Abstract
Background:
Abiotrophia defectiva is a fastidious aerobic gram-positive bacterium which is part of the normal flora of the human oral cavity. It is an unusual cause of peritoneal dialysis–related peritonitis.
Case Presentation:
We present a case of a man in his fifties with end-stage renal failure secondary to polycystic kidney disease who presented with a cloudy peritoneal fluid effluent and a cell count of 35 620 × 106 cells/L with 90% polymorphonuclear cells. The fluid was cultured per unit protocol, and the organism was identified as Abiotrophia defectiva. Post–peritonitis dialysis technique review revealed frequent lapses in the use of facemask and hand washing during cycler connection and disconnection. The patient responded well to vancomycin; however, he subsequently developed ultrafiltration failure and symptoms of fluid overload and uremia and was transferred to home hemodialysis.
Conclusions:
Abiotrophia defectiva is an unusual cause of peritoneal dialysis–related peritonitis. The organism is a normal commensal of the oral cavity and may cause peritonitis in patients with nonadherence to dialysis technique. In our case, the infection was followed by peritoneal membrane failure and transfer to hemodialysis. It remains to be seen if peritonitis with Abiotrophia defectiva heralds a worse outcome.
Keywords: peritoneal dialysis, peritonitis, microbiology, technique failure
Abstract
Mise en contexte:
Une des causes inhabituelles de péritonites en situation de dialyse péritonéale est attribuée à Abiotrophia defectiva, une bactérie à Gram positif aérobie et exigeante qui fait partie de la flore normale de la cavité buccale.
Présentation du cas:
Nous présentons le cas d’un patient âgé de 51 ans atteint d’insuffisance rénale terminale à la suite d’une maladie polykystique des reins (MPR). Le patient présentait des effluents de liquide péritonéal troubles dont le compte cellulaire évalué à 35 620 × 106 cellules/L comportait 90 % de cellules polymorphonucléaires. Le fluide a été recueilli selon le protocole de l’unité de dialyse et le microorganisme responsable de l’infection identifié comme étant Abiotrophia defectiva. L’examen de la technique de dialyse après la péritonite a révélé de fréquentes lacunes dans le port du masque ou dans le lavage des mains au moment du branchement ou du débranchement du cycleur. Le patient a bien répondu au traitement antibiotique par la vancomycine, mais a tout de même connu une défaillance de microfiltration et développé des symptômes de surcharge liquidienne ainsi que d’urémie à la suite de sa péritonite, ce qui a nécessité son transfert sur hémodialyse à domicile.
Conclusions:
La bactérie Abiotrophia defectiva est une cause inhabituelle de péritonite reliée à la dialyse péritonéale. Ce microorganisme est un commensal normal de la cavité buccale et peut causer des péritonites chez les patients qui ne suivent pas les consignes strictes de cette technique de dialyse. Dans le cas présenté, l’infection a été suivie d’une défaillance de la membrane péritonéale qui a nécessité le transfert du patient sur hémodialyse. Il reste à démontrer si les cas de péritonites par Abiotrophia defectiva s’avèrent annonciateurs d’un pronostic aussi pessimiste.
Introduction
Abiotrophia defectiva is a species of fastidious aerobic gram-positive bacteria previously classified as a nutritionally variant streptococcus. It was included in its own genus in 1995 and revised in 2000.1,2 It is part of the normal human flora of the oral cavity. However, its pathogenic potential is described in rare circumstances in literature. We describe a case of peritoneal dialysis (PD)–associated peritonitis with this uncommon organism.
Case
Our patient was a man in his fifties with End Stage Renal Disease (ESRD) due to autosomal dominant polycystic kidney on PD for 3 years. His comorbidities included hypertension and dyslipidemia. Two years prior to the current presentation, he had an episode of coagulase-negative Staphylococcus and subsequently Klebsiella oxytoca peritonitis. These were treated with vancomycin and ceftazidime, respectively, as per unit protocol.
