pannexin1 channels in osteoblasts: who is the “hemichannel”? J Membr Biol. 2012;245(7):401–9. doi: 10.1007/s00232-012-9462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell. 2009;20(11):2697–708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK acts in parallel to PKCdelta to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol. 2012;302(7):C1035–44. doi: 10.1152/ajpcell.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell. 2005;16(1):64–72. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with betaarrestin: a prerequisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112(10):2920–30. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31(7):1075–81. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niger C, Lima F, Yoo DJ, Gupta RR, Buo AM, Hebert C, et al. The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter. Bone. 2011;49(4):683–92. doi: 10.1016/j.bone.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem. 2003;278(27):24377–87. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- 73.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277(10):8648–57. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 74.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280(8):7317–25. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 75•.Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109(9):3359–64. doi: 10.1073/pnas.1115967109. Systematically demonstrated the role of Cx43 in the mechano-activation of osteocytes by the direct physical interaction of the Cx43 C-terminus with integrins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Pacheco-Costa R, Davis HM, Sorenson C, Hon MC, Hassan I, Reginato RD, et al. Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone. 2015;81:632–43. doi: 10.1016/j.bone.2015.09.011. Revealed an important role of the Cx43 C-terminus in the trabecular compartment of female mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammond MA, Berman AG, Pacheco-Costa R, Davis HM, Plotkin LI, Wallace JM. Removing or truncating connexin 43 in murine osteocytes alters cortical geometry, nanoscale morphology, and tissue mechanics in the tibia. Bone. 2016;88:85–91. doi: 10.1016/j.bone.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]