Abstract
Background:
Early initiation of chronic dialysis (starting dialysis with higher vs lower kidney function) has risen rapidly in the past 2 decades in Canada and internationally, despite absence of established health benefits and higher costs. In 2014, a Canadian guideline on the timing of dialysis initiation, recommending an intent-to-defer approach, was published.
Objective:
The objective of this study is to evaluate the efficacy and safety of a knowledge translation intervention to promote the intent-to-defer approach in clinical practice.
Design:
This study is a multicenter, 2-arm parallel, cluster randomized trial.
Setting:
The study involves 55 advanced chronic kidney disease clinics across Canada.
Patients:
Patients older than 18 years who are managed by nephrologists for more than 3 months, and initiate dialysis in the follow-up period are included in the study.
Measurements:
Outcomes will be measured at the patient-level and enumerated within a cluster. Data on characteristics of each dialysis start will be determined by linkages with the Canadian Organ Replacement Register. Primary outcomes include the proportion of patients who start dialysis early with an estimated glomerular filtration rate greater than 10.5 mL/min/1.73 m2 and start dialysis in hospital as inpatients or in an emergency room setting. Secondary outcomes include the rate of change in early dialysis starts; rates of hospitalizations, deaths, and cost of predialysis care (wherever available); quarterly proportion of new starts; and acceptability of the knowledge translation materials.
Methods:
We randomized 55 multidisciplinary chronic disease clinics (clusters) in Canada to receive either an active knowledge translation intervention or no intervention for the uptake of the guideline on the timing of dialysis initiation. The active knowledge translation intervention consists of audit and feedback as well as patient- and provider-directed educational tools delivered at a comprehensive in-person medical detailing visit. Control clinics are only exposed to guideline release without active dissemination. We hypothesize that the clinics randomized to the intervention group will have a lower proportion of early dialysis starts.
Limitations:
Limitations include passive dissemination of the guideline through publication, and lead-time and survivor bias, which favors delayed dialysis initiation.
Conclusions:
If successful, this active knowledge translation intervention will reduce early dialysis starts, lead to health and economic benefits, and provide a successful framework for evaluating and disseminating future guidelines.
Trial Registration:
Keywords: knowledge translation, chronic kidney disease, end-stage renal disease, dialysis, intent-to-defer, early dialysis, cluster randomized trial, randomized controlled trial
Abrégé
Mise en contexte:
Malgré l’absence d’avantages probants pour la santé des patients et en dépit de coûts plus élevés liés à la dialyse, la décision d’amorcer un tel traitement au moment où la fonction rénale du patient est encore relativement élevée (dialyse hâtive) est en forte hausse depuis une vingtaine d’années au Canada et partout dans le monde. Toutefois, les lignes directrices canadiennes publiées en 2014 à ce sujet recommandent plutôt de retarder le démarrage de la dialyse.
Objectifs de l’étude:
Cette étude a pour but d’évaluer l’efficacité et la sécurité d’une intervention au niveau de l’application des connaissances qui favoriserait le démarrage tardif de la dialyse chronique dans la pratique.
Cadre et type d’étude:
Il s’agit d’un essai clinique randomisé en deux groupes parallèles avec échantillonnage par grappes (clusters). Cinquante-cinq cliniques multidisciplinaires traitant des patients en insuffisance rénale chronique et provenant de partout au Canada participent à l’étude.
Patients:
L’étude porte sur des patients adultes suivis par un néphrologue depuis plus de trois mois et ayant démarré la dialyse au cours de la période de suivi.
Mesures:
Les données recueillies seront mesurées au niveau des patients et analysées par regroupement (clusters). Les paramètres de démarrage pour chaque début de dialyse seront établis par la consultation du registre canadien des insuffisances et des transplantations d’organes (RCITO). Les issues primaires sont i) la proportion de patients qui auront démarré la dialyse avec un débit de filtration glomérulaire estimé de plus de 10,5 mL/min/1,73 m2 (dialyse hâtive); ii) la proportion de patients pour lesquels l’amorce aura été faite au cours d’une hospitalisation ou lors d’une admission aux urgences. Les issues secondaires qui seront mesurées incluent : le taux de variation dans le moment du démarrage de la dialyse, le taux d’hospitalisations, le nombre de décès et les coûts associés aux soins prédialyse (lorsque l’évaluation est possible). On voudra également établir un rapport trimestriel des nouveaux cas de démarrages de dialyses, et savoir à quel point les éléments de transmission des connaissances seront acceptés dans la pratique.
