Abstract
Background:
Obesity is associated with increased surgical complications and long-term cardiovascular mortality. Studies of access in kidney transplantation have found a bias against obese patients on the wait-listing.
Objective:
To determine the current state of clinical practice for the management of obesity in kidney transplantation.
Design:
A survey in two versions, PDF and traditional paper, composed of categorical questions.
Setting:
A pan-Canadian survey of transplant nephrologists and surgeons.
Methods:
The survey PDF was distributed electronically to the Kidney Group of the Canadian Society of Transplantation. A shorter, hardcopy version was distributed subsequently at a national transplant meeting.
Results:
There were 47 responses, including almost every Canadian adult transplant program. Most (81%) reported the use of a body mass index limit for access to the waiting list. However, only 40% reported a strict enforcement. There were several instances of intra-hospital disagreements regarding the use of a policy, among the centers with multiple responses. The body mass index limit was most commonly 40 kg/m2 (62%), followed by 35 kg/m2 (36%). Despite the body mass index limit, few centers (30%) reported having a weight management program. The reported experience with bariatric surgery was small, though nearly all replied that they would refer to a bariatric specialist in the future.
Limitations:
This national survey provides a broad assessment of clinical practice. The distinction between an official policy and informal clinical tendencies is difficult. The results cannot be used to support any specific limit or policy.
Conclusions:
This survey found that the body mass index limit for access to the kidney transplant waiting list was common in Canada. Several inconsistencies suggest a lack of official policy. To achieve equity in access, clear guidelines for obesity should be established and enforced. Bariatric surgery has the promise of rapid weight loss. Resource allocation to study obesity in transplant patients will be essential.
Keywords: kidney transplantation, waiting list, obesity, weight loss, bariatric surgery
Abrégé
Mise en contexte:
L’obésité est associée à l’augmentation de complications survenant lors d’une intervention chirurgicale ainsi qu’à la mortalité à long terme des suites d’une maladie cardiovasculaire.
Objectif de l’étude:
L’étude visait à dresser l’état actuel des pratiques préconisées en matière de prise en charge pour les patients souffrant d’obésité dans le contexte de la transplantation rénale.
Cadre et type d’étude:
L’étude a consisté en un sondage pancanadien effectué en deux parties—sous forme de document PDF et papier—auprès des néphrologues et des chirurgiens pratiquant des greffes de reins. Le sondage était constitué de questions catégorielles.
Méthodologie:
Le document PDF a été distribué par voie électronique au Kidney Group of the Canadian Society of Transplantation. Une version imprimée et écourtée du sondage a par la suite été distribuée lors d’une assemblée nationale de spécialistes en transplantation rénale.
Résultats:
Nous avons obtenu 47 réponses provenant de presque tous les programmes canadiens en transplantation rénale. La grande majorité (81%) des répondants a rapporté l’utilisation d’une valeur limite de l’indice de masse corporelle (IMC) pour avoir accès aux listes d’attente pour une greffe. Par contre, seulement 40% des répondants ont mentionné appliquer ce paramètre de façon rigoureuse. En plusieurs occasions, nous avons remarqué qu’il existait des désaccords intrahôpitaux, au sein des centres ayant fourni plus d’un exemplaire du sondage, au sujet du recours à une politique quelle qu’elle soit. La valeur limite d’IMC la plus souvent mentionnée était 40 kg/m2 (62% des répondants), suivie par la valeur limite de 35 kg/m2 (36% des répondants). Malgré l’existence d’une valeur limite d’IMC, peu de centres (30%) ont rapporté détenir un programme formel de contrôle pondéral. Peu de centres ont mentionné avoir fait l’expérience de la chirurgie bariatrique quoique la majorité ait répondu vouloir consulter des spécialistes en chirurgie bariatrique dans le futur.
Limites de l’étude:
Ce sondage national procure une évaluation générale des pratiques cliniques en vigueur. La distinction entre une politique officielle et les tendances cliniques informelles demeure toutefois difficile. Les résultats colligés lors de la présente étude ne peuvent en aucun cas être utilisés pour définir quelconque limite ou politique.
Conclusions:
Ce sondage nous a permis de constater qu’il était répandu au Canada d’établir une valeur limite d’IMC pour permettre l’accès aux listes d’attente pour une greffe de rein. Le manque d’uniformité en la matière suggère toutefois l’absence d’une politique officielle. Des lignes directrices claires concernant l’obésité devraient être établies et mises en application afin de parvenir à un accès équitable. La chirurgie bariatrique promet une perte de poids rapide. L’allocation de ressources favorisant l’étude des patients obèses nécessitant une greffe de rein sera essentielle.
