Abstract
The human dopamine transporter gene (hDAT) encodes the dopamine transporter in dopamine (DA) neurons to regulate DA transmission. hDAT expression varies significantly from neuron to neuron, and from individual to individual so that dysregulation of hDAT is related to many neuropsychiatric disorders. It is critical to identify hDAT-specific cis-acting elements that regulate the hDAT expression. Previous studies showed that hDAT Intron 1 displayed inhibitory activity for reporter gene expression. Here we report that the hDAT Intron 1 contains a 121-bp fragment that down-regulated both SV40 and hDAT promoter activities by 80% in vitro. Subfragments of 121-bp still down-regulated the SV40 promoter but not the hDAT promoter, as supported by nuclear protein-binding activities. Collectively, 121-bp is a silencer in vitro that might coordinate with transcriptional activities both inside and outside 121-bp in regulation of hDAT.
Keywords: DAT, SLC6A3, Medication target, Promoter activity, Cell type-dependent, Primate-specific
Introduction
Dopamine (DA) transmission plays essential roles in brain physiological states (voluntary movement, motivation, cognition, attention, working memory and behavioral organization) and pathological processes (attention deficit/hyperactivity disorder (ADHD), schizophrenia, bipolar disorder, Parkinson’s disease and substance abuse). As a critical regulator of DA transmission, the dopamine transporter (DAT) regulates the spatio-temporal components of DA transmission, by sequestering DA into presynaptic neurons to terminate transmission. Therefore, DAT contributes to voluntary movement, reward and mnemonic functions of the brain and efficacy of therapeutic drugs targeted to DAT. Expression of the human DAT gene (hDAT) throughout the brain is highly circumscribed, varies among individuals and can be regulated by endogenous and exogenous factors [1–3]. Abnormal hDAT expression may confer risks for various neuropsychiatric diseases [4].
Only one in a million neurons is a DA neuron and hDAT is expressed in DA neurons only and at levels of great differences among different brain regions [5, 6]. Apparently, regional specificity of quantitative hDAT expression is finely controlled. Recent in vitro studies have shown that hDAT expression in DA cell lines is activated by common TFs including NurrI, ZFP161 and Sp [7–11]. However, cis-acting elements such as Intron 1 [12, 13] for controlling hDAT activity represent a rarely explored subject. We report an in vitro dissection of cis-acting activities that regulate hDAT promoter and represent an hDAT-specific medication target for correcting abnormal hDAT activity.
Materials and methods
Cell lines and RNA isolation
All human cell lines including four DA cell lines SK-N-AS, SH-SY5Y, BE(2)-M17, IMR-32 and one non-DA cell line HEK293T were purchased from ATCC and maintained at 37°C, 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal calf serum, penicillin (100 U/ml), and streptomycin (100 U/ml) (Invitrogen, Carlsbad, CA, USA).
Total RNA was isolated from cells with TRI Reagent solution (Ambion, Austin, TX, USA) according to the manufacturer’s protocol and subsequently, subjected to DNase treatment.
Quantitation of mRNA levels by qRT-PCR
To prepare cDNA, reverse-transcription used Superscript II reverse transcriptase (Qiagen, Valencia, CA, USA) and oligo dT as the primer. Quantitative PCR reactions used the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and two pairs of primers listed in Table S1. GAPDH was used as an internal control. Relative expression levels were calculated by using an empirical 1.777−ΔΔCT method where the amplification efficiency value of 1.777 ± 0.183 (N = 10) was a qRT-PCR-based average for the hDAT primers [14].
Reporter construction
pGL3-hDAT7.9kb was generated by cloning a 7,777 bp BsaBI/EcoRV fragment into the SmaI site of pGL3-enhancer. BsaBI is located 5,930 bp upstream and EcoRV, 1,847 bp downstream of the transcription start site. PCR using primers 869f and 869r was carried out to clone a 869 bp fragment from a lambda clone (a gift of Dr. A. P. Kouzmenko of University of Melbourne, Australia), digested by BamHI and replaced the 748 bp BamHI/BglII fragment of pGL3-hDAT7.9kb, generating pGL3-hDAT7.9kb. Insertion orientation was confirmed by DNA sequencing with primer Luc170r. pGL3-hDAT7.9kb+121bp covered −5930 to +1968 and pGL3-hDAT7.9kb covered −5930 to +1847, assuming the transcription start site as +1 according to Genbank accession number NM_001044 (18-OCT-2009).
