Abstract
When making decisions regarding vascular access creation, the clinician and vascular access team must evaluate each patient individually with consideration of life expectancy, timelines for dialysis start, risks and benefits of access creation, referral wait times, as well as the risk for access complications. The role of the multidisciplinary team in facilitating access choice is reviewed, as well as the clinical evaluation of the patient.
Keywords: vascular access, fistula, graft, vessel mapping, arteriovenous access evaluation, cardiac remodeling, fistula maturation, cannulation
Abrégé
Au moment de prendre la décision de créer un accès vasculaire, le médecin traitant et l’équipe qui le soutient se doivent d’évaluer chaque patient de façon individuelle et tenir compte de plusieurs facteurs. Ces derniers incluent l’espérance de vie du patient, l’échéancier à respecter pour le démarrage de la dialyse, les risques et les avantages liés à la création d’un accès vasculaire, les temps d’attente à prévoir pour la consultation, de même que les risques de complications encourus à la suite de la procédure. Ce chapitre évalue le rôle de facilitateur que joue l’équipe multidisciplinaire dans la prise de décision de créer un accès vasculaire, de même que l’examen clinique du patient.
Arteriovenous Access Considerations
When making decisions regarding vascular access creation, the clinician and vascular access team must evaluate each patient individually, weighing issues such as life goals and expectancy, timelines for dialysis start, risks and benefits of access creation, referral wait times, as well as the risk for access complications.
Patient Choice
Eligibility must be considered not only in physical terms (eg, patient and vessel characteristics) but also in terms of patient’s life circumstances, goals, and preferences. Ideally, the patient’s decision should be based on an understanding of the risk and benefit profile of the various access types in relation to the patient characteristics.
Life Expectancy and Comorbidities
Vascular access selection is complex, and several patient algorithms1,2 have been developed to assist in this selection of the most appropriate type of vascular access. A young patient with low comorbidity, appropriately sized vessels, a long life expectancy on hemodialysis (HD), and sufficient time for maturation prior to use should consider a fistula as the first access.
Patients with a shortened life expectancy or high comorbidity may be more appropriate for a graft or a catheter. Some comorbidity, like severe heart failure or significant peripheral vascular disease, may lead to negative patient outcomes, reduce the success of arteriovenous creation, and/or lead to increased risk of complications such as worsening heart failure or ischemic steal.3,4
While most patients should be considered candidates for arteriovenous access creation, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative, KDOQI guidelines acknowledge that patients who are expected to live less than 1 year are acceptable patients for chronic catheter use.5 For example, the median survival of patients 80 years old or greater in the United States is 1.3 years.6 Depending on the judgment of the nephrologist, they may not be offered fistula creation although a graft may be considered. Life expectancy in the elderly may also be affected by a higher likelihood of severe comorbidities such as metastatic cancer, severe congestive heart failure, or severe vascular disease.
Despite these concerns in the elderly, it should be recognized that fistula creation can be successful. Studies show that fistulas created in the predialysis period in older patients are used to start dialysis in approximately half of the patients,7 with many patients dying before needing dialysis. Primary patency (time from access creation until the first access thrombosis or any intervention to maintain patency8,9) is generally less than 50% at 1 year. However, secondary (or assisted) patency (time from access creation until access abandonment) ranges from 75% to 92% in single-center studies.10-12 Elderly patients may particularly benefit from Doppler mapping and more lead time to facilitate maturation prior to use. Claudeanos et al13 in a small study of patients aged 80 years or older reported that the median survival after fistula creation was 26 months and that 21% of dialysis time was spent using a catheter. These results suggest that fistula creation in the elderly can be successful, but patients should be selected and monitored carefully.
Center Specific Variation
There appears to be variation in the prevalence of fistula use, which is not directly related to patient characteristics. It is likely that program factors, such as infrastructure and program culture or philosophy regarding vascular access, impact access choice and access placement.
Moist et al14 reported that prevalent catheter use in Canada increased by 10% between 2001 and 2004. Prevalent catheter use by province ranged from approximately 30% to 60%, and the variation was not explained with adjustment for baseline factors within the Canadian Organ Replacement Register.14 Catheter use was strongly associated with mortality. Variation of fistula use in US dialysis facilities, where on average 38% of patients were dialyzing with a fistula, showed 7.1% of the variation was attributable to the facility after case-mix adjustment.15
Suitable Vasculature
Patients require suitable vessels for arteriovenous access creation. Both fistula and graft maturation require an adequate cardiac output (CO) to deliver required blood flow, an adequate arterial conduit, adequate vein size and compliance, as well as unobstructed outflow veins. Veins that are scarred or damaged by previous intravenous catheters, central venous catheters (peripherally inserted central catheter, [PICC] or traditional ones), pacemakers, or cardiac implanted electronic devices (CIED) can develop stenosis or occlusion of the cephalic and basilic veins16 that prohibits arteriovenous access creation.
Timing of Arteriovenous Access Creation
The timing of arteriovenous access creation is complex. Patients with chronic kidney disease, CKD have varying rates of progression to end-stage kidney disease (ESKD) with death as competing risk.17,18 ESKD risk equations use multiple factors to predict risk of CKD progression and may assist in the timing of access creation,19 but whether their use in clinical practice can improve patient outcomes has not been reported. Predialysis arteriovenous access creation is complicated and difficult to properly time, contributing to high incident use of catheters.
The earlier in the course of predialysis care a fistula is created, the more time there is for the fistula to mature, but also, the more likely it will not be used because of the competing risks of death, lack of progression of kidney disease, or failure of the fistula prior to use. Some guidelines recommend evaluating patients for fistula creation at glomerular filtration rate (GFR) of 15 to 20 mL/min/1.73 m2 if they have progressive kidney disease.20 The literature suggests that fistulas are often created at much lower GFRs and therefore may not be ready for use at the start of dialysis.21,22 A recent study in Ontario found the timing varied significantly with only 40% of fistulas being placed in the 3- to 12-month window before the start of HD.18 De Silva et al also found that 50% of elderly patients who had undergone predialysis fistula creation required catheters to be placed to start HD.23 Grafts require a shorter maturation time than fistula: from 3 to 4 weeks after placement for a standard graft, to same-day cannulation for an early-cannulation graft.
Impact of Primary Failure
Primary failure (see “Predictors of Primary Failure” section in MacRae et al24) is an important consideration affecting access recommendations and choice. Primary failure occurs when a fistula either thromboses before its use or lacks suitability for use on dialysis. There is no standardized definition, but it has been defined by reliability of cannulation, adequate blood flow on dialysis, appropriate clearance, and whether catheter-free use is achieved. The rate of primary failure varies from 25% TO 60%,25,26 which should be taken into account with access decisions. Grafts have a much lower rate of primary failure: from 12% to 20%27,28 (see “Key Relevant Arteriovenous Access Patency Rates” section in MacRae et al24).