What Was Known Before
Although kidney transplant guidelines suggest that candidates lose weight to achieve a body mass index (BMI) below 30 kg/m2, there are no evidence-based recommendations for a BMI limit or the actual treatment of obesity.
What This Adds
This survey has found that in practice a BMI limit for candidates is common, yet there is poor enforcement and a lack of consensus on the details for the management of obesity.
Introduction
Morbid obesity constitutes a substantial health care burden in the renal failure population,1 as it is with the general population.2 The impact of obesity on surgical complications during kidney transplantation3,4 should not come as a surprise. Most importantly, despite being recognized as a risk factor for long-term cardiovascular death,5,6 it remains undertreated in transplant candidates and recipients alike.
A bias against obesity has been consistently observed in studies of access to kidney transplantation. A study of the United Network for Organ Sharing (UNOS) database demonstrated an increasingly longer time to transplantation and a decreasing likelihood of receiving a transplant with each class of obesity.7 Similarly, a study of the United States Renal Data System (USRDS) found that a greater BMI mediated the association between diabetes and reduced access to transplantation, including a longer time to listing, a longer time to living donor transplantation, and a longer time to transplantation after listing.8 There could be multiple reasons behind such a systemic bias, from official policy to individual tendencies in clinical practice.
Little literature has been published describing policies on obesity and kidney transplantation. A survey of the American Society of Transplant Surgeons (ASTS), that had been cited but never published, reported that 66 of 67 kidney transplant centers had a BMI limit in the range of 35 to 45 kg/m2.9 A survey on policy and listing in the United Kingdom found that the majority of programs (60%) excluded patients with a BMI exceeding a median limit of 35 kg/m2.10 A worldwide survey of mainly European nephrologists reported that the majority (63%) had a BMI limit for referral to a transplant center,11 which was most commonly 35 kg/m2 (29%), followed by 30 kg/m2 (27%) and 40 kg/m2 (7%).
Practice guidelines of the American Society of Trans-plantation (AST) and Canadian Society of Transplantation (CST) recommend weight loss for transplant candidates above a BMI of 30 kg/m2,12,13 yet scant details regarding the “how, when, and how much” are given. No suggestions are made for the use of limits, and as such these are left by default to individual programs if they so choose. Bariatric surgery is only mentioned as an unproven treatment in this population.12 The King’s College Hospital has reported the only prospective clinical trial of a comprehensive weight management plan using the relatively restrictive BMI limit of 30 kg/m2.14 Our own institutional policy at the Hôpital Maisonneuve-Rosemont includes a BMI limit for listing of 36.0 kg/m2, referral to bariatric surgery at a BMI of 40 kg/m2, and clinical surveillance every 3 months for all potential candidates with a BMI greater than 32 kg/m2.
This study presents the results of a survey of transplant specialists in Canada that was conducted to understand the practice patterns and policies in place for the management of obesity of a kidney transplant candidate, and to gauge the current experience with bariatric surgery.
Methods
Survey Design
In 2013, the kidney transplant group at Hôpital Maisonneuve-Rosemont sought to establish an official policy toward obesity in the evaluation of kidney transplant candidates. In an effort to establish a broad perspective of the current practice patterns across Canada, a survey was designed to gauge the multiple facets of obesity management in transplant candidates.
The survey was created with Microsoft Word and the questions were developed in 5 sections: identification of the respondent (4 questions), assessment of the candidate for eligibility for kidney transplantation (6 questions), perception of the impact of morbid obesity on renal transplantation (8 questions), bariatric surgery and kidney transplantation (5 questions), and personal opinion (7 questions). The questions pertaining to the assessment of the candidate were formulated to determine which of the criteria were used in the evaluation and surveillance of patients. This group of clinical questions, though not necessarily exhaustive, includes those that our group encountered during the development of our local policy and was felt to be clinically relevant. The section of “Perception and Personal Opinion” was designed to probe the clinical opinion of respondents regarding the risks associated with obesity, the expectations for successful weight loss, and the impact on the eligibility of patients for kidney transplantation. The questions regarding bariatric surgery were formulated to gauge the experience of the transplant specialist with patients who had had bariatric surgery, prior to or before kidney transplantation. The survey was subsequently reduced to a short form, a single page, in view of its distribution as a hardcopy version (18 questions total). The sections on perception of obesity and personal opinion were eliminated or shortened to limit the survey to 1 page and hopefully decrease the perceived effort required to complete the survey. These questions were deemed expendable because of the relatively homogeneous responses obtained in the initial survey. Almost all questions were multiple choice using a set of check-boxes, except for questions regarding the BMI limit, which required a written numeric response. Additional comments were permitted at the end of each survey. This study was approved retroactively by the Ethics in Research Committee at the Centre de Recherche de l’Hôpital Maisonneuve-Rosemont (study number: 2017-345).