Méthodologie:
Nous avons randomisé 55 cliniques multidisciplinaires en traitement de l’insuffisance rénale (clusters) au Canada à recevoir, ou non, une intervention de transmission des connaissances portant sur les lignes directrices Canadiennes du démarrage de la dialyse. L’intervention consiste en une visite médicale individuelle où l’information pertinente et des outils pédagogiques, tant pour le patient que pour le médecin traitant, sont distribués. Le suivi est assuré par rétroaction et par des vérifications ponctuelles (audits). Les groupes contrôles sont quant à eux mis au fait des nouvelles recommandations sans toutefois recevoir d’outils pédagogiques ni être soumis à la diffusion active de l’information. Nous émettons l’hypothèse que la proportion de dialyses hâtives diminuera au sein des cliniques ayant été randomisées dans le groupe où une intervention sera effectuée.
Limites de l’étude:
La première limite consiste en la possible diffusion passive des nouvelles lignes directrices uniquement par voie de publication. En outre, les biais liés à la survie et au délai d’exécution favorisent les démarrages tardifs de dialyse.
Conclusion:
Une intervention réussie au niveau de la transmission des connaissances contribuera à réduire le nombre d’amorces de dialyse hâtives. Dès lors, on peut penser que cela aura une incidence sur les coûts reliés à cette procédure et des avantages pour la santé des patients. Enfin, cette étude pourrait constituer un cadre favorable pour procéder à l’évaluation et à la diffusion de futures lignes directrices.
What Was Known Before?
Kidney failure requiring dialysis is a major public health problem with a rising incidence and prevalence in Canada and worldwide. Over the past 2 decades, early dialysis starts has risen rapidly, despite the absence of established health benefits and higher costs.
What Does This Study Add?
The Canadian clinical practice guideline on the optimal timing of dialysis initiation will be conveyed to health care providers through knowledge translation activities and uptake will be encouraged. Success of the study will result in reduced early dialysis starts, health and economic benefits, and creation of a network for the evaluation and dissemination of future guidelines for chronic kidney disease care in Canada.
Background
Kidney failure, also known as end-stage renal disease (ESRD), is a major public health problem with a rising incidence and prevalence in Canada, and worldwide.1 In 2010, approximately 2.6 million individuals worldwide received treatments for kidney failure. In most of the western hemisphere, patients are typically treated with in-center hemodialysis, delivered in 4-hour treatment sessions thrice weekly.2 There is a high cost associated with delivering dialysis and it has continued to rise; it now exceeds Can $80,000 per patient per year, and comprises more than 2% of the health care budget. These costs are even more impressive when one considers that only 0.1% of Canadians have kidney failure.3,4
Over the past 2 decades, there has been a strong trend toward earlier initiation of dialysis (that is, starting dialysis at a higher level of remaining kidney function) in patients whose kidneys are progressively failing (ie, progressive chronic kidney disease [CKD]).5,6 In a recent Canadian study that examined trends in dialysis initiation, the average estimated glomerular filtration rate (eGFR) at initiation of dialysis increased from 9.3 mL/min/1.73 m2 in 2001 to 10.2 mL/min/1.73 m2 in 2007, and the secular increase in the starting eGFR has continued. Similarly, the proportion of patients starting dialysis “early” (defined in clinical trials by an eGFR > 10.5 mL/min/1.73 m2) increased from 28% to 36% in the same time period, and is now greater than 40%.6,7 After accounting for patient characteristics that influence dialysis initiation, it is estimated that the average patient started dialysis approximately 5 months (147 days) earlier in 2007 when compared with 1997. For patients older than 75 years who represent the fastest growing segment of the dialysis population, this mean difference in timing was estimated at 8 months (233 days).8
There are several proposed reasons for the trend toward earlier initiation of dialysis. Most focus on the aging population of patients with advanced CKD and suggest that the frail elderly patient with multiple comorbid conditions develops symptoms that mimic uremia (eg, fatigue and lower appetite), for which dialysis therapy is offered.9 As such, these patients are early starts and have a worse prognosis, in large part due to their underlying age and comorbidity.10 In fact, mounting evidence from multiple observational studies and a large randomized trial indicates that the early initiation of dialysis may be harmful or at minimum not beneficial.6,7,11-13
It is important to appreciate that the general trend toward earlier initiation of dialysis may not be uniform across all jurisdictions. Recently, we conducted a large retrospective cohort study examining the variation in the timing of dialysis initiation across Canada.14 Although adjustments for case mix and other patient- and dialysis facility-level characteristics decreased the magnitude of the between-center variation, a significant effect remained. These results suggest that some centers are more likely to initiate dialysis earlier in patients than others, and this tendency cannot be explained by measured characteristics of both patients and their dialysis facilities.