The Role of the Multidisciplinary Team in Access Choice
Individualized, patient-centered planning for dialysis access is the preferred model of care. Decision making requires input from the multidisciplinary team, including the vascular access nurse or nurse educator, nephrologist(s), surgeon(s), radiologist(s), patient, and family members. The process begins with timely referral to nephrologists and patient education, followed by suitable investigations and interventions in preparation for the desired dialysis access, including surgical referral where appropriate. After access creation, the multidisciplinary team coordinates evaluation, use, and maintenance of the dialysis access. This is facilitated by regular and inclusive multidisciplinary communication.
Table 1 lists proposed roles for the multidisciplinary team. Each can be performed in conjunction with other multidisciplinary team members. This will require center-specific modification.
Table 1.
Role of Multidisciplinary Team Members.
| Team member | Role pre-creation | Role post-creation |
|---|---|---|
| Nephrologist | Educate patients, often with the CKD educator regarding CKD progression and RRT modality options Educate patient re: choice of dialysis access based on clinical circumstances (comorbidities, rate of progression) Discuss risks and benefits of peritoneal catheter and hemodialysis vascular access. Provide timely referral to the surgeon and/or interventionist. |
Monitor, along with the Vascular Access coordinator, the access after creation for signs of complications and facilitate interventions to maintain long-term function. Manage vascular access complications (eg, catheter-related malfunction or infection or fistula or graft complications?). |
| Surgeon/interventional radiologist or nephrologist | Evaluate re: choice of vascular access based on patient and vessel characteristics (optimally, in conjunction with information provided by the nephrologist regarding the patient’s anticipated time to initiation of dialysis). Discuss surgical and interventional risks and benefits for each access with patient/family. |
Create the vascular access and manage immediate perioperative complications including revisions as required. Perform facilitative and/or corrective procedures to attain and/or maintain patency, eg, coil embolization, angioplasty, thrombolysis. |
| Peritoneal and/or vascular access coordinator | Facilitate communication between nephrologist, surgeon, radiologist and patient/family. Coordinate peritoneal dialysis or hemodialysis vascular access management (eg, booking of diagnostic tests, communicates with patient re: dialysis access appointments, etc). |
Monitor patient’s dialysis access on a regular basis and informs nephrologist and/or surgeon/interventionist of concerns. Key “point person” for patient when access issues arise. |
| Patient and family | Provide information about patient’s life circumstances (social, occupational, cultural, spiritual, functional, etc). Provide information about patient dialysis access preferences, life goals, and concerns. Ask questions to ensure they understand various dialysis access options to their satisfaction. |
Provide information regarding any changes in life circumstances or preferences |
Note. RRT = renal replacement therapy.
The role of the vascular access team is to advise the patient on the options for vascular access. Considerations for the type of vascular access should include the patient’s comorbidities, vessel characteristics, patient preferences, life circumstances, and goals as described previously. Together with the patient, the vascular access team should plan out the dialysis access lifeline options.
Evaluation for Arteriovenous Vascular Access Creation
An adequate evaluation of the patient increases the likelihood of a successfully created and functioning arteriovenous access.29 A patient evaluation should include history and physical examination and when needed, the appropriate vessel imaging. Knowledge of vessel anatomy is an important requirement for a vascular access preoperative assessment. See Atlas of Dialysis Vascular Access: http://c.ymcdn.com/sites/www.asdin.org/resource/resmgr/imported/atlas%20of%20dialysis%20access.pdf.
Vessel Anatomy of the Arm
A basic understanding of the anatomy of vessels utilized to create the vascular access is crucial both for the preoperative vascular access assessment as well as the proper handling and care of an access during dialysis therapy.
The venous system of an extremity includes superficial and deep veins. The superficial system is most important for access creation.
The superficial vein in the upper extremity that is preferred and most commonly utilized for fistula creation is the cephalic vein.
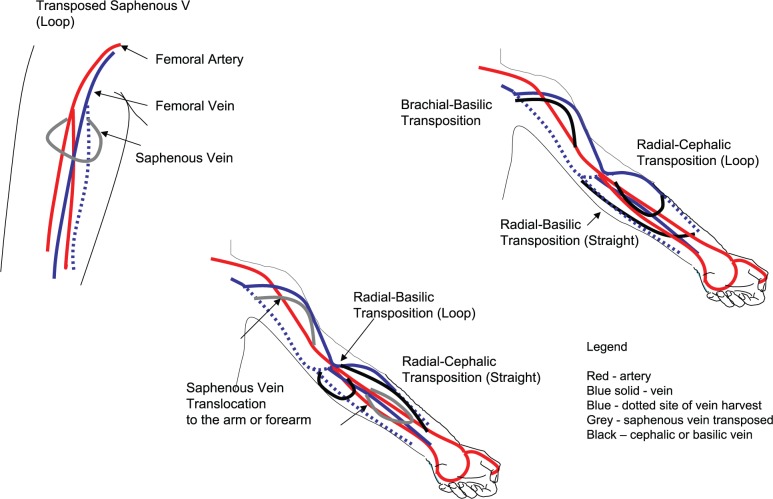
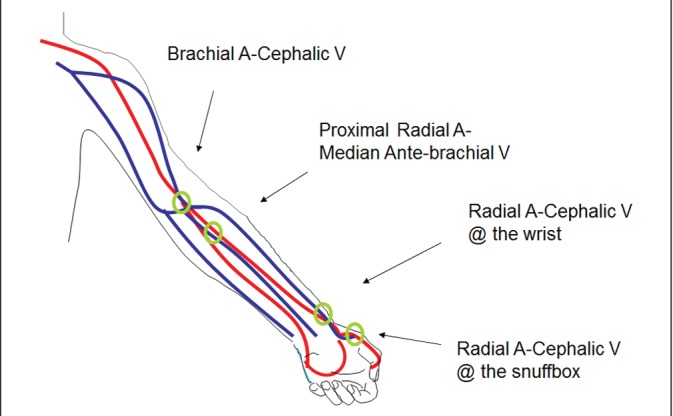
The radiocephalic fistula at the wrist is considered the first choice HD access and utilizes the forearm segment of the cephalic vein (see Figure 1).
The brachiocephalic fistula at the elbow utilizes the upper arm segment of the cephalic vein and generally is the second choice site for fistula creation.
The other superficial veins in the forearm (the basilic vein on the ulnar side and the median basilic vein near the elbow) are occasionally used for fistula creation.
The deep veins in the forearm are not ideal for fistula creation. The deep veins in the upper arm are the brachial and basilic veins that run parallel to the brachial artery.
The basilic vein in the medial aspect of the upper arm is the most common deep vein utilized for fistula creation. The basilic vein is mobilized from its usual location and transposed superficially through the deep fascia in the upper arm to create the “transposed basilic vein” fistula (see Figure 2).
The brachial veins in the upper arm are used for dialysis access as a last resort. The brachial veins and the basilic vein join and continue as the axillary vein until the outer border of the first rib. The axillary vein continues as subclavian vein from the outer border of the first rib and extends to the sternal end of the clavicle.
