Abstract
Purpose
To estimate the associations of individual pre-fracture characteristics with total health care costs after hip fracture, using Study of Osteoporotic Fractures (SOF) cohort data linked to Medicare claims.
Methods
Our study population was 738 women age 70 and older enrolled in Medicare Fee for Service (FFS) who experienced an incident hip fracture between 1/1/1992 and 12/31/2009. We assessed pre-fracture individual characteristics at SOF study visits, and estimated costs of hospitalizations, skilled nursing facility and inpatient rehabilitation stays, home health care visits, and outpatient utilization from Medicare FFS claims. We used generalized linear models to estimate the associations of predictor variables with total health care costs (2010 U.S. Dollars) after hip fracture.
Results
Median total health care costs for one year after hip fracture were $35,536 (inter-quartile range $24,830 to $50,903). Multi-variable adjusted total health care costs for one year after hip fracture were 14% higher ($5,256, 95% C.I. $156 to $10,356) in those with walk speed <0.6 meter/sec compared to ≥1.0 meter/sec; 25% higher ($9,601, 95% C.I. $3,314 to $16,069) in those with body mass index ≥30 kg/m2 compared to 20 to 24.9 mg/kg2; and 21% higher ($7,936, 95% C.I. $346 to $15,526) for those with 7 or more compared to no comorbid medical conditions.
Conclusions
Pre-fracture poor mobility, obesity, and multiple comorbidities are associated with higher total health care costs after hip fracture in older women. Studies to investigate if targeted health care interventions for these individuals can reduce the costs of hip fractures are warranted.
Keywords: Hip fracture costs, total health care costs, walk speed, obesity, multimorbidity
INTRODUCTION
Osteoporotic fractures cause substantial morbidity among older persons, and are projected to cost $25 billion in the United States by 2025.[1] Hip fractures account for 50% to 70% of fracture costs,[1, 2] but these costs are highly variable at the individual level. Reducing the incidence, morbidity, and costs of hip fractures remain important health policy goals. A necessary step in making progress toward these goals is to identify individual patient characteristics that are associated with higher hip fracture costs.
Studies of hip fracture costs thus far have focused primarily on the associations of post-acute care use,[3–6] post-fracture rehabilitation intensity,[7, 8] or identified health care structural characteristics that influence post-acute care use after hip fracture.[3, 9] These studies, based on claims data, have limited ascertainment of individual patient characteristics such as age, sex, location of residence, and claims-based estimates of co-morbidity. Prospective epidemiologic studies have assessed unique patient characteristics that predict incident hip fracture[10], and many of these patient attributes may be related to aspects of health care utilization. In particular, slow walk speed[11] predicts higher hospital[12] and nursing home utilization[13, 14] among community-dwelling adults but its association with total health care costs in the general or selected subsets of the aged population has not been assessed.
Identification of characteristics associated with high health care costs after hip fracture is important for several reasons. Pre-fracture characteristics that predict high health care costs after hip fracture may help direct the design, and improve the cost-effectiveness, of various health care interventions. For example, pharmacologic treatments to reduce risk of hip fracture may be particularly cost-effective for osteoporotic individuals with characteristics that predict high health care costs after hip fracture; this may be an important consideration for newer but more expensive pharmacologic fracture prevention therapies. If pre-fracture characteristics that are potentially modifiable (such as slower walk speed and greater depressive symptoms)[15] are associated with both a higher incidence of hip fracture and higher post-fracture costs, then interventions that improve these risk factors are even more likely to be cost-effective. Finally, even non-modifiable risk factors that aid identification of individuals with high post-fracture costs could help target programs to coordinate and improve the efficiency of health care for these individuals, ultimately reducing their health care burden and costs if a hip fracture occurs.
Our primary objective was to estimate the associations of pre-fracture characteristics, (including measures of physical performance, depressive symptoms, cognitive function, body mass index (BMI), and comorbidity burden) with total health care costs for the year after hip fracture among older women. Our secondary objective was to estimate the associations of pre-fracture characteristics with component costs, specifically acute hospital costs, skilled nursing facility costs, and outpatient costs.
METHODS
The Study of Osteoporotic Fractures (SOF) recruited 9,704 community-dwelling Caucasian women age 65 or older between 1986 and 1988 from population based listings in four geographic regions of the United States; Baltimore, MD; Minneapolis, MN; Portland, OR; and a rural area (Monongahela Valley) near Pittsburgh, PA.[16] SOF study visits were carried out every 2–5 years through 2010. At the sixth SOF study visit between 1996 and 1998, 662 African-American women age 65 and older were also recruited.[17] Using validated methods detailed in previous publications,[18, 19] successful matches to Medicare claims were achieved for 92% (n=9,228) of women enrolled in SOF as of January 1, 1991, when outpatient Medicare claims first became available. Since the far majority of all individuals who have a hip fracture are admitted to hospital for surgical treatment, we used the U.S. Medicare Provider Analysis and Review (MedPAR) file to identify SOF participants with an incident hip fracture between January 1, 1992 and December 31, 2009 by the following criteria; a) a discharge diagnosis of hip fracture (ICD-9 code 820.0x or 733.14); b) a hip fracture surgical treatment code (ICD-9 codes 78.55, 79.15, 79.35, 81.51, 81.52); and c) not having a treatment code indicating surgical removal of hardware or revision arthroplasty (78.65 or 81.53). The date of the hip fracture was the admission date for the hospital stay during which their hip fracture was surgically treated. Of the 1170 women who met these criteria, 738 (63%) were enrolled in Medicare Fee for Service parts A and B for the year before and year after their hip fracture, and this subset comprised our analytic cohort (figure 1). The remaining 432 participants were enrolled in Medicare Advantage during part or all of the first year before or year after their hip fracture, and therefore their claims were not available to us.