A 121 bp fragment was broken down into four subfragments and each subfragment was prepared by oligonucleotide (oligo) annealing to be boarded in HindIII and NcoI ends, followed by insertion into the HindIII/NcoI sites of Promega’s pGL3-Promoter Vector, generating four SV40 subfragment plasmids pGL3-SV40-I, pGL3-SV40-II, pGL3-SV40-III and pGL3-SV40-IV. To replace the SV40 promoter in the subfragment plasmids with hDAT promoter, a 5,330 bp BsaAI/EcoRV hDAT fragment was isolated from the pGL3-hDAT7.9kb construct by BsaAI/HindIII digestion (HindIII located in the reporter vector), inserted into the SmaI/HindIII sites of the subfragment plasmids, generating four hDAT subfragment plasmids pGL3-hDAT5.3kb-I, pGL3-hDAT5.3kb-II, pGL3-hDAT5.3kb-III and pGL3-hDAT5.3kb-IV. Restriction enzymes were purchased from New England Biolabs (NEB, Ipswich, MA, USA).
Transfection and luciferase activity measurements
Transfection procedure has been described before except using a 24-well format in this study [15]. All transfection used plasmid DNAs mixed from at least two-three independent isolations (each with OD ratio of>1.7) in order to ensure that quality of DNA preparations did not complicate promoter activity signals. Transfection efficiency (mean ± SD) was 0.67 ± 0.05 for SK-N-AS, 0.46 ± 0.05 for IMR-32 and 0.78 ± 0.06 for HEK293T on average, based on using a plasmid of similar size and carrying GFP gene (4′-6-Diamidino-2-phenylindole or DAPI staining for total cells) and immunocytochemical staining with anti-luciferase antibodies [16]. Two days after transfection, cells were harvested and luciferase (Luc) activity measured by using a Luciferase Assay System Kit (Promega, Madison, WI, USA) in Bio-Tek Synergy HT/KC4 and a 96-well format, according to manufacturer’s instructions. Cell number in each well was estimated by protein amount based on Bio-Rad’s Protein Assay Reagent. Arbitrary unit of promoter activity is calculated as Luc activity readout/protein. Data are all presented as mean ± SEM.
Electrophoretic mobility shift assay (EMSA)
35S-Labeling of double-stranded (ds) oligos. 20 μM of (equimolar concentrations of forward and reverse) oligoswere annealed in 200 μl of Annealing Buffer (10 mM Tris, pH 7.8, 50 mM NaCl and 1 mM EDTA) by incubation at 95°C for 2 min, followed by 45–60 min cooling down to room temperature and aliquots stored at −80°C. Labeling was carried out in a 30 μl solution containing 0.5 μl of 20 μM annealed ds oligos, 3 μl of 10× Reaction Buffer 2 (Invitrogen), 6 μl of 35S-dATP (60 pmol, PerkinElmer Life and Analytical Sciences, Boston, MA, USA) and 1 μl of Large Fragment of DNA Polymerase I (NEB) and 19.5 μl of H2O, incubated at room temperature for 28 min before cooling down on ice. To remove the unincorporated 35S-dATP, the labeling mixtures were loaded into CHROMA SPIN-10 columns (Clontech, Mountain View, CA, USA), the columns centrifuged at 700×g for 5 min and the elute collected as 35S-ds oligos.
DNA binding
Each 17 μl of binding reaction was prepared by (EMSA Accessory Kit, EMD4Biosciences, Gibbstown, NJ, USA) mixing 5 μl of 4× binding buffer (80 mM HEPES, pH 8.0, 0.4 M KCl, 80% glycerol, 0.8 mM EDTA and 2 nM DTT), 1 μl of 500 ng/μl sonicated salmon sperm DNA (average size ~500 bp), 1 μl of poly(dI):poly(dC) (0.01 U dissolved in PBS), and 3 μl of yeast extract and 7 μl of sterile H2O, followed by mixing with 1 μl of 35S-labeled ds oligo (0.03 pmol) and incubation on ice for 2–3 h before loading for electrophoresis.
EMSA
6% non-denaturing DNA retardation gels (20 ml gel solution containing 4 ml of 29:1 acrylamide:bisacrylamide solution, 1 ml of 10× TBE, 15 ml of ddH2O, 40 μl of 20% ammonium persulfate and 12 μl of TEMED) was prepared 1 day before pre-running in 0.5× TBE at 120 V for 0.5 h. After loading the binding reaction solutions, electrophoresis was carried out at 120 V for 1 h. Gels were dried on filter papers and exposed to X-ray film for 3–5 days for autoradiography.