In early 2014, the Canadian Society of Nephrology (CSN), in collaboration with the Canadian Kidney Knowledge Translation and Generation Network (CANN-NET), developed and published the first Canadian guideline on the timing of dialysis initiation.15 The principle recommendation of the guideline was adoption of an intent-to-defer strategy for the initiation of chronic dialysis: Patients older than 18 years with an eGFR less than 15 mL/min/1.73 m2 are to be monitored closely by a nephrologist, and dialysis initiated with the first onset of a clinical indication or a decline in eGFR less than or equal to 6 mL/min/1.73 m2. This recommendation weighed the evidence from the observational studies and the Initiating Dialysis Early and Late (IDEAL) randomized trial, and concluded that early initiation of dialysis was not beneficial for patient outcomes or system resources.12 The guideline was delivered as a strong recommendation supported by evidence of moderate quality. In an effort to reduce early dialysis initiation across Canada, this study utilizes knowledge translation (KT) activities to convey and encourage uptake of the guideline information to health care providers, and evaluate the efficacy and safety of the KT intervention.
Methods
Study Design
We are conducting a multicenter, 2-arm parallel design cluster randomized trial comparing the effect of an active KT strategy (active KT intervention group) versus simple guideline release without active dissemination (control group) on the timing of dialysis initiation in patients originating from 55 advanced CKD clinics (clusters) across Canada. This cluster randomized trial meets The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials.16 The cluster randomized trial design was adopted due to the type of intervention being implemented. Specifically, the intervention is being applied at the level of the clinic, as it is not feasible to apply at the patient level without experimental contamination (ie, nephrologists who care for different patients, but are located at the same clinic, will share information regarding clinical practice and patient care). Informed consent was not required for this trial. The study interventions and data collection processes pose minimal risk; the intervention involves an already published clinical practice guideline, and the data required for this study are already routinely collected. This study has been approved by the Health Research Ethics Board at the University of Manitoba and the Conjoint Health Research Ethics Board at the University of Calgary.
Data Sources
Data on the eGFR at dialysis initiation and the number of inpatient starts will be verified by linkages with the Canadian Organ Replacement Register (CORR). CORR is a validated registry that includes the following information on all patients with ESRD in Canada: demographics, comorbidities, vascular access, dialysis modality, transplantation, and mortality.17 Data are collected by dialysis facilities and are centrally housed with the Canadian Institute for Health Information (CIHI). New chronic dialysis start data from CORR can be linked to CIHI’s Discharge Abstract Database and National Ambulatory Care Reporting System to confirm elective outpatient versus urgent/emergent inpatient or emergency room starts.17,18
Study Population
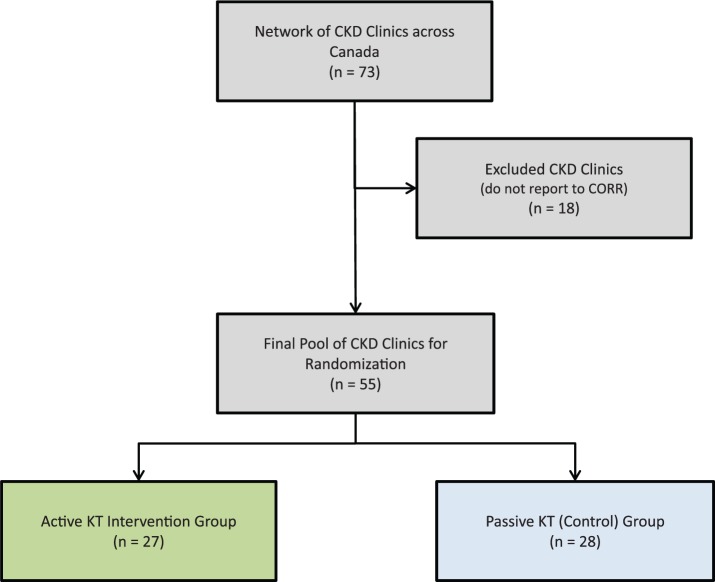
There are 73 CKD clinics (clusters) across Canada who provide care to nearly all patients with severe CKD and are responsible for the elective initiation of dialysis. Each clinic is associated with one or more dialysis facilities, forming a cluster. Of the clinics in the network, 55 were randomized to the study (Figure 1). Eighteen clinics were excluded from randomization as they do not submit data to CORR. The clinics are geographically separated (not located in the same office building) and are staffed by adult nephrologists who do not see outpatients in another multidisciplinary clinic. Individual clinics that send new dialysis starts to the same dialysis facility were combined to create unique measurement clusters. All patients older than 18 years who are managed by nephrologists for more than 3 months, and initiate dialysis in the follow-up period are included in the study.
Figure 1.
Study flow diagram.