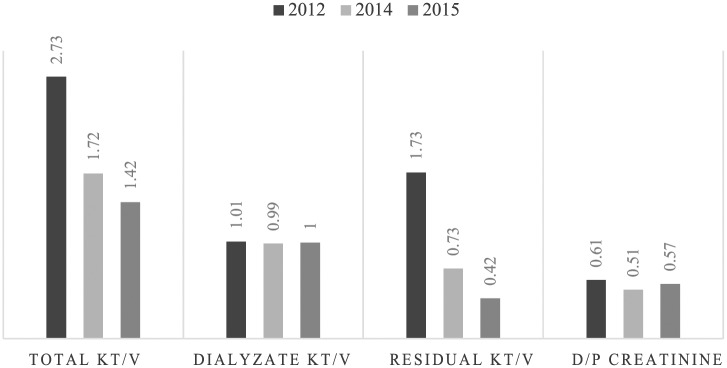
His surgical history was significant for a previous umbilical hernia which was repaired at the time of PD catheter insertion 3 years prior to current presentation, and this was subsequently complicated by an incisional hernia. He presented to the PD clinic with an overnight history of cloudy dialysis effluent. His blood pressure was 120/80 mm Hg, pulse was 104 beats/min, exit site was unremarkable, and he was afebrile. Mild diffuse rebound tenderness was elicited over the abdomen. The initial peritoneal fluid cell count was 35 620 × 106 cells/L of which 90% were polymorphs. Our local empiric peritonitis protocol was initiated consisting of ceftazidime 1 g intra peritoneal (IP), to dwell over 6 hours daily and vancomycin 1 gm IP over 6 hours every 5 days. Blood cultures were negative at 48 hours. His dialysis prescription at the time of his current presentation was cycler-assisted PD with 4 overnight exchanges of 2 L of 2.5% solution, with a last fill of 1 L of icodextrin solution as a day dwell. He had no uremic symptoms, and his blood pressure control was good. The results of his peritoneal equilibration test (PET) and adequacy are tabulated in Figure 1. The previous episodes of peritonitis did not affect the peritoneal membrane transporter status. His most recent kt/v before this incident episode was 1.42 (with the dialysate kt/v remaining at around 1, whereas the residual kt/v had dropped to 0.42).
Figure 1.
Peritoneal equilibration test results.
Post–peritonitis dialysis technique review revealed frequent lapses in the use of facemask and hand washing during cycler connection and disconnection. The PD effluent was cultured according to the standard operating protocols at the Provincial Laboratory for Public Health, Edmonton, Alberta. The specimen was inoculated into enrichment broth (BACTEC vial; Becton Dickinson, Mississauga, Ontario, Canada) as well as onto solid media for culture of aerobic and anaerobic organisms. The broth was positive for bacterial growth on day 3 of incubation. Twenty-four hours after subculture of the broth to solid media, fine alpha hemolytic colonies were observed on 5% sheep’s blood agar, satelliting around the Staphylococcus aureus streak and fine green colonies were seen on the chocolate agar plates, indicating a nutritionally fastidious organism. The gram stain of these colonies revealed gram-positive cocci in chains which tested catalase negative. The organism was identified as Abiotrophia defectiva with 99.9% probability on the Vitek Matrix-Assisted Light Desorption Ionisation–Time of Flight Mass Spectrometer (bioMérieux, Durham, NC, USA). The identification of this organism on this instrument has been verified in house according to the verification criteria in Cumitech 31A.3 No other organisms grew aerobically or anaerobically from this specimen.
Susceptibility testing was performed according to the Clinical & Laboratory Standards Institute (CLSI) M45 document using the gradient diffusion method (Etest; bioMérieux). Minimum inhibitory concentrations (MICs) were interpreted according to CLSI M45-A2 document.4 The isolate was susceptible to ceftriaxone MIC 0.19 mg/L, vancomycin MIC 0.38 mg/L, and intermediate to penicillin MIC 0.5 mg/L.