Results and discussion
Endogenous hDAT expression in human cell lines
To study regulatory elements of the hDAT promoter, human cell lines that express endogenous hDAT were assessed among five human cell lines including four DA cell lines SK-N-AS, (BE2)-M17, IMR-32, SH-SY5Y and a non-neuronal cell line HEK293T by using quantitative real-time PCR (qRT-PCR). As a result, SK-N-AS expressed the highest levels of endogenous hDAT mRNA among the five cell lines and (BE2)-M17 the second highest (9.5% of SK-N-AS levels; Fig. 1). IMR-32 expressed 3.7% of the SK-N-AS levels. SH-SY5Y and HEK93 expressed <3% of the SK-N-AS levels. These data are consistent with the previous report that SK-N-AS expressed robust levels of hDAT [17]. We therefore used human DA cell line SK-N-AS as a main system and two to four other cell lines for hDAT regulation analysis that mimic different DA neurons to some extents.
Fig. 1.
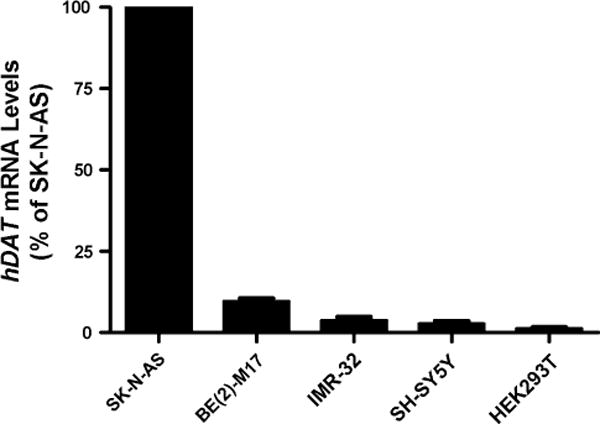
Endogenous hDAT expression in five human cell lines including four DA cell lines SK-N-AS, BE(2)-M17, IMR-32, SH-SY5Y and one non-neuronal cell line HEK293T, based on qRT-PCR that used two different pairs of hDAT primers (N = 3)
Addition of an intronic 121-bp fragment decreased hDAT promoter activity
In a study on hDAT promoter regulation, we cloned a 7.9 kb promoter fragment (covered −5930 to +1847, assuming the transcription start site as +1 according to Genbank accession number NM_001044 entered on PRI 18-OCT-2009) into Promega’s pGL3-enhancer (a promoterless firefly luciferase reporter vector) by unique restriction sites BsaBI and EcoRV. This reporter plasmid is now termed pGL3-hDAT7.9kb. We used the pGL3-enhancer, instead of the pGL3-Basic vector because the 7.9 kb in the later vector displayed very low activity and generated very unreliable results. The 7.9 kb was selected because that was the maximal size the vector could harbor. In order to cover more in the 3′ end (or Intron 1), we extended the 3′ end by adding 121 bp, generating pGL3-hDAT7.9kb+121bp (see “Materials and methods” section for construction). In order words, addition of a 121-bp into the 3′ end of the hDAT promoter fragment in pGL3-hDAT7.9kb resulted in pGL3-hDAT7.9kb+121bp (Fig. 2a). When assaying the promoter activity of these reporter plasmids, we found that pGL3-hDAT7.9kb expressed more promoter activity than pGL3-hDAT7.9kb+121bp consistently among three cell lines: 3.97-fold in SK-N-AS, 3.34-fold in SH-SY5Y and 4.42-fold in HEK293T (Fig. 2b–d). These data suggested that the 121 bp fragment inhibited the hDAT promoter.
Fig. 2.