Abbreviations: CKD, chronic kidney disease; CORR, Canadian Organ Replacement Register; KT, knowledge translation.
Study Objective, Aims, and Hypothesis
The objective of the study is to determine the efficacy and safety of a KT intervention targeted at kidney care providers on reducing the proportion of early dialysis starts in patients receiving nephrology care in Canada.
Overall, we aim to reduce early dialysis starts in patients who are not symptomatic. Although this is currently not tracked, starting with an eGFR greater than 10.5 mL/min/1.73 m2 is a reasonable proxy of a clinic’s attitude toward starting dialysis.
The primary aim will focus on efficacy to compare the impact of an active KT intervention versus simple guideline release without active dissemination on the proportion of patients, managed by nephrologists for more than 3 months, initiating dialysis with an eGFR greater than 10.5 mL/min/1.73 m2 across 55 CKD clinics in Canada.
The secondary aim will focus on safety to compare the impact of an active KT intervention versus simple guideline release without active dissemination on the proportion of all incident dialysis patients starting dialysis as inpatients in hospital or in an emergency room setting, across 55 CKD clinics in Canada.
The duration of the follow-up period after intervention will be 12 months. We hypothesize that clinics randomized to the active KT intervention will start a greater proportion of patients on dialysis later (eGFR < 10.5 mL/min/1.73 m2) compared with the clinics randomized to simple guideline release without active dissemination (control group).
Cluster Randomization
In this stratified cluster randomized study, the unit of observation is the patient (ie, outcomes will be measured at the level of an individual patient) and the unit of randomization is at the level of the multidisciplinary CKD clinic (cluster). Stratified randomization was carried out by the study statistician using software for random number generation with allocation concealment using R statistical software. The stratification variables included were province/region and size of the CKD clinic (<200 patients, 200-600 patients, >600 patients seen in the prior year). Clinics were randomized to either the active KT intervention group or the control group. A simulation of the randomization was completed to ensure balance on prespecified cluster level variables. Approximately 1000 iterations were performed, and a randomization scheme that resulted in good balance on all measured cluster factors was selected. Randomization assignments were concealed until the planned implementation of the intervention for a given region.
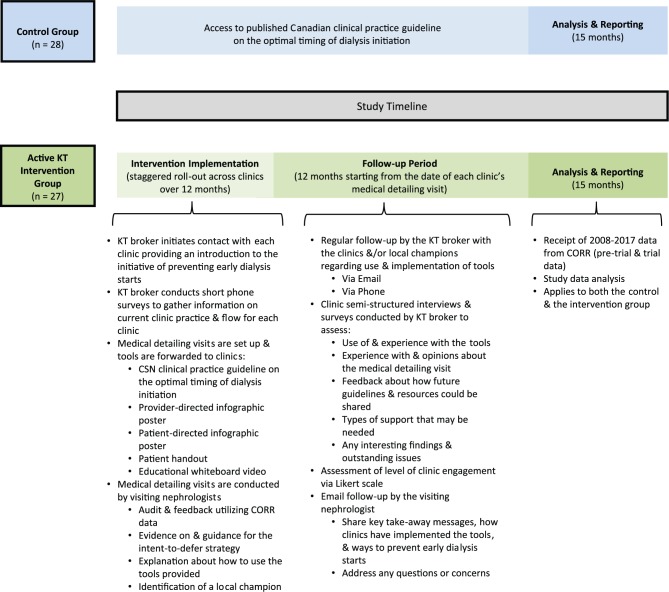
Active KT Intervention Group
The active KT intervention is comprised of multiple components: initial contact by the KT broker (who is an individual with expertise in communicating findings to knowledge users), the Canadian clinical practice guideline on the optimal timing of dialysis initiation, a provider-directed infographic poster, a patient-directed infographic poster, a patient handout, a whiteboard video, audit and feedback delivered by a subject-matter expert (nephrologist) during a single comprehensive in-person medical detailing visit, follow-up by the KT broker, and follow-up by the nephrologist who conducted the medical detailing visit. The study timeline and intervention are outlined in Figure 2.
Figure 2.
Study intervention outline and timeline.
Abbreviations: KT, knowledge translation; CORR, Canadian Organ Replacement Register; CSN, Canadian Society of Nephrology.
Clinics have been contacted via e-mail by the KT broker and were provided an introduction to the initiative of preventing early dialysis starts. After this initial contact, the KT broker conducted a short phone call with each of the clinics to learn more about their current practice and clinic flow. The guideline, provider- and patient-directed infographic posters, patient handout, and whiteboard video were then disseminated to the clinics. Following this, medical detailing visits were scheduled for a time and date convenient for each of the clinics and the visiting nephrologist.