The patient received vancomycin for a total of 3 weeks, and repeat PD fluid cell count on day 3 of treatment decreased to 656 × 106 cells/L (62% polymorphs), with complete resolution of symptoms and clear dialysate. There was no evidence of systemic dissemination of the infection. Over the next 3 months, the patient developed ultrafiltration failure and symptoms suggestive of uremia like nausea, vomiting, poor appetite and energy, in addition to signs and symptoms of fluid overload. A formal PET was not performed. There was no improvement despite intensification of the dialysis prescription, and ultimately, the patient was deemed to have failed PD and transferred to home hemodialysis.
Discussion
Abiotrophia defectiva is the causative organism in 5% of cases of infective endocarditis. This organism has also been reported as a rare causative agent of meningitis, otitis media, ocular infections, pancreatic abscess, prosthetic joint infections, breast implant infections, and osteomyelitis.5-8 This organism has been reported as causative agent of PD peritonitis once before, and we describe the second only reported case and the first in North America.9 In previous retrospective studies,10-12 streptococcal peritonitis was associated with lower risk of relapse, catheter removal, and permanent transfer to hemodialysis as compared with nonstreptococcal peritonitis, but Abiotrophia was not specifically identified at that time. There is no direct evidence connecting PD membrane failure to the specific organism except for the history and temporal course of events. It remains to be seen if PD-related peritonitis with Abiotrophia defectiva heralds a worse outcome.
Footnotes
Ethics Approval and Consent to Participate: Formal research ethics board approval was not sought for this case report.
Consent for Publication: Patient has consented for publication and this has been documented in patient’s chart.
Availability of Data and Materials: Further data is available on file.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kawamura Y, Hou XG, Sultana F, Liu S, Yamamoto H, Ezaki T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int J Syst Bacteriol. 1995;45(4):798-803. [DOI] [PubMed] [Google Scholar]
- 2. Collins MD, Lawson PA. The genus Abiotrophia (Kawamura et al.) is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol. 2000;50(pt 1):365-369. [DOI] [PubMed] [Google Scholar]
- 3. Clark RB, Lewinski M, Loeffelholz MJ, Tibbetts RJ. Cumitech 31A, Verification and Validation of Procedures in the Clinical Microbiology Laboratory. Washington DC: American Society of Microbiology Press; 2009. [Google Scholar]
- 4. Wayne PA. CLSI, Methods for Antimicrobial Dilution and Disk Susceptibility of Infrequently Isolated or Fastidious Bacteria; Approved Guideline. 2nd ed. CLSI Document M45-A2. Wayne, Pennsylvania 19083-1898: Clinical & Laboratory Standards Institute; 2010. [Google Scholar]
- 5. Cassir N, Grillo J-C, Argenson J-N, Drancourt M, Levy P-Y. Abiotrophia defectiva knee prosthesis infection: a case report. J Med Case Reports. 2011;5:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos JN, dos Santos LS, Vidal LMR, et al. A case report and literature overview: Abiotrophia defectiva aortic valve endocarditis in developing countries. Infection. 2014;42(3):579-584. [DOI] [PubMed] [Google Scholar]
- 7. Tena D, Solís S, Lainez S, et al. Meningitis caused by Abiotrophia defectiva: case report and literature review. Infection. 2013;41(2):571-570. [DOI] [PubMed] [Google Scholar]
- 8. Cerceo E, Christie JD, Nachamkin I, Lautenbach E. Central nervous system infections due to Abiotrophia and Granulicatella species: an emerging challenge? Diagn Microbiol Infect Dis. 2004;48(3):161-165. [DOI] [PubMed] [Google Scholar]
- 9. Arslan U, Guney I, Yuksekkaya S, Atalay H, Dagý HT. First case of peritonitis due to Abiotrophia defectiva. Perit Dial Int. 2006;26(6):725-726. [PubMed] [Google Scholar]
- 10. Shukla A, Abreu Z, Bargman JM. Streptococcal PD peritonitis—a 10-year review of one centre’s experience. Nephrol Dial Transplant. 2006;21(12):3545–3549. [DOI] [PubMed] [Google Scholar]
- 11. O’Shea S, Hawley CM, McDonald SP, et al. Streptococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 287 cases. BMC Nephrol. 2009;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho Y, Johnson DW. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 2014;64(2):278-289. [DOI] [PubMed] [Google Scholar]