Survey Participants and Dissemination
The distribution of the survey was aimed to attain a nation-wide participation of kidney transplant specialists, nephrologists, and surgeons. Transplant coordinators, nurse specialists, or practitioners were initially invited to respond to the survey, but no responses were obtained. The survey was sent by e-mail as an attachment to the members of the Adult Kidney Group of the Canadian Society of Transplantation (72 members, including nephrologists, transplant surgeons, and allied health professionals). A French language version was sent to members of the Comité de Rein-Pancréas of Transplant Québec (10 members), which includes one nephrologist and one surgeon from each of the five kidney transplant centers in Québec. There was likely only a small overlap of the mailing lists of the adult kidney group of the CST and the Comité de Rein-Pancréas of Transplant Québec, although the exact number is unknown as the adult kidney group’s mailing list was kept confidential from the survey researchers. The surveys were sent twice by e-mail within a 2-week interval in May 2013. Completed surveys were returned by e-mail or facsimile. The short-form survey was distributed at the meeting of Canadian Transplant Forum in Montreal, Canada, in November 2013. At this meeting, there were approximately 80 attendees, including nephrologists, coordinators, nurses, and surgeons.
Data Analysis
All respondent data were collected in an Excel spreadsheet and expressed as the number and percentage. Data analysis was performed using standard descriptive statistical methods. Two respondents had completed both versions of the survey, of which the short-form version was discounted.
Results
In total, 47 responses were collected representing 17 of the 18 adult transplant centers in Canada. The respondent centers were located in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, and Nova Scotia. The first version received 31 responses for an approximate return rate of 32%. The short-form version collected a further 16 responses of the approximately 80 attendees of the Canadian Transplant Forum (20%). The only transplant center not represented was a small volume (<20 transplants annually) hospital without a living donor program. Multiple responses were returned from 12 centers. The specialties represented included nephrologists (n = 35) and transplant surgeons (n = 10), while there were two responses that were completed collectively by the center’s transplant group.
Official Policy Regarding Morbid Obesity for Candidates
The majority of transplant specialists (81%) reported that their center had an official policy for obesity in the candidate selection process for kidney transplantation. Most transplant centers had at least one response that reported the use of a policy (89%). However, there was a significant level of intra-center disagreement about whether there was a policy or not (42% of centers with multiple survey responses), either between individuals or, between an individual and the group response.
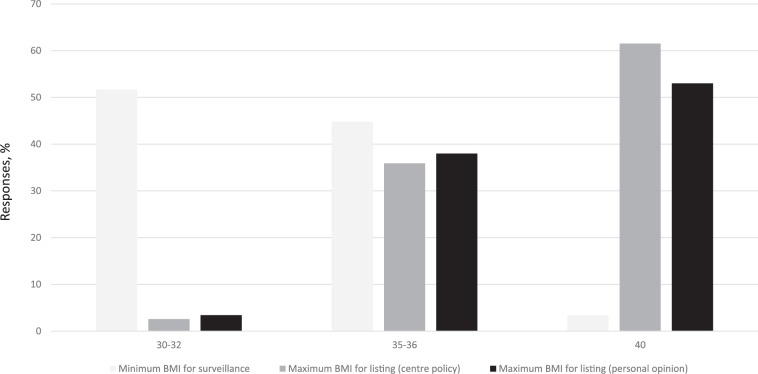
The maximum BMI limit in the transplant candidate was most commonly reported at 40 kg/m2 (62%) followed by 35 kg/m2 (36%; Figure 1). However, less than half (40%) reported that their limit was strictly enforced and just below two thirds stated that patients were regularly monitored for obesity. The surveillance started at a lower BMI, 30 kg/m2 most commonly (52%) then 35 kg/m2 (45%). The majority of respondents (63%) would place a candidate on hold if the maximum was exceeded during surveillance.
Figure 1.
The BMI limits for obesity in kidney transplant candidates.
Note. BMI = body mass index.
Almost all specialists (94%) reported that obesity was taken into consideration during the selection process. In the comments section, numerous respondents noted the use of several other anthropometric criteria: abdominal circumference (n = 13), fat distribution (abdominal vs hip; n = 9), and waist to hip ratio (n = 3). Other criteria mentioned included exercise tolerance or physical functioning (n = 3), co-morbidities (n = 2), muscle content (n = 2), and compliance (n = 1). Assessment of the impact of obesity was also a reason for consultation of a transplant surgeon (78%), with referrals being requested most commonly at a BMI of 35 kg/m2 (64%).