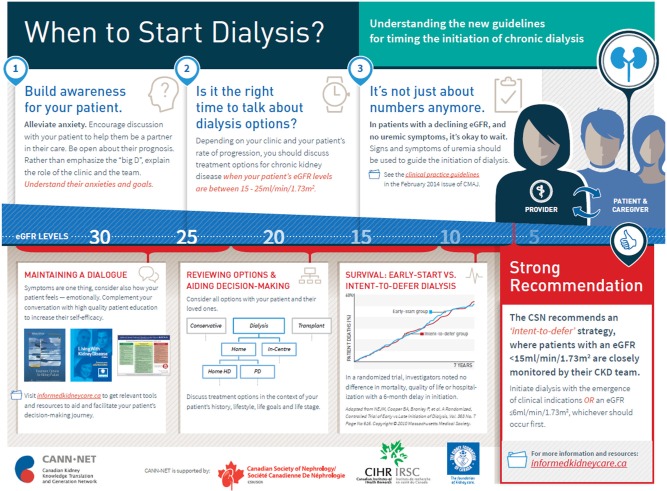
The Canadian clinical practice guideline on the optimal timing of dialysis initiation highlights evidence for and describes the intent-to-defer approach: Patients older than 18 years with an eGFR less than 15 mL/min/1.73 m2 are to be monitored closely by a nephrologist, and dialysis initiated with the first onset of a clinical indication or a decline in eGFR less than or equal to 6 mL/min/1.73 m2.15 The guideline is emphasized in each of the other intervention components.
The provider-directed infographic poster (Figure 3), patient-directed infographic poster (Figure 4), and patient handout (Figure 5) all recommend an intent-to-defer dialysis initiation strategy for outpatients with progressive CKD. These tools highlight the importance of symptoms over “numbers” (ie, relying solely on eGFR) in determining the optimal timing of dialysis initiation. Clinics have been advised to place the posters on prominent clinic wall space in patient and staff areas, and to make the patient handout available in patient waiting areas and/or education classes.
Figure 3.
Provider-directed infographic outlining the intent-to-defer dialysis initiation strategy for outpatients with progressive CKD.
Abbreviation: CKD, chronic kidney disease.
Figure 4.

Patient-directed infographic recommending an intent-to-defer dialysis initiation strategy.
Figure 5.
Patient handout (2 pages) recommending an intent-to-defer dialysis initiation strategy.
The educational whiteboard video, an innovative method of KT, explains the concepts of kidney failure, treatment with dialysis, and stresses the importance of symptoms over numbers in determining the appropriate timing of dialysis initiation. It has been provided to clinics as a hardcopy upon request and is accessible online via the YouTube website or through www.knowingkidneys.ca. Clinics have been advised to use the video in new staff orientation sessions, patient waiting areas, and patient education classes and were informed that it could be disseminated to patients within teaching materials.
Each clinic received a comprehensive in-person medical detailing visit from one of the study investigators (a nephrologist). The visiting nephrologist prepared for each visit by reviewing clinic information collected by the KT broker from the phone call made after initial contact, and by evaluating the clinic’s data from CORR (if it was accessible to the visiting nephrologist). CORR provides reports for each center outlining the proportion of patients starting dialysis early (eGFR > 10.5 mL/min/1.73 m2) for all incident dialysis patients originating from the clinic; comparisons from provincial and national averages were also included. During each visit in a standardized 1-hour presentation to clinic medical and allied health staff, the visiting nephrologist highlighted the clinical practice guideline, shared evidence supporting the intent-to-defer strategy, reviewed the clinic’s CORR data and provided tailored and active feedback on the clinic’s performance, and provided guidance on implementation of the intent-to-defer strategy. An interactive session with clinic staff followed the presentation, where supporting visual aids (ie, provider-directed infographic poster, patient-directed infographic poster, patient handout, and whiteboard video) were described, and information regarding how the clinics might use and implement the aids was provided. In addition, local barriers to implementing the intent-to-defer strategy were discussed, including usual cases when dialysis might be initiated early (ie, fluctuation in kidney function, symptoms that mimic uremia such as old age, or comorbid conditions). A local champion (a nephrologist or CKD clinic team member who is a local opinion leader) who was willing to advocate for guideline adherence at the clinic was identified at or shortly after the visit.