A graft made from synthetic material like polytetrafluoroethylene (PTFE) is used for access creation if the native vessels are not suitable for creating a fistula. The forearm loop, upper arm straight, and thigh loop grafts are commonly used configurations for creating a dialysis access.
Figure 1.
Fistula creation.
Source. Modified from Spergel et al.30
Note. Typical sites for fistula creation in the arm are highlighted.
Figure 2.
Atypical fistula creation.
Source. Modified from Spergel et al.30
Note. Atypical sites for fistula creation are highlighted.
History and Physical Examination
To determine the type of dialysis access most suitable for a patient, a general history and physical examination is required. The patient’s history can be broadly categorized by their (1) medical history, (2) current active medical issues, and (3) specific access-focused history. A patient’s medical history will provide necessary details regarding the eligibility of a patient for peritoneal dialysis (PD) or HD. For example, surgeries affecting the peritoneum may contraindicate PD.
An HD access-focused history is unique and should be performed each time a patient is assessed for a new HD access. This history will provide insight for risk of developing complications such as failure of a fistula to mature or the development of steal syndrome, and may guide the surgeon to pursue a preemptive intervention or to consider an alternate access. An access history focuses on the vessels, reviews the type and nature of previous vascular procedures (eg, PICCs, CIEDs), and obtains previous access creations or interventions required to facilitate or maintain access patency and reason(s) for previous access loss). In addition, a history of comorbidities such as heart failure with low ejection fraction or unstable angina is important, given the increased cardiac demands placed by an AV access.
The physical exam should include the following:
Any physical evidence (scars) from a prior central venous catheter
Swelling of collateral veins in the neck, arms, chest
CIED such as a permanent cardiac pacemaker. The wires associated with these devices are a high-risk factor for causing central vein stenosis.31 It is important to avoid access creation ipsilateral to potentially damaged central veins, as such may occur with transvenous pacemakers.
Arterial evaluation to ensure adequate blood flow and an intact dual blood supply to the hand. This includes pulse examination (axillary, brachial, radial, and ulnar), Allen test, and bilateral upper extremity blood pressure. A difference of 20 mm Hg or greater is suggestive of subclavian artery stenosis in the lower pressure arm.32
Vessel (vein and artery) mapping can be accomplished using duplex ultrasonography and venography, but mapping the extremity superficial veins should first be attempted by physical exam.
Vein anatomy, the anatomical course and continuity of the vein is examined in both the forearm and upper arms. Forearm vein anatomy can be augmented by using a blood pressure cuff inflated to a pressure about 5 mm Hg greater than the measured arterial diastolic pressure to dilate the veins. The blood pressure cuff should be left in place for no more than 5 minutes at a time. Other maneuvers, such as use of warm water may be effective in dilating veins. Unfortunately, in patients with obesity and deep veins, physical examination alone may be insufficient to view superficial veins along the length of the arm.
Vessel Mapping
Vessel mapping33-35 is associated with increased fistula creation36; however, a high primary fistula failure rate persists.37 A meta-analysis38 did not demonstrate any increase in fistula creation, maturation, or functional ability to be used for dialysis with vessel mapping. The extent to which ultrasound mapping is used varies by center and surgical expertise; however, there is general agreement to use ultrasound mapping in patients who are at high risk for failure to mature and those with obesity. Table 2 summarizes the criteria used for suitable vein and artery anatomy in access planning.
Table 2.
Evaluation for Arteriovenous Access Creation.
| Vein anatomy | Artery anatomy | Central vein anatomy |
|---|---|---|
| Physical exam | ||
| Compressible/distensible | Compliant | Absence of collateral veins on chest or abdomen |
| Absent occluded segments | Palpable pulses | Absent pacemaker |
| Length of vein sufficient for cannulation (≥15 cm) | Difference of<20 mm Hg between arms | |
| Straight vein segment | Patent palmar arch | |
| Superficial vein | ||
| Ultrasound | ||
| Absence of stenosis/synechiae (fibrous scars) | Absence of stenosis | Absence of central vein stenosis |
| Absence of intraluminal webs | Normal flow and velocity waveforms | |
| Continuity of outflow vein with central veins | Diameter of artery ≥2 mm at site of anastomosis | |
| Diameter of vein ≥2.5 mm for fistula >4 mm for graft | ||
| Vein depth <1 cm from skin surface | ||
It may be important to image the artery that will be used for the creation of the fistula. If done, the presence of calcification should be documented because this is thought to be a risk for fistula creation and maturation.
Ultrasound does not completely image central veins; where there is a high pre-test probability of stenosis, venography is performed.
Venography
Venography provides a complete assessment of peripheral venous patency and continuity with the central veins and identifies central venous stenosis. Venography should be considered in patients who have a history consistent with central vein stenosis (by physical exam or history of catheters, PICCs or CIEDs). There is concern however over the risk of contrast-induced acute kidney injury, so the volume of dye is often minimized or carbon dioxide venography is substituted in patients who are not on dialysis and in those with residual kidney function.
Anatomy Features for Arteriovenous Access Creation Evaluation
Various anatomy features that are used to evaluate patients for arteriovenous access creation are summarized in Table 2.
Surgical Considerations for Arteriovenous Access
Preoperative evaluation and surgical technique are particularly important in facilitating vessel maturation and preventing primary failure. Formal training in access creation improves vascular access outcomes: Surgeons who created at least 25 fistulae during their training had a lower risk of both primary and secondary fistula failure.39
Postoperative evaluation (ie, meticulous serial physical exams), as well as the appropriate interventions, if necessary (ie, angioplasty, thrombolysis, stenting), is critical in treating primary and secondary failure. However, fistula survival has been shown to vary with the number of interventions required to facilitate fistula maturation and function, with higher numbers of intervention being associated with poorer fistula survival.40
Anesthesia Issues
Native fistulas can usually be constructed under local anesthetic. Transposed fistulas and grafts may require regional nerve blocks or general anesthesia. The type of anesthetic may impact on subsequent vessel dilation and maturation. Brachial plexus blocks result in larger diameter vessels at the time of surgery and in the subsequent fistula for 8 weeks after surgery as compared with fistula created with general anesthetic41 in a small randomized trial. Whether regional nerve block results in improved fistula maturation and use is unknown. A larger randomized trial to determine the effect of brachial plexus block on vessel vasodilation and subsequent primary failure and fistula patency is currently underway (Clinical trials: NCT01706354).
Surgical Factors
The surgical angle of anastomosis of the artery to the vein is likely an important determinant of fistula maturation. It appears that the angle of the anastomosis also affects wall shear stress; more acute anastomotic angles promote neointimal hyperplasia and subsequent stenosis formation.42 Observational data suggest that the type of material used to create the anastomosis may impact access outcomes with vascular clips (vs sutures) having superior primary patency.43,44 Intraoperative blood flow of less than 120 mL/min determined at the time of anastomosis appears to be predictive of primary failure in a study cohort.45
Hemodynamics of Fistula Creation
Arteriovenous Vessel Remodeling
Creating an anastomosis between an artery and a vein sets in motion a complex biologic process of vascular remodeling that hopefully results in a mature fistula.