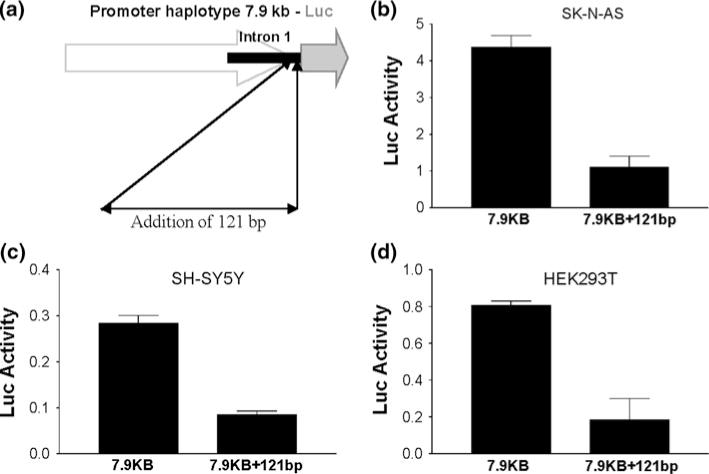
Promoter activity decreased by addition of (extending) a 121-bp fragment into Intron 1. a Schematic of 7.9 kb-Luc expression constructs and addition of a 121-bp fragment. b–d Addition of 121-bp fragment decreased the promoter activity in SK-N-AS, SH-SY5Y and HEK293T. Luc activity (arbitrary units) represents promoter activity of 7.9 kb hDAT fragment before (7.9 kb for pGL3-hDAT7.9kb) and after (7.9kb+121bp for pGL3-hDAT7.9kb+121bp) adding 121-bp. P < 0.0001 for SK-N-AS (N = 6) and SH-SY5Y (N = 14) or P = 0.0342 for HEK293T (N = 2 each in duplicate) all by t tests
Down-regulation of SV40 promoter by the 121-bp fragment independently of insertion location
Possibilities for the intronic 121-bp fragment to inhibit the hDAT promoter may include destabilization of the Luc RNA by the 121-bp fragment (because this fragment became a 5′ part of the Luc transcript) or this fragment served directly as a transcription factor (TF) binding site. To differentiate these two possibilities, we examined whether this fragment still inhibited Luc gene expression when inserted outside the Luc gene in Promega’s reporter vector, pGL3-Promoter Vector. To do so, the 121-bp fragment was cloned into two different locations to generate two reporter plasmids pGL3-SV40+121bp (Fig. 3a): before and after SV40 promoter. We then transiently expressed these pGL3-SV40+121bp plasmids to examine 121-bp fragment regulation of SV40 promoter activity in two human cell lines, SK-N-AS and BE(2)-M17. The 121-bp fragment down-regulated the SV40 promoter activity by 30–40% when inserted either after or before the promoter in SK-N-AS (Fig. 3b); the down-regulation was 27–31% when inserted after or before the promoter in BE(2)-M17 (Fig. 3c). Importantly, the difference in down-regulation between “after” and “before” promoter was statistically significant in neither of these cell lines, suggesting that the 121-bp fragment likely served as a TF binding site and did not affect Luc mRNA activity. Overall, the down-regulation of the SV40 promoter was less significant than that of the hDAT promoter, suggesting that the 121-bp fragment be more effective on the native promoter.
Fig. 3.
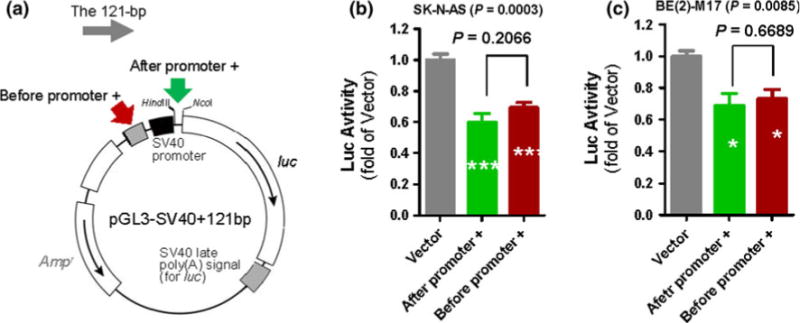
Down-regulation of SV40 promoter activity by hDAT intronic 121-bp fragment. a Cloning of the 120-bp fragment into two different locations (indicated by two arrows) in pGL3-Promoter Vector (Vector), resulting in pGL3-SV40+121bp. 121-bp inhibition of SV40 promoter activity, based on Luc activity from Vector versus pGL3-SV40+121bp in two different human cell lines SK-N-AS and BE(2)-M17 (b, c). Arrows indicate two different insertion locations. ANOVA and Tukey tests: *P < 0.05; ***P < 0.001, compared to Vector (N = 4, each in duplicate)
We thus reported the identification of a unique 121-bp fragment, located from +1848 to +1968 near the 3′ end of the 2.2 kb Intron 1, as a cis-acting inhibitory element for the 7.9 kb hDAT promoter activity. Not found in the rest of the human genome, this sequence is conserved in chimpanzee (96% identity) but is not in rodents (only 61% in a localized and gapped 70 nucleotides around I and II), suggesting a possibility of species-related regulation of the DAT gene. Cis-acting elements that confer neuron specificity of hDAT expression are unlikely located in the DNA fragments examined in this study. The 7.9 kb hDAT promoter was expressed in all types of cells tested, including SY-SY5Y and HEK293T that expressed very little endogenous hDAT, suggesting that unlike the zebrafish gene [18], cis-acting elements that confer neuron specificity in humans are not located in this 7.9 kb. The 121-bp fragment did not displayed the largest inhibitory activity in non-neuronal cells (Fig. 4), suggesting that this 121-bp fragment does not contribute to the neuron specificity either.