Furthermore, the KT broker will be in regular contact with the clinics and/or the local champions via e-mail and/or phone calls during the follow-up period. During this time, the KT broker will conduct semistructured interviews and surveys to assess the use of and experience with the tools, experience with and opinions about the medical detailing visit, feedback about how future guidelines and resources could be shared, types of support that may be needed, and any interesting findings and outstanding issues. Each clinic’s level of engagement will also be assessed using a Likert scale. Afterward, the KT broker will continue to check-in on the clinics periodically and encourage them to contact the KT broker anytime with any questions or concerns they may have. In addition, the visiting nephrologist will follow-up with each clinic. Key take-away messages, how clinics have implemented the tools, and ways to prevent early dialysis starts will be shared, and any questions or concerns will be addressed.
Control Group (Simple Guideline Release Without Active Dissemination)
All clinics will have access to the Canadian clinical practice guideline on the optimal timing of dialysis initiation. It was published with open access in the Canadian Medical Association Journal (CMAJ) and presented at the annual meeting of the CSN.15 The guideline was not actively disseminated to the clinics in this group.
Outcomes
Primary outcomes
Outcomes for this trial will be measured at the patient level and will be enumerated within a cluster. The primary efficacy outcome will be the proportion of all incident dialysis patients, originating from the randomized clinic clusters and followed by a nephrologist for more than 3 months, who start dialysis with an eGFR greater than 10.5 mL/min/1.73 m2 calculated using the Modification of Diet in Renal Disease (MDRD) study equation, in the follow-up period. This will be an outcome for the study because early initiation of dialysis may be associated with harm. As a secondary efficacy outcome, the rate of change in early dialysis starts will be analyzed to assess whether the effect of the active KT intervention dissipates over time, and for nonlinear effects.
The primary safety outcome will be the proportion of all incident dialysis patients, originating from the randomized clinic clusters, who start dialysis in hospital as inpatients or in an emergency room setting within the follow-up period. This will be an outcome for the study as delaying the initiation of dialysis may result in a greater number of crash starts: Patients may become ill and present to the emergency room or be admitted to hospital immediately to start dialysis treatment (which otherwise would have begun in the outpatient dialysis facility were they to have started early). Although the IDEAL Study did not show this,12 we are including this as we are now studying the intent-to-defer strategy though a more pragmatic trial that has fewer constraints.
Secondary outcomes
A major limitation of observational studies and clinical trials examining the effect of timing of dialysis initiation on mortality is survivor bias; patients who die before dialysis are omitted from these analyses, and their outcomes and costs are therefore ignored. We will aim to address this issue by examining the outcomes of all patients followed in the nephrology clinics using provincial data linkages, wherever available (presently Ontario, Manitoba, and Alberta) to examine rates of hospitalizations, deaths, and cost of predialysis care in both study arms.
In these analyses, all patients enrolled in the participating multidisciplinary clinics will be included. We will also determine a quarterly proportion of new starts from each clinic (new starts/total number of patients followed in the clinic) and determine the differences in this proportion between the 2 study arms. Additional secondary outcomes will include the acceptability of the KT materials provided to the clinic measured using semistructured interviews and surveys.
Power Calculation
Recently, a trend toward dialysis initiation at higher levels of residual renal function has been reported, with upward of 40% of patients initiating chronic dialysis early, contributing to substantial practice pattern variation in the timing of dialysis initiation in Canada.6,7 We expect a 10% absolute risk reduction in early dialysis starts with the implementation of the active KT intervention (corresponding to 30% of patients initiating chronic dialysis early in the intervention group). Sood et al found an adjusted facility-level intraclass correlation coefficient (ICC) of 0.031 in their work.14 Given these parameter estimates, and at the .05 level of significance and estimating 3696 new dialysis users across Canada in our 55 clusters, we would have over 90% power, using a power calculation for proportions that includes inflating the variance for the facility-level correlation. We maintain approximately 80% power if our ICC is doubled (0.062) or if we find only an 8% absolute risk reduction. We expect the stratified design will increase our power. However, we are unable to calculate our power based on the stratified design because this requires strata specific rates of events, which are unavailable.
Statistical Analysis
We are employing a stratified cluster randomized trial design, where the unit of observation is the patient and the unit of randomization is the CKD clinic. A cluster randomized design was chosen for multiple reasons, including practical/administrative convenience of applying the active KT intervention, and reducing the potential for treatment contamination. We chose to stratify clusters based on region to account for additional clustering within regions and to facilitate subgroup analyses. We also chose to stratify clusters based on facility size in order to ensure balance across the 2 arms of the trials because facility size may be associated with the outcome of interest.
Given the publication of the IDEAL Study,12 and the release of the CSN timing of dialysis guideline,15 which is available to all clinics, we will evaluate secular trends in dialysis initiation in both groups using an interrupted time series design as a sensitivity analysis. Analysis of pretrial and trial data, specifically data from 2008 to 2017, will allow us to assess for changes in clinical practice prior to and after the implementation of our KT intervention.