Weight Loss Program Specific for the Chronic Renal Failure and Transplant Patient
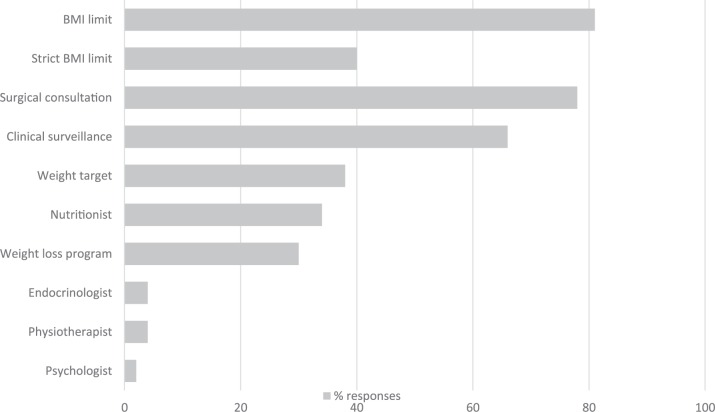
Given that a large majority of specialists reported a maximum limit for BMI, relatively few (30%) reported having a weight loss program available for obese patients. The most commonly cited interventions used, though not necessarily as part of a program, were a target weight (38%) and a consultation with a nutritionist (34%). Clinical surveillance was used at intervals of 3, 6, or 12 months. A time limit to achieve the weight loss goal was uncommon (13%), as was the consultation of other specialists and allied health professionals such as physiotherapists (4%), endocrinologists (4%), and psychologists (2%; Figure 2).
Figure 2.
Weight loss strategies for kidney transplant candidates.
Note. BMI = body mass index.
Bariatric Surgery for Transplant Candidates and Recipients
Although most transplant specialists reported a previous experience (76%) with patients having had or undergoing bariatric surgery, fewer than half had personally referred a patient (47%). Mostly, the reported experience with bariatric surgery tended to be small, less than 5 cases per specialist (75% of those who reported any experience at all). No respondent reported an experience of more than 10 cases. Of the respondents who consulted bariatric surgery routinely, the referral BMI was split evenly between 35 and 40 kg/m2.
The experience of transplant specialists was mainly with patients having had bariatric surgery prior to wait-listing (and likely prior to referral for transplantation; 93% of respondents), whereas fewer had had experience with patients undergoing bariatric surgery while on the waiting list (20%) or after transplantation (17%). The types of bariatric surgery performed were most commonly sleeve gastrectomy (n = 11), gastric banding (n = 7), and Roux-en-Y gastric bypass (n = 7), while a significant proportion were unable to identify the type of surgery (n = 9). Noted post-bariatric complications included dysphagia (n = 3), nephrolithiasis (n = 3), anastomotic leak (n = 2), deep vein thrombosis or pulmonary embolus (n = 2), and pneumonia (n = 1). Interestingly, there were no reports of acute on chronic renal failure or dehydration.
A common concern about bariatric surgery is the effect on the pharmacokinetics of immunosuppressive medication. Four survey respondents noted that they had had an experience with an increase in the dosing of immunosuppressive medication after bariatric surgery: tacrolimus (n = 3), mycophenolate mofetil (n = 1), enteric-coated mycophenolate sodium (n = 1), and prednisone (n = 1).
When queried regarding management in the future, nearly all specialists indicated that they would refer patients for bariatric surgery. Most were comfortable with the surgery in the context of pre-transplantation (93%), hemodialysis (93%), and pre-dialysis (89%). Slightly fewer would refer a patient who was on peritoneal dialysis (76%) or post-transplantation (74%). Only one respondent felt that the risks of bariatric surgery were too high in this population.
Perception and Personal Opinion
The perception of obesity among transplant specialists was relatively homogeneous. For a BMI above 36 kg/m2, 97% of respondents would counsel the patient to lose weight by diet and exercise. All felt that obesity leads to increased risk of intra-operative and post-transplant complications, decreased patient survival (93%), and decreased graft survival (77%). Few (7%) responded that weight loss while on dialysis was associated with too much risk, and 27% felt that attempting weight loss in the chronic renal failure patient was futile.
The majority (88%) felt that morbid obesity should be a contra-indication for kidney transplantation, and that the limit, if judged by BMI, should be 40 kg/m2 (53%) or 35 kg/m2 (38%; Figure 1), though there was still a significant portion who had replied “don’t know” (n = 11). Almost all felt that patients should be required to lose some weight prior to transplantation (97%), whereas relatively few felt that they should be required to lose all excess weight prior to listing (17%). The majority (72%) also felt that many patients are not referred to transplantation due to morbid obesity. Only a minority (33%) replied that morbidly obese patients would be unfairly discriminated against if a BMI limit were used.