The first step is appreciating the physiologic changes that result from fistula creation. The typical arterial flow in the radial and brachial artery prior to creation is approximately 25 mL/min and 50 mL/min, respectively. The typical flow in the cephalic vein is 28 mL/min with shear stress of 5 to 10 dyne/cm.2,46 After the fistula is created, blood flow rapidly increases 10 to 20 folds.47,48 Typically, fistulas attain 40% to 60% of maximal blood flow within 1 day of creation, and maximal blood flow is approached within 4 weeks.
The cardiac output increases in response to baroreceptor-induced changes (see “Cardiac Hemodynamic Changes With Arteriovenous Access Creation” section). This dramatically increased pressure and flow in the vein causes increased shear stress (~24.5 dyne/cm2), which is sensed by the endothelial cells. Although the exact pathways are not fully understood, mediators such as nitric oxide and metalloproteinases are important to induce vasodilation, vein wall thickening, and vascular remodeling.49 The goal of this vessel dilation and remodeling is to reduce pressure and shear stress in the vascular system to accommodate the increased flow from the fistula. For example, the diameter of the cephalic vein at the wrist typically increased from 2.3 to 3.2 mm to 5.8 to 6.6 mm after creation, and shear stress is reduced to approximately 10 dyne/cm2 2 to 3 months after fistula creation.46
Not all studies show that vein size correlates with maturation, but in general, cephalic vein diameter greater than or equal to 2 to 2.5 mm at the wrist and greater than or equal to 4 mm at the elbow is considered acceptable.35,50 Upper arm fistulas are more likely to mature, as are fistulas in men. An ongoing multicenter study funded by the National Institutes of Health (NIH) will be instructive as it will be looking at measures of vascular function (flow-mediated nitroglycerin-mediated brachial artery dilation, carotid-femoral and carotid-radial pulse wave velocity, vein compliance)51 as well as the traditional predictors of maturation. Vein segments will also be sampled during fistula creation for structural, cellular, and molecular markers including baseline measurement neointimal hyperplasia.
Cardiac Hemodynamic Changes With Arteriovenous Access Creation
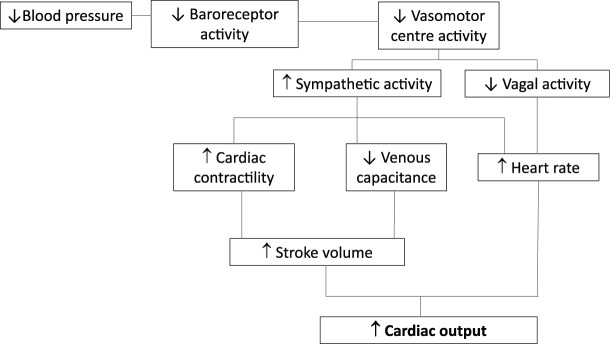
Fistula creation results in cardiac hemodynamic changes that are characterized by a hyper-dynamic circuit. Upon anastomosis of a high-pressure artery to a low-resistance vein, there is an immediate increase in blood flow and a decrease in systemic vascular resistance. The transient decrease in blood pressure, caused by fistula creation, decreases carotid baroreceptor activity, which inhibits the vagal nerve and increases the sympathetic activity (Figure 3). The increased sympathetic activity has multiple effects: an increased heart rate, increased contractility, and a decrease in vein capacitance (via increased vein tone). Both the increased contractility and decreased vein capacitance produce an increased stroke volume, which along with the higher heart rate causes an increase in cardiac output. The vasoconstriction of veins is equivalent to an increase in the blood volume of the circuit.52
Figure 3.
Hemodynamics of fistula creation.
Studies consistently show an increase in the cardiac output by 15% to 20% after fistula creation.53,54 In addition, there is a significant increase in both atrial natriuretic peptide (ANP) and brain natriuretic peptide within 2 weeks of fistula creation reflecting increased left atrial (LA) and left ventricle (LV) stretch from the increased volume state. The increase in ANP has been correlated to the increase in cardiac output.54 Echocardiographic changes support the concept of an increased blood volume with increased inferior vena cava diameter, increased LA size, and increased left ventricle end diastolic volume (LVEDV) and dimension (LVEDD) 1 week after fistula creation. Similar echo findings are seen on the right heart with increases in right ventricle end diastolic dimension (RVEDD).54
A prospective magnetic resonance (MR) study among CKD stage 5 patients demonstrated significant increases in LA size, LVEDV, LV mass, and cardiac output at 6 months after fistula creation.55 However, interpretation of these findings must be tempered by the lack of a control group, which makes it difficult to separate the effect of a progressive decline in kidney function (and subsequent volume overload) from the effect of fistula creation.
The presence of an arteriovenous access is thought to lead to LA and LV remodeling. In fact, LV hypertrophy (LVH) is an adaptive response to the increased cardiac workload imposed by the access. Reverse modeling, with LVH regression and LV cavity size decrease, is consistently demonstrated in transplant patients with fistula ligation. A cardiac MR study showed significant decreases in both LV and RV cavity size as well as cardiac output with fistula ligation.56
Right Ventricle Remodeling and Pulmonary Hypertension
Given the interdependence of the LV and RV, it is not surprising to see corresponding changes in the right heart with fistula creation. Similar to the increase in LV workload, there is an increase in RV systolic performance as well as increased right atrial size and RVEDV.55 The increase in blood volume as well as the increased RV performance may lead to increased pulmonary flow and possibly increased pulmonary pressure resulting in pulmonary hypertension. There may be a relationship between the degree of pulmonary hypertension and the age and flow rate of the fistula.57
The systolic pulmonary artery pressure (PAP) is determined by the cardiac output as well as the pulmonary vascular resistance. Pulmonary hypertension, which occurs when the PAP exceeds 30 mm Hg, can be defined as mild (<45 mm Hg), moderate (45-65 mm Hg), or severe (>65 mm Hg58). Overall, the prevalence of pulmonary hypertension in HD patients is estimated to be approximately 40%.59 The mechanism is postulated to be chronic vasoconstriction in the pulmonary vascular bed from an imbalance of endothelin (ET-1) and nitric oxide. Fistula creation may be associated with endothelial dysfunction in the pulmonary circuit,56 which may promote pulmonary hypertension.
Not all studies support the concept that fistula creation promotes pulmonary hypertension. Unal et al58 found that fistula creation had no significant effect on the development of pulmonary hypertension, and they found no correlation between access flow and pulmonary hypertension. Furthermore, the prevalence of pulmonary hypertension increases as GFR declines among CKD patients, possibly reflecting the tendency to increased volume overload with progressive loss of kidney function.60
The hemodynamic changes associated with graft placement are less pronounced than those with fistula creation. The resistance properties of a graft are higher than those of a fistula and thus the immediate impact on cardiovascular remodeling is postulated to be different.