For the randomized component of our study, dichotomous outcomes (such as the proportion of patients who start dialysis at an eGFR > 10.5 mL/min/1.73 m2) will be compared using an adjusted 2-sample t test. To adjust for individual and cluster level covariates as well as correlation within clusters, we will use generalized estimating equations to obtain the population average effect of the intervention in the presence of clustering. To assess the intervention by cluster, we can implement a multilevel (hierarchical) model, which provides a random effect for each cluster. Subgroup analysis will be conducted for patients who start dialysis in the first 6 months post intervention versus the remainder of the study period to determine if the effectiveness of the active KT intervention changes over time. For continuous outcomes (such as mean eGFR at dialysis initiation), mixed-effects generalized linear models will be utilized. These models will allow us to examine the effects of the active KT intervention, while adjusting for potential confounding variables and accounting for the clustering of observations within dialysis facilities. Analyses will be performed using Stata, R statistical software version 3.1, and SAS Enterprise Guide. A 2-sided P value less than .05 will be considered statistically significant.
Discussion
In recent years, a trend toward dialysis initiation at higher levels of residual renal function has been noted, with upward of 40% of patients initiating chronic dialysis early, contributing to substantial practice pattern variation in the timing of dialysis initiation in Canada.6,7 This study aims to evaluate the efficacy of an active KT intervention to reduce early dialysis starts, which was developed using high-quality evidence from the literature. Additional important aims of this study include an evaluation of the potential adverse effects of delayed initiation of dialysis on unplanned/crash dialysis starts and predialysis patients’ outcomes and health care resources.
Multifactorial KT strategies delivered in a high intensity standardized fashion have been shown to be more effective than a single intervention delivered without follow-up.19 In addition, there exists compelling evidence to support the individual components of our KT intervention. In particular, the effect of audit and feedback in reducing practice pattern variation (median 4.3% [0.5%-16%] absolute increase in health care provider compliance with desired practice) for management of chronic conditions (nondialysis) has been well validated in recent Cochrane reviews.20,21 Meta-regression from these reviews shows that audit and feedback is most effective when baseline performance is low, the source is a supervisor or colleague, it is delivered in both verbal and written formats, and when it includes both explicit targets and an action plan.19 Furthermore, detailing by a medical expert/key opinion leader has been shown to greatly enhance the effect of audit and feedback interventions, and there is an evidence base for local opinion leaders being important in KT.22 Given the collective support, we believe our active KT intervention (which addresses patient, health care provider, and system-level factors) is likely to be effective in reducing the proportion of early dialysis starts, when compared with the control group, and that an absolute reduction of 10% in early dialysis starts is justified.
We acknowledge that the study has limitations, with the first being the passive dissemination of the guideline through publication. The Canadian clinical practice guideline recommending an intent-to-defer strategy for dialysis initiation has been published in CMAJ.15 If the guideline is widely adopted, it may decrease the proportion of early starts in the control group. In addition, practices may have changed even before guideline publication as a result of the publication of the IDEAL Study. To account for the possible change in physician/team behavior due to peer-reviewed publication alone (ie, without a KT strategy), we will examine trends in dialysis initiation in both groups using an interrupted time series design as a sensitivity analysis. A second limitation is that the lead-time and survivor bias favors delayed dialysis initiation. It is possible that early initiation is associated with harm as patients with advanced CKD and patients with a delayed initiation plan may die before starting dialysis, and therefore only survivors are able to defer dialysis initiation. However, we believe this to be improbable, given that the IDEAL Study did not find any evidence of harm with an intent-to-defer strategy.12 Nonetheless, we will examine for unintended consequences of an intent-to-defer strategy (crash starts) and hospitalizations and death predialysis with our outcomes.23,24 Another factor to consider is the potential occurrence of a rapid decline in early starts in the active KT intervention group, followed by regression of intervention effect. It is possible that there will be an acute decline in early dialysis starts in the months immediately following the medical detailing visit, with a dissipation of the effect over time. As such, we have incorporated multiple follow-ups from the KT broker, identified a local champion at each site, and will analyze the rate of change in early dialysis starts as a secondary efficacy outcome.
With the successful completion of this study, there will be potential economic, health, and scientific benefits. If the active KT intervention is shown to be effective in reducing early dialysis starts, we can implement it nationally within 12 months of trial completion, and therefore successfully translate our findings. A modest impact of a 10% absolute risk reduction in early starts will yield a minimum cost savings of Can$12 million from a payer perspective annually. These cost savings would be primarily driven by a reduction in dialysis days from later dialysis starts, which have major implications on patient morbidity, mortality, and health-related quality of life. Also, other aspects of clinical practice, such as appropriateness of care, may be evaluated in the future to yield further cost savings. Finally, our study will provide a framework for future KT interventions and will have utilized a novel and important national network of CKD clinics across Canada. The study will demonstrate how such a network can be used and how it has the potential to transform the kidney clinical research environment in Canada.