Fig. 4.
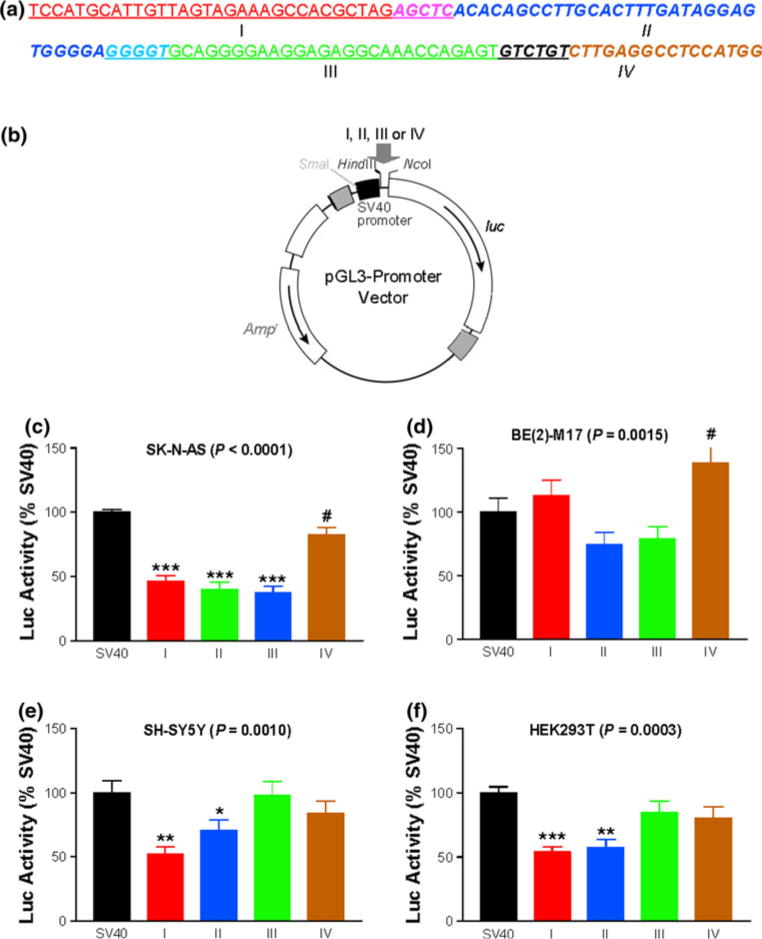
Inhibition of SV40 promoter activity by subfragments of 121-bp. a DNA sequences of four subfragments (I–IV) throughout 121-bp. b Reporter pGL3-Promoter Vector. Gray arrow indicates subfragment insertion site. c–f Influences of subfragments on SV40 promoter activity in four different cell lines. SV40 pGL3-Promoter Vector; I pGL3-SV40-I; II pGL3-SV40-II; III pGL3-SV40-III; IV pGL3-SV40-IV. Indicated P values are based on ANOVA: *P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test: #P < 0.05, comparing to SV40 (N = 4–5, each in duplicate)
Regulation of the SV40 promoter by subfragments of the 121-bp
TFs usually recognize <20 bp DNA. To identify which regions of the 121-bp displayed the inhibitory activity, we broke down the 121-bp into four subfragments, I–IV (Fig. 4a) and analyzed their individual regulatory activity on the SV40 promoter after cloning by replacing the 33 bp HindIII/NcoI fragment of the Promega’s pGL3-Promoter Vector (Fig. 4b). Subfragment I had 36 bp; II, 41 bp; III, 38 bp and IV, 22 bp with some overlapping between subfragments to reduce the possibility of losing TF binding activity (Fig. 4a).