Cardiac Remodeling and Patient Selection
Fistula creation is associated with cardiac hemodynamic effects that are generally well tolerated. However, based on the expected physiology of an increased demand on cardiac output, some nephrologists consider severe heart failure as a relative contraindication to fistula placement. For those patients with a significant cardiac history, a lower flow forearm access is expected to have a lower increase in cardiac output61 and may be a preferred approach (see “Access Flow and Heart Failure” section in MacRae et al62).
Clinical Evaluation of Fistula Maturation
The maturation status of a fistula is primarily assessed clinically. The factors required for adequate maturation include an appropriate blood flow and diameter and adequate vein length for cannulation. The presence of a palpable, soft, continuous thrill and a biphasic bruit at the anastomosis implies that the flow is adequate.63 The vessel should be easy to palpate, easy to compress, and should collapse with arm elevation (indicating no central vein stenosis). There should be no indications of stenosis (eg, swelling of the arm), obvious collaterals, or signs of steal.51
KDOQI5 has established simple “rules of 6’s” for the determination of a mature fistula. Six weeks after creation, the flow of a fistula should be 600 mL/min, fistula diameter should be 0.6 cm, fistula depth should be less than 0.6 cm below the skin, and the fistula should have at least 6 cm of straight segment for cannulation. However, these numbers are not based on direct evidence. Robbin et al64 conducted an observational study in 69 patients who underwent ultrasound determination of fistula flow and diameter at months 2 and 4 post creation. Fistulas with a diameter greater than 0.4 cm or flow greater than 500 mL/min successfully supplied adequate blood flow for hemodialysis. If both flow and diameter criteria were combined, this further increased the predictive value of successful dialysis use to 95%. In addition, this study found that an experienced nurse could predict fistula maturity and suitability for dialysis with 80% accuracy. The authors also showed that there was no difference in the flow rates or fistula diameter at month 4 compared with month 2. Therefore, a routine maturation assessment should be obtained by month 2 in order to assess for appropriateness for cannulation.
Aspects of Cannulation
Cannulation technique is an important aspect of long-term AV access patency. Each blood vessel puncture may incite local trauma and subsequent venous neointimal hyperplasia and stenosis formation.65 Over time, repeated punctures in the same area result in vessel wall weakness with subsequent aneurysm.66 Furthermore, needle infiltration (estimated to occur in approximately 35% of cannulations [Charmaine Lok MD MSc, personal written communication]) produces a hematoma, which is itself associated with an increased risk of fistula thrombosis67 and access loss. Thus, every HD program should ensure that nurses are adequately trained with respect to knowledge and skills, and that they maintain their skills through continuous practice. Considering the importance of cannulation for AV survival, programs need to ensure adequate support and dedicated time to allow for AV access education with focus on assessment and cannulation.
Needle Technique Options
There are 3 types of needle technique that are in use: area wall, rope ladder, and buttonhole. Area wall refers to needling the same section of the fistula or graft, which over time can lead to aneurysm growth. Parisotto et al68 showed that the highest risk of access failure is associated with this cannulation, and therefore, this technique should be avoided. Rope ladder, the recommended needling technique, refers to the rotation of needle sites in a ladder formation along the entire length of the fistula or graft. Buttonhole, only used in fistula, refers to needling at the same site over the course of 6 to 9 sessions with a sharp needle, which, over time, forms an epithelialized track of tissue in which blunt buttonhole needles can then be inserted. Buttonhole needling is contraindicated in grafts.
The buttonhole technique, first described in 1977,69 garnered a great deal of enthusiasm due to reported reduction in pain with needling and reduced aneurysm growth seen in observational studies. More recently, randomized trials demonstrate no difference in pain70-72 with needling and conflicting results on fistula survival.72,73 Vaux72 demonstrated significantly better access survival at 1 year with buttonhole (100% vs 86% access survival, P = .005) with no increased infection risk while MacRae73 showed no difference in access survival but significantly increased risk of Staphy-lococcus aureus infections with buttonhole technique (incidence rate ratio [RR], 63; 95% confidence interval [CI], 22-180; P < .001). The main difference between the trials was the technique to create the buttonhole track: repeated sharp cannulation in the MacRae trial (which was associated with significant difficulty) and the use of a biohole polycarbonate peg in the Vaux trial. The reader is referred to Atkar et al74 for a summary of the trial design and outcomes of the randomized buttonhole trials.
The main concern with buttonhole needling is the increased infection risk, especially for S aureus bacteremia. Further evidence on this question is provided by a systematic review of 15 buttonhole (4 randomized, 11 observational) studies of more than 1600 patients which found an increased risk of infection with buttonhole compared with rope ladder techniques (RR, 3.18; 95% CI, 2.12-4.77).75 Wong et al,76 although unable to quantify a risk of infection due to heterogeneous definitions, did report a trend to increased infections with buttonhole in a meta-analysis of 5 randomized and 18 observational studies. Canadian Society Nephrology guidelines recommend use of topical antimicrobial prophylaxis for buttonhole technique77 (very low quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation, GRADE criteria). Given the increased infection risk with buttonhole cannulation, this technique should be considered only when there is consistent application of cannulation protocols including the use of face mask, skin disinfection with chlorhexidine-alcohol, and regular technique audits for both staff and patients who self-needle.
Potential advantages of buttonhole include a reduction in aneurysm growth72,78 and fewer hematomas.71,76 Indications for buttonhole include a fistula with only a short length for cannulation that would otherwise be abandoned, fistula with aneurysm, or in a home dialysis patient with fear of needling despite teaching. Given the high risk of S aureus infection, mechanical heart valves or indwelling implanted devices are contraindications. Readers are referred to http://www.ishd.org/7-the-care-and-keeping-of-vascular-access-for-home-hemodialysis-patients for details on cannulation techniques.
Impact of Needle Size and Direction of Placement
The needle gauge and direction of needle placement impact the flow dynamics in the blood vessel. Studies have shown that the needle alters fluid dynamics for up to 10 cm downstream of the vessel with a change in wall stress and turbulent flow.79,80 The change to turbulent flow is associated with decreased nitric oxide production and decreased nitric oxide release from endothelial cells.79 Smaller gauge needles may incite less vessel wall trauma but have higher blood flow velocity which may alter shear dynamics.81
It is generally recommended for the venous needle to be placed in the antegrade position, in the same direction as the blood flow. Antegrade needle placement reduces hematoma formation and reduces the tendency for pseudoaneurysm development upon needle withdrawall82 and may be associated with improved access survival according to observational data68 (see “Infiltration” section). Recent flow dynamic studies show that retrograde arterial needle placement is associated with positive flow dynamics.81
The optimal blood pump speed is unknown; however, there is an association between higher pump speed and subsequent AV access loss.83 Most programs will initiate needling at a low pump speed with the smallest gauge needle and slowly advance to a speed that will permit adequate clearance in a reasonable time frame.
What About Other Features of Needling Technique?