The rates of unanswered questions were between 4.5% and 9%, except for the section concerning bariatric surgery. Questions such as, a previous experience with bariatric surgery (29%), the post-bariatric surgery complications (16%), and modification of immunosuppression as a result of bariatric surgery (23%) had experienced higher rates of non-response.
Discussion
The prevailing practice among kidney transplant specialists in Canada is in favor of obesity as a contra-indication for renal transplantation. This survey found that most transplant centers had at least one specialist who reported the use of a maximum limit of BMI for transplant candidates, furthermore, most respondents reported the use of a maximum BMI. In terms of personal opinion, the majority also agreed that morbid obesity should be a contra-indication for transplantation. In the United States, a survey of 67 ASTS kidney transplant centers found that almost all (99%) reported using a BMI limit, though the results have never been formally published.9
The objective of this survey was also to determine the range of clinical practice in Canada by comparing each center’s official policy. However, the questions were not specifically developed to distinguish an official policy versus a bias or tendency, such as a “case-by-case” basis. There are several indirect observations that suggest a lack of official policy across Canada. Foremost, the majority of respondents (57%) reported that their BMI limit was not strictly enforced. Also, of the centers with multiple respondents, a significant proportion had internal disagreements about whether there was an official policy. In a UK study on access to transplantation, up to 25% of centers had an internal disagreement regarding the limit on obesity.10 Furthermore, in this survey, both centers where a group answer had been provided, also had separate individual respondents who differed in their answers to the questions of the BMI limit, monitoring, and management. Among the respondents who answered negatively to having an official policy, more than half (5/9) still provided details regarding a maximum acceptable BMI for listing and surveillance. This suggests an informal policy more so than an official one. Perhaps in hindsight, one weakness of the survey is the lack of a definition for an “official policy.” In our personal experience, the official policy should be used by all specialists in a center, nephrologist, surgeon, nurses, and other health professionals. It should govern the management of obese candidates, including the criteria of obesity and risk, and limits for listing and surveillance. Practically, the development of the official policy should be recorded in the minutes of a multidisciplinary meeting and subsequently, clearly written and discussed with obese patients. As a whole, the numerous instances of inconsistent results in this survey suggest that very few centers employed an official policy defined this way. This survey should provide the impetus for transplant centers to develop and formalize a policy on obesity.
The danger of a poorly defined or an informal policy is the potential for inequity in the access to transplantation. Patients share the same dialysis units and because obesity is common and difficult to hide, an inconsistently enforced policy could fuel a sentiment of injustice. This could be avoided with a defined and universally enforced policy regarding obesity. Any institutional policy will have to be determined by the consensus of each transplant center’s own multidisciplinary group.
In Canada, the most common BMI limits used were 40 kg/m2 followed by 35 kg/m2. Both are likely based on the grading for obesity as defined by the National Institute of Health and the World Health Organisation. The same limits have also been reported in the United Kingdom and the United States. The median limit reported in the United Kingdom was 35 kg/m2,10 whereas the range reported from a survey of ASTS kidney transplant program was between 35 and 45 kg/m2. Several retrospective case series have described the details of a transplant center’s policy for obesity (Table 1). Generally, the BMI limits used fall within the range observed in this survey. At one extreme, the University of Chicago has reported using no limit on obesity and applying a robotic technique for kidney transplantation to minimize post-operative wound infections.15 This type of approach obviously does not address the long-term risks associated with obesity but adapts the surgical technique to minimize the short-term consequences of obesity. Interestingly, this center has also performed a case of simultaneous kidney transplantation and sleeve gastrectomy.16 Tulane University has a maximum BMI for listing of 42 kg/m2 and requires a close clinical follow-up of every 3 months.17 The policy at the University of California at San Francisco sets the BMI limit at 40 or 35 kg/m2 with 1 co-morbid disease of the metabolic syndrome. With such a policy any patient who is ineligible for kidney transplantation has an indication for bariatric surgery.18 At Stanford University, patients with a BMI greater than 40 kg/m2 are refused and referred for bariatric surgery, whereas those with a BMI between 35 and 39 kg/m2 are considered with the proviso of weight loss to achieve a BMI below 35 kg/m2.19 The Houston Methodist Hospital used a BMI limit of 38 kg/m2 for listing, but required a BMI below 35 kg/m2 at time of transplantation.20
Table 1.