As a result, in SK-N-AS, subfragments I, II, III and IV reduced the SV40 promoter activity by 53.6, 60.2, 63.1 and 17.4%, compared to the vector (Fig. 4c). In BE(2)-M17, subfragments II and III did not reduce the activity in a statistically significant manner; subfragment IV increased the activity by 38.3%, reaching statistical significance only by t tests, not by ANOVA posttest Tukey (Fig. 4d). In SH-SY5Y, subfragments I and II reduced the SV40 promoter activity by 48.1 and 29.4%; neither of III and IV regulated the SV40 activity (Fig. 4e). Similar to SH-SY5Y, in HEK293T, subfragments I and II reduced the SV40 promoter activity by 46.0 and 42.5%; neither of III and IV regulated the SV40 activity (Fig. 4f). In summary, I and II displayed the most consistent inhibitory activities and IV might display activating activity, depending on cell types.
We also examined regulation of the 5.3 kb hDAT promoter by the subfragments but did not observe any significant inhibitions in SK-N-AS or HEK293T (data not shown). An interesting question is how the inhibitory activity on the hDAT promoter disappeared after the 121-bp broke down into four smaller elements. One explanation is that the inhibitory activity of the 121-bp was achieved by coordination among the TFs that bind to the individual subfragments. It has been reported that a TF can function differently depending on the environments [19–22]. TF scan has predicted that 121-bp could be recognized by more than 30 TFs, suggesting that multiple TFs might regulate this fragment. To better understand the intronic and significant regulation of hDAT, future studies will need to identify these TFs so that we may further examine whether these TFs recognize each other and/or undergo different posttranslational modifications in response to external or internal regulations. Cell type- as well as promoter-dependences were observed in subfragment regulation, suggesting that same subfragments may regulate different promoters in different ways and in different cells [23, 24]. These data thus cautioned that in vitro assays on transcriptional regulations need to be careful in choosing target promoters and that the assays should not depend upon single easy-to-assess (high activity) promoters such as the SV40 promoter.
Nuclear protein binding to the subfragments
To examine whether any potential TFs could bind to the subfragments, we have carried out EMSAs and investigated whether nuclear proteins isolated from the four cell lines bind to double-strand oligos of the four subfragments. We prepared double-strand oligos with five extra “T” at 5′ end of each forward strand, followed by filling-in in the presence of 35S-dATP to label the oligos. Labeled oligos were each mixed with nuclear proteins isolated from three DA cell lines (SK-N-AS, BE(2)-M17, SH-SY5Y) and one non-DA cell line (HEK293T). It turned out that subfragments II and IV both displayed binding activities for all four cell lines (see arrows in lanes 3, 11, 19, 27 and 7, 15, 23 and 31 of Fig. 5). Subfragment IV displayed a binding activity that could not be inhibited completely by including 30-fold excess of the unlabeled oligo in the binding mixture (see two lanes under “IV”). Such information suggests that this particular protein is expressed abundantly but has low affinity for IV. Nevertheless, those binding activities are consistent with the inhibitory effects of both subfragments on the SV40 promoter in most of the cell lines. Subfragment I showed two bands for SK-N-AS but lacked evident binding activity for other cell lines (see lanes 9 vs. 10, 17 vs. 18 and 25 vs. 26). Subfragment III did not show any binding activity.
Fig. 5.
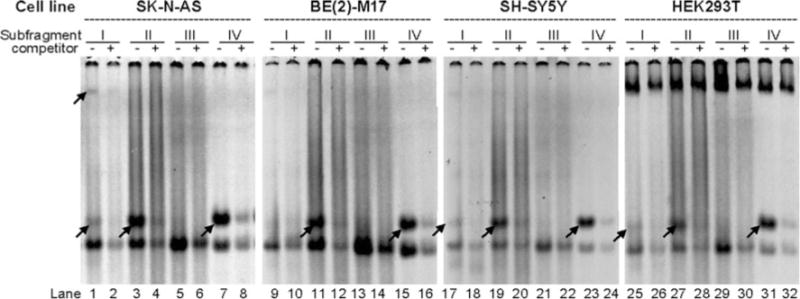
EMSA of nuclear protein binding to four subfragments (I–IV) of 121-bp. Nuclear proteins were isolated from SK-N-AS, BE(2)-M17, SH-SY5Y and HEK293T, as indicated on top. DNA sequences of I–IV are shown in Fig. 4a and ds oligos were labeled by 35S-dATP. Competitor is 30-fold excess of unlabeled double-strand oligo. Arrow specific binding activity by comparing to next lane with competitor; lowest band poly(A/T, generated by 35S-A filling-in)-binding activity (control not shown)
This hDAT 121-bp may evolve into an HTS platform to develop hDAT-based genetic medicine. In humans, lower hDAT expression levels seem to correlate with higher risks for diseases, as reviewed before [4]. Consistently, animal studies have also shown that reduced DAT expression is a risk factor. For example, mice carrying a reduction in DAT expression display liability for drug addiction [25–28]. Both clinical and pre-clinical findings suggest that up-regulation of hDAT, perhaps via modulation of the 121-bp, may alleviate related brain disorders such as substance abuse in humans.