Acknowledgments
We thank Selina Allu and Sarah Gil (Division of Nephrology, Health Sciences Centre, Calgary, Alberta, Canada) and Michelle Di Nella (Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada) for conducting and/or assisting with study activities. We also thank Kelsey Uminski (University of Manitoba, Winnipeg, Manitoba, Canada) for contributing minor manuscript edits.
Footnotes
Abbreviations: CANN-NET, Canadian Kidney Knowledge Translation and Generation Network; CKD, chronic kidney disease; CMAJ, Canadian Medical Association Journal; CORR, Canadian Organ Replacement Register; CSN, Canadian Society of Nephrology; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IDEAL Study, Initiating Dialysis Early and Late (IDEAL) Study; KT, knowledge translation.
Ethics Approval and Consent to Participate: Informed consent was not required for this trial. This study has been approved by the Health Research Ethics Board at the University of Manitoba and the Conjoint Health Research Ethics Board at the University of Calgary.
Consent for Publication: Not applicable.
Availability of Data and Materials: Data have not yet been collected and analyzed; there is no data to share.
Authors’ Contributions: EMTC prepared and edited the manuscript, and conducted study activities. BJM, AXG, MMS, SJK, DN, GEN, SDS, MB, SD, and AA contributed to the design of the trial, reviewed and edited the manuscript, and conducted study activities. NT contributed to the design of the trial, reviewed, edited, and approved the manuscript, and conducted study activities.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by the Manitoba Health Research Council 2014 Operating Grant.
References
- 1. Meguid El, Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331-340. [DOI] [PubMed] [Google Scholar]
- 2. Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20(12):2587-2593. [DOI] [PubMed] [Google Scholar]
- 3. Manns BJ, Mendelssohn DC, Taub KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ. 2007;7(2-3):149-169. [DOI] [PubMed] [Google Scholar]
- 4. Komenda P, Copland M, Makwana J, Djurdjev O, Sood MM, Levin A. The cost of starting and maintaining a large home hemodialysis program. Kidney Int. 2010;77(11):1039-1045. [DOI] [PubMed] [Google Scholar]
- 5. Rosansky SJ, Cancarini G, Clark WF, et al. Dialysis initiation: what’s the rush? Semin Dial. 2013;26(6):650-657. [DOI] [PubMed] [Google Scholar]
- 6. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM. Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol. 2013;8(2):265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Hare AM, Choi AI, Boscardin WJ, et al. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med. 2011;171(18):1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707. [DOI] [PubMed] [Google Scholar]
- 10. Zaman T, Filipowicz R, Beddhu S. Implications and importance of skeletal muscle mass in estimating glomerular filtration rate at dialysis initiation. J Ren Nutr. 2013;23(3):233-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crews DC, Scialla JJ, Liu J, et al. Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol. 2014;25(2):370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. [DOI] [PubMed] [Google Scholar]
- 13. Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171(5):396-403. [DOI] [PubMed] [Google Scholar]
- 14. Sood MM, Manns B, Dart A, et al. Variation in the level of eGFR at dialysis initiation across dialysis facilities and geographic regions. Clin J Am Soc Nephrol. 2014;9(10):1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nesrallah GE, Mustafa RA, Clark WF, et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186(2):112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weijer C, Grimshaw JM, Eccles MP, et al. ; Ottawa Ethics of Cluster Randomized Trials Consensus G. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9(11):e1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moist LM, Richards HA, Miskulin D, et al. A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol. 2011;6(4):813-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sood MM, Hemmelgarn B, Rigatto C, et al. Association of modality with mortality among Canadian Aboriginals. Clin J Am Soc Nephrol. 2012;7(12):1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6:CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Does telling people what they have been doing change what they do? A systematic review of the effects of audit and feedback. Qual Saf Health Care. 2006;15(6):433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamtvedt G, Young JM, Kristoffersen DT, Thomson O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003;(3):CD000259. [DOI] [PubMed] [Google Scholar]
- 22. Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;(8):CD000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boulware LE, Tangri N, Ephraim PL, et al. Comparative effectiveness studies to improve clinical outcomes in end stage renal disease: the DEcIDE patient outcomes in end stage renal disease study. BMC Nephrol. 2012;13:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crews DC, Scialla JJ, Boulware LE, et al. Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. Am J Kidney Dis. 2014;63:806-815. [DOI] [PMC free article] [PubMed] [Google Scholar]