Published Policies for Obesity in Kidney Transplant Programs.
| Hospital center | Maximum BMI | Weight loss program | Clinical surveillance | Indications for bariatric surgery | Conditional listing |
|---|---|---|---|---|---|
| Cincinnati | 38a | (Not indicated) | (Not indicated) | >40, or >35 with co-morbidity | (Not indicated) |
| Illinois | None | Noneb | None | None | No restrictions |
| Tulane | 42 | Nutritionist, weight contract | 3 month × 1 year | >45 | Inactive for 1 year |
| UCSF | 40 35c |
(Not indicated) | (Not indicated) | Failure of conservative | (Not indicated) |
| Stanford | 40 35-40d |
(Not indicated) | (Not indicated) | >40 | 35-40 with proviso of weight loss to <35 |
| Methodist | 38 | (Not indicated) | (Not indicated) | >40 | Proviso of a BMI <35 at time of transplantation |
| UCLA | 40 35-39e |
Supervised for individuals deemed suitable | (Not indicated) | >40 or failure of conservative |
(Not indicated) |
| King’s College | 30 | Diet, exercise, behavior therapy, Orlistat | Monthly × 6 months then biannually | (not indicated) | (Not indicated) |
| Hôpital Maisonneuve-Rosemont | 36.0 | Nutritionist, regular exercise | 3 months | >40 | None |
Note. BMI = body mass index; UCSF = University of California at San Francisco; UCLA = University of California at Los Angeles.
Relative contra-indication, considered with co-morbidities.
No weight loss program is used for the transplant candidate, though a robotic-assisted kidney transplant could be utilized to apply a minimally invasive approach to the operation and diminish the incision-related complications.
With diabetes.
By evaluation of the transplant surgeon.
With two co-morbidities and central obesity.
The evidence to support any particular BMI limit is not extensive. A USRDS study of wait-listed end-stage renal disease patients (1995-1999) found that at a BMI greater than 41 kg/m2,21 the survival benefit of renal transplantation was lost, though the statistical power was quite low because of the small numbers of patients in this weight class. A more recent study of the same database (1997-2007) was able to demonstrate a significant short-term survival benefit at 1 year for patients with a BMI greater than 40 kg/m2, albeit at a relatively lower benefit relative to the non-obese weight groups.22 It should be noted that a survival benefit observed at 1 year does not capture the increased long-term cardiovascular risk associated with obesity. In another database study, there was a significant increase in long-term risks of death (due to cardiovascular disease and infections) with a functioning graft and of death-censored graft loss associated with a BMI above 36 kg/m2.23 Pragmatically, the options remain 35 and 40 kg/m2, depending on whether a center wishes to be more exclusive or inclusive. The theoretical benefits of the more strict, 35 kg/m2, limit could be a lower incidence of post-operative complications and lower long-term cardiovascular mortality. A higher limit of 40 kg/m2 would result in more patients gaining access to transplantation with the associated short-term improvement in survival (vs remaining on dialysis), but at the cost of higher rates of peri-operative complications and long-term cardiac mortality. Future studies should attempt to define the inflection point where the risk associated with obesity begins to affect the long-term survival of the graft and the patient as compared with a non-obese recipient.
Whether BMI is the most appropriate metric for the risk associated with obesity in the chronic renal failure or transplant populations is unknown, as no other measure has been studied as extensively. In this survey, numerous other anthropometric criteria were suggested by the respondents. Most were measures of an abdominal distribution or central-type obesity, such as abdominal circumference and waist to hip ratio, and more subjectively, physical examination by a transplant surgeon. These measures have also been shown previously to correlate with worse cardiovascular outcomes24-27 and, obviously, a more difficult exposure during the transplant operation. Other suggested criteria, such as total fat content or total muscle mass are more difficult to use clinically. Muscle wasting, or sarcopenia, has been shown to be a strong predictor of poor outcome in chronic renal failure patients,28 though it may be more a measure of malnutrition than of obesity. Measurements such as abdominal circumference and waist to hip ratio, can be used in combination with the BMI, to gauge the prognosis of a patient’s body habitus, and certainly must be included in any future clinical studies. In general, BMI despite its limitations, is still the most commonly used measure in the clinic and in research. The National Institute of Health’s definition of obesity29 and the indications for bariatric surgery30 are also graded by BMI. It is easily calculated with precision from routine clinical information. The bulk of controversies regarding BMI as a prognostic measure of obesity in obesity may derive from the general definition of obesity as a BMI greater than 30 kg/m2, and the relatively small number of patients in study populations in whom the BMI exceeds 35 or 40. Relatively little data exist to support the use of other metrics, and extensive research will be required to develop risk stratification in association with the other measures of obesity in the chronic renal failure or transplant patient.