In conclusion, this 121-bp is a silencer of hDAT promoter. 121-bp-mediated inhibition appears to involve a transcriptional complex, providing a novel platform to screen for hDAT modulators.
Supplementary Material
Acknowledgments
We are grateful to Dr. A. P. Kouzmenko for providing a genomic clone. This research was supported by U.S. National Institutes of Health grant R01DA021409 (to Z.L.), by a Foundation for University Yong Key Teacher by The Education Department of Henan Province China, 2008 (to YZ), and National Nature Science Fundation of China 81100956 (to YZ).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11033-011-1339-4) contains supplementary material, which is available to authorized users.
Contributor Information
Ying Zhao, Department of Psychiatry, Harvard Medical School, Boston, MA, USA; Division of Alcohol and Drug Abuse, McLean Hospital, Mailstop 318, 115 Mill Street, Belmont, MA 02478, USA; School of Pharmacy, Xinxiang Medical University, Xinxiang, Henan, China.
Yanhong Zhou, Department of Psychiatry, Harvard Medical School, Boston, MA, USA; Division of Alcohol and Drug Abuse, McLean Hospital, Mailstop 318, 115 Mill Street, Belmont, MA 02478, USA.
Nian Xiong, Department of Psychiatry, Harvard Medical School, Boston, MA, USA; Division of Alcohol and Drug Abuse, McLean Hospital, Mailstop 318, 115 Mill Street, Belmont, MA 02478, USA; Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Zhicheng Lin, Department of Psychiatry, Harvard Medical School, Boston, MA, USA; Division of Alcohol and Drug Abuse, McLean Hospital, Mailstop 318, 115 Mill Street, Belmont, MA 02478, USA.
References
- 1.Hitri A, Hurd YL, Wyatt RJ, Deutsch SI. Molecular, functional and biochemical characteristics of the dopamine transporter: regional differences and clinical relevance. Clin Neuropharmacol. 1994;17:1–22. doi: 10.1097/00002826-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ohyama K, Sogawa C, Sogawa N, Morita K, Dohi T, Kitayama S. Nicotine stimulates transcriptional activity of the human dopamine transporter gene. Neurosci Lett. 2010;471:34–37. doi: 10.1016/j.neulet.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga-Barke EJ. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: developmental continuities in gene–environment interplay. Am J Med Genet B. 2009;150B:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Canales JJ, Björgvinsson T, Thomsen M, Qu H, Liu QR, Torres GE, Caine SB. Monoamine transporters vulnerable and vital doorkeepers. Prog Mol Biol Transl Sci. 2011;98:1–46. doi: 10.1016/B978-0-12-385506-0.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48:969–977. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- 6.Hurd YL, Pristupa ZB, Herman MM, Niznik HB, Kleinman JE. The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience. 1994;63:357–362. doi: 10.1016/0306-4522(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee KH, Kwak YD, Kim DH, Chang MY, Lee YS. Human zinc finger protein 161, a novel transcriptional activator of the dopamine transporter. Biochem Biophys Res Commun. 2004;313:969–976. doi: 10.1016/j.bbrc.2003.11.183. [DOI] [PubMed] [Google Scholar]
- 8.Sacchetti P, Brownschidle LA, Granneman JG, Bannon MJ. Characterization of the 5′-flanking region of the human dopamine transporter gene. Brain Res Mol Brain Res. 1999;74:167–174. doi: 10.1016/s0169-328x(99)00275-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Michelhaugh SK, Bannon MJ. Valproate robustly increases Sp transcription factor-mediated expression of the dopamine transporter gene within dopamine cells. Eur J Neurosci. 2007;25:1982–1986. doi: 10.1111/j.1460-9568.2007.05460.x. [DOI] [PubMed] [Google Scholar]
- 10.Xing G, Zhang L, Heynen T, Li XL, Smith MA, Weiss SR, Feldman AN, Detera-Wadleigh S, Chuang DM, Post RM. Rat nurr1 is prominently expressed in perirhinal cortex, and differentially induced in the hippocampal dentate gyrus by electroconvulsive vs. kindled seizures. Brain Res Mol Brain Res. 1997;47:251–261. doi: 10.1016/s0169-328x(97)00056-9. [DOI] [PubMed] [Google Scholar]