Tourniquet use
This is a technique used to increase dilation of the blood vessel and stabilize the site, which may improve the successful needle placement and minimize infiltrations. In addition, the tourniquet may minimize pain. A multinational observational study68 showed that use of a tourniquet at the time of cannulation is associated with improved access survival as compared with patient compression of the fistula at the time of cannulation.
Bevel up
There is some uncertainty regarding the placement of the needle bevel. Some recommend placing the bevel down in order to prevent the tip of the needle from piercing the back wall and causing an infiltration. Rotating or flipping the needle should be actively discouraged due to the potential trauma to the vessel.81 In the absence of direct evidence, readers are recommended to follow their unit-specific protocols.
Use of ultrasound to assist with needling
Ultrasound is a tool to assist with cannulation, but it requires specialized skill and training. It can be helpful to improve visualization of the vessel, identify depth, determine the diameter, highlight possible areas of stenosis, identify location of valves, and diagnose other complications such as hematomas and pseudoaneurysms. Many nurses may find this device initially awkward to use until familiarity develops. Teaching and mentorship programs should be established if ultrasound is used.
Steel versus Teflon needle use
Steel needles are most commonly used in North America due to cost. Teflon needles (also called angiocatheters) may be used and can be considered for new or fragile arteriovenous access and are frequently used for nocturnal84 or restless patients with frequent movement. However, there is no evidence to preferentially support the use of 1 type of needle. Cannulating with a Teflon catheter has a different technique than a steel, and nurses should be trained how to cannulate successfully using this type of needle.
The cannulation of an AV access is a skill that deserves careful attention and adequate staff resources to facilitate. The following links include details on cannulation technique:
http://www.ishd.org/7-the-care-and-keeping-of-vascular-access-for-home-hemodialysis-patients
http://esrdncc.org/ffcl/change-concepts/change-concept-8/cannulation-of-the-av-fistula/
Infiltration
Often referred to as a “blow,” infiltration occurs when the needle has been dislodged from inside the fistula or graft during needle insertion or during a dialysis treatment. It occurs when the tip of the needle slips out of the fistula, passes through the wall of the fistula allowing blood to infuse into the surrounding tissue due to poor hemostasis. Risk factors include cannulator experience, immature fistulas, deep vessel depth, stenotic accesses, hastened hemostasis, anticoagulant therapy, peripheral arterial and vascular disease, and age.67 Every infiltration is associated with an increased risk of interventions including prolonged catheter dependence.67
An infiltration is often associated with pain, warmth, and bruising on examination. The bruise can be quite extensive and involve the entire arm and even track into the thoracic region. Often the bruit is abnormal and the fistula is firm to the touch. Mild infiltrations are best treated with repeat 20-minute applications of ice packs for the first 24 hours followed by a warm compress. It is best to avoid the affected area when cannulating. Severe infiltrations may require a temporary HD catheter in order to rest the access until the swelling and bruising dissipate.
Summary
Patients should undergo careful assessment by the vascular access team to determine if they are eligible for AV access creation.
Potential candidates should have further evaluation by a surgeon who may also wish to perform Duplex US venous ± arterial mapping to determine eligibility. Venography is used for concerns of central vein occlusion.
Eligible candidates should be offered fistula creation, but the nephrologist should carefully consider baseline comorbidity and anatomical and other relevant factors, and the risks of the procedure should be clearly explained to the patient. The final decision to proceed with arteriovenous access creation should be made by a multidisciplinary team (nephrologist, surgeon, vascular access nurse) and the patient/family.
An individualized approach that takes into consideration the patient’s chronologic and physiologic age, comorbidities, anatomic factors, and patient concerns is suggested.
Fistula creation decreases baroreceptor activity, which results in a series of events that increases cardiac output.
Over time, fistula creation is associated with cardiac remodeling and LV hypertrophy.
It is unclear if creation of a fistula facilitates the development of pulmonary hypertension.
Cannulation technique impacts access survival; infiltration and subsequent hematoma are associated with increased risk of access thrombosis.
Area wall technique should be avoided as it leads to aneurysm formation.
Buttonhole is associated with increased risk of infection; measures should be in place to counteract this risk, including the use of topical antimicrobial prophylaxis.
The optimal needle gauge and blood pump speed are unknown.
Acknowledgments
The authors thank Kirsten Campbell for her editing and management skills and Adam Bass, Rob Quinn, and Pietro Ravani for their expertise.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval and consent to participate was not required for this trial.
Consent for Publication Availability: Consent for publication was obtained from all authors.
Availability of Data and Materials: There is no data to share.
Author Contributions: J.M.M. conceived, designed, and coordinated the review; drafted the manuscript; and critically revised the manuscript. M.O., E.C., C.D., S.H., M.K., C.L., R.L., and L.M. helped design and draft the manuscript and provided critical review. J.K. helped coordinate the review and provided critical review. L.M.M. helped design and coordinate the review and provide critical review. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Vascular Access Work Group was supported by the Canadian Society of Nephrology through an unrestricted educational grant from Roche Pharmaceuticals, Canada.