This survey found that most transplant centers did not have a weight loss management program. Although nearly all specialists counseled patients to lose weight, the effectiveness of such an approach is generally negligible.31 The use of a structured weight loss program can result in successful long-term weight loss maintenance of about 20% at 5 years.32 However, this survey has found a surprisingly low level of involvement of allied health professionals. Only 34% replied that they were using nutritionists, 4% physiotherapists, and 2% psychologists, and as a whole reflects the dearth of support and resource allocation dedicated to treating obesity. The only weight loss program prospectively studied consisted of a low-fat renal-specific diet, regular exercise, cognitive behavioral therapy, and Orlistat, an oral lipase inhibitor.14 Intensive monthly clinical surveillance for 6 months was followed by biannual clinical visits until the study completion at 2 years. Significantly more weight loss was achieved in the weight management group, exclusively during the intensive phase, as compared with “usual care.” As a result, significantly more (35% vs 6%) achieved the necessary weight loss for listing. This type of program is labor intensive and its cost effectiveness is unknown. Furthermore, Orlistat has never achieved widespread acceptance as a treatment of obesity and its relative contribution to this weight loss program is unknown. The effectiveness of any weight management program needs to be studied prospectively to determine its efficacy, though patients clearly need more support than what is currently being offered.
In general, the reported experience among Canadian transplant specialists with bariatric surgery was relatively minor (all under 10 cases). Only a few reported referring patients regularly. Several respondents noted in the comments that their access to bariatric surgery was limited or, not available at all in their practice region. Interestingly, despite a relatively novice experience, almost all respondents indicated that they would consider bariatric surgery for obese transplant candidates in the future. This may be the result of the obvious success bariatric surgery has achieved in the non-transplant population coupled with the lack of effective alternatives. The National Institutes of Health (NIH) indications for bariatric surgery (BMI > 40 alone or, a BMI > 35 with diabetes, hypertension, or dyslipidemia)33 apply to almost all chronic renal failure patients who exceed currently used BMI limits. Theoretically, surgical weight loss could help a patient rapidly achieved the BMI limit for access to transplantation. The benefits should also include the long-term improvements that have already been shown for diabetes, hypertension, and quality of life. Although the current published experience consists only of small retrospective series, the potential is tantalizing. From this survey, almost all transplant specialists were willing to try. Obesity must be considered as a modifiable risk factor, and treatment should be sought much like revascularization for coronary atherosclerosis.
The response rate was relatively low but not unexpected for a survey of an exploratory nature. As there was at least one response for nearly every transplant center, this survey provides a general idea of the practice patterns concerning obesity in kidney transplantation in Canada. There was no verification of the validity of the responses, such as the adherence to the limits cited by each specialist. This could be done by studying the BMI of a cohort of recent kidney transplant recipients at each center. These survey results from 2013-2014 also reflect clinical practice during that period and may have changed since. It is also important to note that the results of this survey do not support any particular policy or limit, but rather they demonstrate indirectly a lack of policy in practice. An increased number of responses from all centers would perhaps have permitted a better understanding of intra-center differences or even the differences between surgeons and nephrologists. The proportion of unanswered questions was not unusual. The details pertaining to the type of bariatric surgery seem to be largely unknown to the transplant specialist. This may not be surprising as the patient likely had the surgery in the past, prior to referral for transplantation, and often at a different hospital. It should be highlighted that transplant specialists be better informed of the nature of any prior bariatric surgery, in particular the mechanism used for weight loss, whether restrictive, malabsorptive, or both, as these may have an impact on absorption of drugs and are associated with certain metabolic complications.
Conclusion
This survey has found that the vast majority of kidney transplant centers in Canada try to use a BMI limit for candidates. This limit appears to be inconsistently enforced and variable between different centers. Furthermore, there is a general lack of support for patients in their effort to lose weight and gain access to the transplant list. The current level of experience with bariatric surgery in transplant patients is relatively small, yet promising enough that almost all specialists appear willing to try.
Equity balanced with utility are the pillars that should govern access to transplantation. In the short term, transplant centers need to formalize their local policy for obesity and provide consistent rules governing access for all potential local candidates. The policy should cover the limit, evaluation, surveillance, and management of obesity. Future prospective research should focus on the determination of the long-term risk associated with morbid obesity. Furthermore, prospective studies should evaluate the effect of weight loss treatment on the peri-operative risk and the long-term cardiovascular complications to maximize the long-term utility of the kidney graft. Obesity should be considered a modifiable risk factor and should not be left untreated as a potentially effective treatment is already in common use in the general population.
Acknowledgments
Drs Brigitte Goulet and Lynne Senecal who provided assistance in the translation of the survey.
Footnotes
List of Abbreviations: UNOS, United Network for Organ Sharing; BMI, body mass index; NIH, National Institutes of Health; UK, United Kingdom; ESRD, end-stage renal disease; ASTS, American Society of Transplant Surgeons; USRDS, United States Renal Data System.
Ethics Approval and Consent to Participate: This was not applicable.
Consent for Publication: This was not applicable.
Availability of Data and Supporting Materials: This was not applicable.