- 11.Yokoro K, Yanagidani A, Obata T, Yamamoto S, Numoto M. Genomic cloning and characterization of the mouse POZ/zinc-finger protein ZF5. Biochem Biophys Res Commun. 1998;246:668–674. doi: 10.1006/bbrc.1998.8675. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood TA, Kelsoe JR. Promoter and intronic variants affect the transcriptional regulation of the human dopamine transporter gene. Genomics. 2003;82:511–520. doi: 10.1016/s0888-7543(03)00142-3. [DOI] [PubMed] [Google Scholar]
- 13.Kouzmenko AP, Pereira AM, Singh BS. Intronic sequences are involved in neural targeting of human dopamine transporter gene expression. Biochem Biophys Res Commun. 1997;240:807–811. doi: 10.1006/bbrc.1997.7754. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Walther D, Yu XY, Li S, Drgon T, Uhl GR. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet. 2005;14:1393–1404. doi: 10.1093/hmg/ddi148. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Zhao Y, Chung CY, Zhou Y, Xiong N, Glatt CE, Isacson O. High regulatability favors genetic selection in SLC18A2, a vesicular monoamine transporter essential for life. FASEB J. 2010;24:2191–2200. doi: 10.1096/fj.09-140368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Bannon MJ. Sp1 and Sp3 activate transcription of the human dopamine transporter gene. J Neurochem. 2005;93:474–482. doi: 10.1111/j.1471-4159.2005.03051.x. [DOI] [PubMed] [Google Scholar]
- 18.Bai Q, Burton EA. Cis-acting elements responsible for dopaminergic neuron-specific expression of zebrafish slc6a3 (dopamine transporter) in vivo are located remote from the transcriptional start site. Neuroscience. 2009;164:1138–1151. doi: 10.1016/j.neuroscience.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, Lin SI, Chen YK, Chiang CS, Liaw GJ. The Torso signaling pathway modulates a dual transcriptional switch to regulate tailless expression. Nucleic Acids Res. 2009;37:1061–1072. doi: 10.1093/nar/gkn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adkins NL, Hagerman TA, Georgel P. GAGA protein: a multi-faceted transcription factor. Biochem Cell Biol. 2006;84:559–567. doi: 10.1139/o06-062. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Lamothe R, Boyle P, Dulude A, Roy V, Lezin-Doumbou C, Kaur GS, Bouarab K, Després C, Brisson N. The transcriptional activator Pti4 is required for the recruitment of a repressosome nucleated by repressor SEBF at the potato PR-10a gene. Plant Cell. 2008;20:3136–3147. doi: 10.1105/tpc.108.061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21:3493–3505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsberg M, Westin G. Enhancer activation by a single type of transcription factor shows cell type dependence. EMBO J. 1991;10:2543–2551. doi: 10.1002/j.1460-2075.1991.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser G, Vogel M, Wolf H, Niller HH. Regulation of the Epstein-Barr viral immediate early BRLF1 promoter through a distal NF1 site. Arch Virol. 1998;143:1967–1983. doi: 10.1007/s007050050433. [DOI] [PubMed] [Google Scholar]
- 25.Hall FS, Sora I, Uhl GR. Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology. 2003;28:620–628. doi: 10.1038/sj.npp.1300070. [DOI] [PubMed] [Google Scholar]
- 26.Morice E, Denis C, Giros B, Nosten-Bertrand M. Evidence of long-term expression of behavioral sensitization to both cocaine and ethanol in dopamine transporter knockout mice. Psychopharmacology. 2010;208:57–66. doi: 10.1007/s00213-009-1707-0. [DOI] [PubMed] [Google Scholar]
- 27.Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering RNA: a phenotypic comparison with knockout animals. Biol Psychiatry. 2007;61:65–69. doi: 10.1016/j.biopsych.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcohol Clin Exp Res. 2002;26:758–764. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