References
- 1. Davidson I, Gallieni M, Saxena R, Dolmatch B. A patient centered decision making dialysis access algorithm. J Vasc Access. 2007;8(2):59-68. [PubMed] [Google Scholar]
- 2. Drew DA, Lok CE, Cohen JT, Wagner M, Tangri N, Weiner DE. Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol. 2015;26(1):183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beathard GA, Spergel LM. Hand ischemia associated with dialysis vascular access: an individualized access flow-based approach to therapy. Semin Dial. 2013;26(3):287-314. [DOI] [PubMed] [Google Scholar]
- 4. Wasse H, Singapuri MS. High-output heart failure: how to define it, when to treat it, and how to treat it. Semin Nephrol. 2012;32(6):551-557. [DOI] [PubMed] [Google Scholar]
- 5. Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(suppl 1):S176-S247. [DOI] [PubMed] [Google Scholar]
- 6. Tamura MK, Tan JC, O’Hare AM. Optimizing renal replacement therapy in older adults: a framework for making individualized decisions. Kidney Int. 2012;82(3):261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hod T, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS. Arteriovenous fistula placement in the elderly: when is the optimal time? J Am Soc Nephrol. 2015;26(2):448-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee T, Mokrzycki M, Moist L, et al. Standardized definitions for hemodialysis vascular access. Semin Dial. 2011;24(5):515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35(3):603-610. [DOI] [PubMed] [Google Scholar]
- 10. Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67(6):2462-2469. [DOI] [PubMed] [Google Scholar]
- 11. Olsha O, Hijazi J, Goldin I, Shemesh D. Vascular access in hemodialysis patients older than 80 years. J Vasc Surg. 2015;61(1):177-183. [DOI] [PubMed] [Google Scholar]
- 12. Nadeau-Fredette AC, Goupil R, Montreuil B, Carignan A, Leblanc M. Arteriovenous fistula for the 80 years and older patients on hemodialysis: is it worth it? Hemodial Int. 2013;17(4):594-601. [DOI] [PubMed] [Google Scholar]
- 13. Claudeanos KT, Hudgins J, Keahey G, Cull DL, Carsten CG., III Fistulas in octogenarians: are they beneficial? Ann Vasc Surg. 2015;29(1):98-102. [DOI] [PubMed] [Google Scholar]
- 14. Moist LM, Trpeski L, Na Y, Lok CE. Increased hemodialysis catheter use in Canada and associated mortality risk: data from the Canadian Organ Replacement Registry 2001-2004. Clin J Am Soc Nephrol. 2008;3(6):1726-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tangri N, Moorthi R, Tighiouhart H, Meyer KB, Miskulin DC. Variation in fistula use across dialysis facilities: is it explained by case-mix? Clin J Am Soc Nephrol. 2010;5(2):307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Ters M, Schears GJ, Taler SJ, et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case-control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Hare AM, Bertenthal D, Walter LC, et al. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int. 2007;71(6):555-561. [DOI] [PubMed] [Google Scholar]
- 18. Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM. Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol. 2012;7(3):466-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559. [DOI] [PubMed] [Google Scholar]
- 20. Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol. 2006;17(3)(suppl 1):S1-S27. [DOI] [PubMed] [Google Scholar]
- 21. Weber CL, Djurdjev O, Levin A, Kiaii M. Outcomes of vascular access creation prior to dialysis: building the case for early referral. ASAIO J. 2009;55(4):355-360. [DOI] [PubMed] [Google Scholar]
- 22. Lopez-Vargas PA, Craig JC, Gallagher MP, et al. Barriers to timely arteriovenous fistula creation: a study of providers and patients. Am J Kidney Dis. 2011;57(6):873-882. [DOI] [PubMed] [Google Scholar]
- 23. DeSilva RN, Patibandla BK, Vin Y, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013;24(8):1297-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacRae JM, Dipchand C, Oliver M, et al. ; on behalf of the Canadian Society of Nephrology Vascular Access Work Group. Arteriovenous access failure, stenosis, and thrombosis. Can J Kidney Health Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(3):464-478. [DOI] [PubMed] [Google Scholar]
- 26. Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allemang MT, Schmotzer B, Wong VL, et al. Arteriovenous grafts have higher secondary patency in the short term compared with autologous fistulae. Am J Surg. 2014;208(5): 800-805. [DOI] [PubMed] [Google Scholar]
- 28. Lee T, Barker J, Allon M. Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol. 2007;18(6):1936-1941. [DOI] [PubMed] [Google Scholar]
- 29. Sidawy AN, Spergel LM, Besarab A, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48(5)(suppl):2S-25S. [DOI] [PubMed] [Google Scholar]
- 30. Spergel LM, Ravani P, Asif A, Roy-Chaudhury P, Besarab A. Autogenous arteriovenous fistula options. J Nephrol. 2007;20(3):288-298. [PubMed] [Google Scholar]
- 31. Saad TF, Ahmed W, Davis K, Jurkovitz C. Cardiovascular implantable electronic devices in hemodialysis patients: prevalence and implications for arteriovenous hemodialysis access interventions. Semin Dial. 2015;28(1):94-100. [DOI] [PubMed] [Google Scholar]
- 32. Conrad MC, Toole JF, Janeway R. Hemodynamics of the upper extremities in subclavian steal syndrome. Circulation. 1965;32(3):346-351. [DOI] [PubMed] [Google Scholar]
- 33. Allon M, Lockhart ME, Lilly RZ, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60(5):2013-2020. [DOI] [PubMed] [Google Scholar]
- 34. Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology. 2000;217(1):83-88. [DOI] [PubMed] [Google Scholar]
- 35. Silva MB, Jr, Hobson RW, III, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302-307; discussion 307-308. [DOI] [PubMed] [Google Scholar]
- 36. Elsharawy MA, Moghazy KM. Impact of pre-operative venography on the planning and outcome of vascular access for hemodialysis patients. J Vasc Access. 2006;7(3):123-128. [DOI] [PubMed] [Google Scholar]
- 37. Wong CS, McNicholas N, Healy D, et al. A systematic review of preoperative duplex ultrasonography and arteriovenous fistula formation. J Vasc Surg. 2013;57(4):1129-1133. [DOI] [PubMed] [Google Scholar]
- 38. Kosa SD, Al-Jaishi AA, Moist L, Lok CE. Preoperative vascular access evaluation for haemodialysis patients. Cochrane Database Syst Rev. 2015(9):CD007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247(5):885-891. [DOI] [PubMed] [Google Scholar]
- 40. Lee T, Ullah A, Allon M, et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol. 2011;6(3):575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sahin L, Gul R, Mizrak A, et al. Ultrasound-guided infraclavicular brachial plexus block enhances postoperative blood flow in arteriovenous fistulas. J Vasc Surg. 2011;54(3):749-753. [DOI] [PubMed] [Google Scholar]
- 42. Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in “side-to-end” fistulae for haemodialysis access. Nephrol Dial Transplant. 2013;28(4):997-1005. [DOI] [PubMed] [Google Scholar]
- 43. Schild AF, Pruett CS, Newman MI, et al. The utility of the VCS clip for creation of vascular access for hemodialysis: long-term results and intraoperative benefits. Cardiovasc Surg. 2001;9(6):526-530. [DOI] [PubMed] [Google Scholar]
- 44. Lin PH, Bush RL, Nelson JC, et al. A prospective evaluation of interrupted nitinol surgical clips in arteriovenous fistula for hemodialysis. Am J Surg. 2003;186(6):625-630. [DOI] [PubMed] [Google Scholar]