Author Contributions: GC was involved in the conception of the project, development of the survey, analysis of the data, and drafting of the article. MS was involved in the development of the survey, analysis of the data, and drafting of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17(5):1453-1459. [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497. [DOI] [PubMed] [Google Scholar]
- 3. Pham PT, Danovitch GM, Pham PC. Kidney transplantation in the obese transplant candidates: to transplant or not to transplant? Semin Dial. 2013;26(5):568-577. [DOI] [PubMed] [Google Scholar]
- 4. Srinivas TR, Meier-Kriesche HU. Obesity and kidney transplantation. Semin Nephrol. 2013;33(1):34-43. [DOI] [PubMed] [Google Scholar]
- 5. Lentine KL, Rocca-Rey LA, Bacchi G, et al. Obesity and cardiac risk after kidney transplantation: experience at one center and comprehensive literature review. Transplantation. 2008;86(2):303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong KA, Campbell SB, Hawley CM, Nicol DL, Johnson DW, Isbel NM. Obesity is associated with worsening cardiovascular risk factor profiles and proteinuria progression in renal transplant recipients. Am J Transplant. 2005;5(11):2710-2718. [DOI] [PubMed] [Google Scholar]
- 7. Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008;19(2):349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patibandla BK, Narra A, DeSilva R, Chawla V, Goldfarb-Rumyantzev AS. Access to renal transplantation in the diabetic population-effect of comorbidities and body mass index. Clin Transplant. 2012;26(3):E307-E315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pondrom S. The AJT report: news and issues that affect organ and tissue transplantation. Am J Transplant. 2012;12(7):1663-1664. [DOI] [PubMed] [Google Scholar]
- 10. Akolekar D, Forsythe JL, Oniscu GC. Impact of patient characteristics and comorbidity profile on activation of patients on the kidney transplantation waiting list. Transplant Proc. 2013;45(6):2115-2122. [DOI] [PubMed] [Google Scholar]
- 11. Stenvinkel P, Ikizler TA, Mallamaci F, Zoccali C. Obesity and nephrology: results of a knowledge and practice pattern survey. Nephrol Dial Transplant. 2013;28(suppl 4):iv99-iv104. [DOI] [PubMed] [Google Scholar]
- 12. Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173(10):1181-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(suppl 2):3-95. [PubMed] [Google Scholar]
- 14. MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55(1):69-76. [DOI] [PubMed] [Google Scholar]
- 15. Oberholzer J, Giulianotti P, Danielson KK, et al. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant. 2013;13(3):721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bariatric News. http://www.bariatricnews.net/?q=news/11404/first-simultaneous-robotic-kidney-transplant-and-lsg. Published 2012.
- 17. Killackey M, Zhang R, Sparks K, Paramesh A, Slakey D, Florman S. Challenges of abdominal organ transplant in obesity. South Med J. 2010;103(6):532-540. [DOI] [PubMed] [Google Scholar]
- 18. Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9(5):653-658. [DOI] [PubMed] [Google Scholar]
- 19. Scandling JD. Kidney transplant candidate evaluation. Semin Dial. 2005;18(6):487-494. [DOI] [PubMed] [Google Scholar]
- 20. Tariq N, Moore LW, Sherman V. Bariatric surgery and end-stage organ failure. Surg Clin North Am. 2013;93(6):1359-1371. [DOI] [PubMed] [Google Scholar]
- 21. Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC. Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int. 2003;63(2):647-653. [DOI] [PubMed] [Google Scholar]
- 22. Gill JS, Lan J, Dong J, et al. The survival benefit of kidney transplantation in obese patients. Am J Transplant. 2013;13(8):2083-2090. [DOI] [PubMed] [Google Scholar]
- 23. Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73(1):70-74. [DOI] [PubMed] [Google Scholar]
- 24. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850-856. [DOI] [PubMed] [Google Scholar]
- 25. Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed). 1984;288(6428):1401-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055-1058. [DOI] [PubMed] [Google Scholar]
- 27. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735-2752. [DOI] [PubMed] [Google Scholar]
- 28. Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86(3):633-638. [DOI] [PubMed] [Google Scholar]
- 29. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S-209S. [PubMed] [Google Scholar]
- 30. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(suppl 2):615S-619S. [DOI] [PubMed] [Google Scholar]
- 31. Treatment of obesity in adults. Council on Scientific Affairs. JAMA. 1988;260(17):2547-2551. [PubMed] [Google Scholar]
- 32. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579-584. [DOI] [PubMed] [Google Scholar]
- 33. Gastrointestinal surgery for severe obesity. Proceedings of a National Institutes of Health Consensus Development Conference March 25-27, 1991, Bethesda, MD Am J Clin Nutr. 1992;55(suppl 2):487S-619S. [DOI] [PubMed] [Google Scholar]