- 45. Saucy F, Haesler E, Haller C, Deglise S, Teta D, Corpataux JM. Is intra-operative blood flow predictive for early failure of radiocephalic arteriovenous fistula? Nephrol Dial Transplant. 2010;25(3):862-867. [DOI] [PubMed] [Google Scholar]
- 46. Corpataux JM, Haesler E, Silacci P, Ris HB, Hayoz D. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant. 2002;17(6):1057-1062. [DOI] [PubMed] [Google Scholar]
- 47. Dixon BS. Why don’t fistulas mature? Kidney Int. 2006;70(8):1413-1422. [DOI] [PubMed] [Google Scholar]
- 48. Lu DY, Chen EY, Wong DJ, et al. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188(1):162-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol. 2013;9(6):348-357. [DOI] [PubMed] [Google Scholar]
- 50. Lauvao LS, Ihnat DM, Goshima KR, Chavez L, Gruessner AC, Mills JL., Sr Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49(6):1499-1504. [DOI] [PubMed] [Google Scholar]
- 51. Vachharajani TJ. Diagnosis of arteriovenous fistula dysfunction. Semin Dial. 2012;25(4):445-450. [DOI] [PubMed] [Google Scholar]
- 52. Tyberg JV. How changes in venous capacitance modulate cardiac output. Pflugers Arch. 2002;445(1):10-17. [DOI] [PubMed] [Google Scholar]
- 53. Ori Y, Korzets A, Katz M, Perek Y, Zahavi I, Gafter U. Haemodialysis arteriovenous access—a prospective haemodynamic evaluation. Nephrol Dial Transplant. 1996;11(1):94-97. [PubMed] [Google Scholar]
- 54. Iwashima Y, Horio T, Takami Y, et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40(5):974-982. [DOI] [PubMed] [Google Scholar]
- 55. Dundon BK, Torpey K, Nelson AJ, et al. The deleterious effects of arteriovenous fistula-creation on the cardiovascular system: a longitudinal magnetic resonance imaging study. Int J Nephrol Renovasc Dis. 2014;7:337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dundon BK, Torpey DK, Nelson AJ, et al. Beneficial cardiovascular remodeling following arterio-venous fistula ligation post-renal transplantation: a longitudinal magnetic resonance imaging study. Clin Transplant. 2014;28(8):916-925. [DOI] [PubMed] [Google Scholar]
- 57. Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10(3):160-166. [DOI] [PubMed] [Google Scholar]
- 58. Unal A, Tasdemir K, Oymak S, et al. The long-term effects of arteriovenous fistula creation on the development of pulmonary hypertension in hemodialysis patients. Hemodial Int. 2010;14(4):398-402. [DOI] [PubMed] [Google Scholar]
- 59. Reque J, Quiroga B, Ruiz C, et al. Pulmonary hypertension is an independent predictor of cardiovascular events and mortality in haemodialysis patients. Nephrology (Carlton). 2016;21(4):321-326. [DOI] [PubMed] [Google Scholar]
- 60. Li Z, Liang X, Liu S, et al. Pulmonary hypertension: epidemiology in different CKD stages and its association with cardiovascular morbidity. PLoS One. 2014;9(12):e114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Begin V, Ethier J, Dumont M, Leblanc M. Prospective evaluation of the intra-access flow of recently created native arteriovenous fistulae. Am J Kidney Dis. 2002;40(6):1277-1282. [DOI] [PubMed] [Google Scholar]
- 62. MacRae JM, Dipchand C, Oliver M, et al. ; on behalf of the Canadian Society of Nephrology Vascular Access Work Group. Arteriovenous access: infection, neuropathy, and other complications. Can J Kidney Health Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Asif A, Leon C, Orozco-Vargas LC, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2(6):1191-1194. [DOI] [PubMed] [Google Scholar]
- 64. Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225(1):59-64. [DOI] [PubMed] [Google Scholar]
- 65. Hsiao JF, Chou HH, Hsu LA, et al. Vascular changes at the puncture segments of arteriovenous fistula for hemodialysis access. J Vasc Surg. 2010;52(3):669-673. [DOI] [PubMed] [Google Scholar]
- 66. Kronung G. Plastic deformation of Cimino fistula by repeated puncture. Nephrol Dial Transplant. 1984;13:635-638. [Google Scholar]
- 67. Lee T, Barker J, Allon M. Needle infiltration of arteriovenous fistulae in hemodialysis: risk factors and consequences. Am J Kidney Dis. 2006;47(6):1020-1026. [DOI] [PubMed] [Google Scholar]
- 68. Parisotto MT, Schoder VU, Miriunis C, et al. Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int. 2014;86(4):790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Twardowski Z, Lebek R, Kubara H. 6-year experience with the creation and use of internal arteriovenous fistulae in patients treated with repeated hemodialysis. Pol Arch Med Wewn. 1977;57(3):205-214. [PubMed] [Google Scholar]
- 70. Chow J, Rayment G, San Miguel S, Gilbert M. A randomised controlled trial of buttonhole cannulation for the prevention of fistula access complications. J Ren Care. 2011;37(2): 85-93. [DOI] [PubMed] [Google Scholar]
- 71. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vaux E, King J, Lloyd S, et al. Effect of buttonhole cannulation with a polycarbonate PEG on in-center hemodialysis fistula outcomes: a randomized controlled trial. Am J Kidney Dis. 2013;62(1):81-88. [DOI] [PubMed] [Google Scholar]
- 73. Macrae JM, Ahmed SB, Hemmelgarn BR. Arteriovenous fistula survival and needling technique: long-term results from a randomized buttonhole trial. Am J Kidney Dis. 2014;63(4):636-642. [DOI] [PubMed] [Google Scholar]
- 74. Atkar RK, MacRae JM. The buttonhole technique for fistula cannulation: pros and cons. Curr Opin Nephrol Hypertens. 2013;22(6):629-636. [DOI] [PubMed] [Google Scholar]
- 75. Muir CA, Kotwal SS, Hawley CM, et al. Buttonhole cannulation and clinical outcomes in a home hemodialysis cohort and systematic review. Clin J Am Soc Nephrol. 2014;9(1):110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wong B, Muneer M, Wiebe N, et al. Buttonhole versus rope-ladder cannulation of arteriovenous fistulas for hemodialysis: a systematic review. Am J Kidney Dis. 2014;64(6):918-936. [DOI] [PubMed] [Google Scholar]
- 77. Nesrallah GE, Mustafa RA, MacRae J, et al. Canadian Society of Nephrology guidelines for the management of patients with ESRD treated with intensive hemodialysis. Am J Kidney Dis. 2013;62(1):187-198. [DOI] [PubMed] [Google Scholar]
- 78. Struthers J, Allan A, Peel RK, Lambie SH. Buttonhole needling of ateriovenous fistulae: a randomized controlled trial. ASAIO J. 2010;56(4):319-322. [DOI] [PubMed] [Google Scholar]
- 79. Huynh TN, Chacko BK, Teng X, et al. Effects of venous needle turbulence during ex vivo hemodialysis on endothelial morphology and nitric oxide formation. J Biomech. 2007;40(10):2158-2166. [DOI] [PubMed] [Google Scholar]
- 80. Unnikrishnan S, Huynh TN, Brott BC, et al. Turbulent flow evaluation of the venous needle during hemodialysis. J Biomech Eng. 2005;127(7):1141-1146. [DOI] [PubMed] [Google Scholar]
- 81. Fulker D, Kang M, Simmons A, Barber T. The flow field near a venous needle in hemodialysis: a computational study. Hemodial Int. 2013;17(4):602-611. [DOI] [PubMed] [Google Scholar]
- 82. Ball LK. Improving arteriovenous fistula cannulation skills. Nephrol Nurs J. 2005;32(6):611-617; quiz 618. [PubMed] [Google Scholar]
- 83. Kimata N, Karaboyas A, Bieber BA, et al. Gender, low Kt/V, and mortality in Japanese hemodialysis patients: opportunities for improvement through modifiable practices. Hemodial Int. 2014;18(3):596-606. [DOI] [PubMed] [Google Scholar]
- 84. Faratro R, Jeffries J, Nesrallah GE, MacRae JM. The care and keeping of vascular access for home hemodialysis patients. Hemodial Int. 2015;19(suppl 1):S80-S92. [DOI] [PubMed] [Google Scholar]