Figure 1. Flow Diagram of Identification of Study Population.
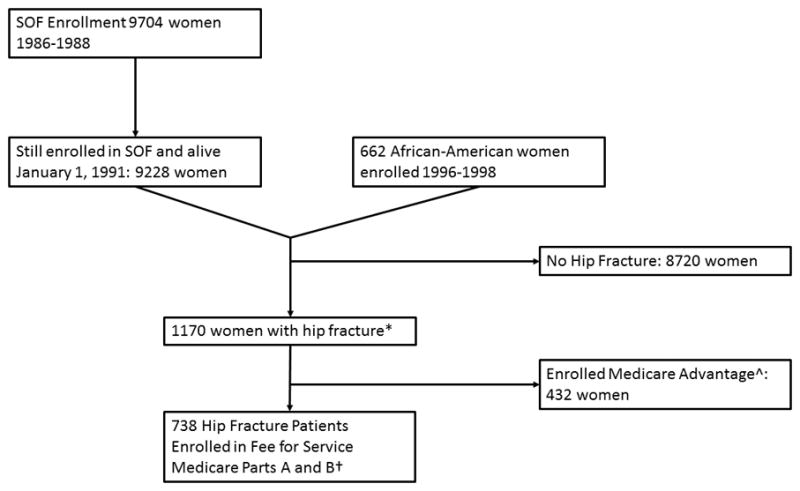
*Hip fracture date between January 1, 1992 and December 31, 2009 inclusive. Identified by self-report to the SOF cohort or by Medicare Claims (required primary discharge diagnosis of hip fracture and hip fracture surgical treatment code and absence of code for surgical removal of hardware)
^Enrolled in Medicare Advantage for part or all of the year before hip fracture date and the year after hip fracture (or until death, whichever came first)
†Enrolled in Medicare Fee for Service Parts A and B for entire year before hip fracture date and the year after hip fracture (or until death, whichever came first)
Total Health Care Costs Year Before and Year after Hip Fracture
Because it is very difficult to accurately ascertain which items of health care utilization after hip fracture are attributable to that fracture,[20] our analytic strategy was to use total healthcare costs the year after hip fracture as our dependent variable and to adjust for pre-fracture total health care costs the year prior to hip fracture. Since health care utilization attributable to hip fracture can occur for at least a year after the event, we defined the episode of care for hip fracture as one year. Total health care costs were calculated as the sum of costs for all acute hospital stays (including the stay during which the hip fracture was surgically treated), skilled nursing facility (SNF) stays paid by Medicare, inpatient rehabilitation facility (IRF) stays, outpatient care, and home health care for one year after and before the hip fracture date. Medication costs were not included. All acute hospital stays, Medicare paid SNF stays, and IRF stays during the year before and year after hip fracture were identified in the MedPAR file. Outpatient utilization was identified in the Carrier and Outpatient Medicare claims files, and home health care visits were identified in the Home Health Care file.
The U.S. Medicare system is a government-administered health insurance system that sets reimbursement payment rates to both inpatient and outpatient providers on behalf of Medicare beneficiaries (U.S. citizens age 65 and older). Medicare tries to base payments for hospital, SNF, IRF and outpatient care providers on the intensity of and true resource costs for those services required for the patient’s medical and surgical acuity.[21] Medicare adjusts final payments to providers for geographic variation in labor and capital price inputs into the costs of delivering health care services. As our aim was to assess impact of individual characteristics on health care costs that were generalizable to the United States as a whole, we used Medicare payments with the geographic price input adjustments removed. Our methods of doing so for acute hospital inpatient stays and outpatient utilization are described fully in previous publications.[19, 22]
In the case of SNF and IRF payments, Medicare uses the health care wage index (WI) to adjust payments for local price inputs. For SNF stays, the local input price adjustment can be removed from the Medicare payment values in the MedPAR file using the following equation:
where PropLABOR-SNF is the proportion of SNF care costs attributable to SNF worker wages and WI is the hospital wage index for that geographic locality.
Similarly for IRF stays, the local input price adjustments can be removed from the Medicare payment values in the MedPAR file using the following equation:
where PropLABOR-IRF is the proportion of IRF care costs attributable to IRF worker wages and WI is the health care wage index for that geographic locality. The proportions of SNF and IRF costs estimated to be attributable to worker wages are published for each fiscal year in the U.S. Federal Register, and the WI for each local geographic location in the U.S. for each fiscal year are available on the U.S. Medicare website.
Costs were calculated and adjusted for health care cost inflation to U.S. 2010 dollars by methods described in detail in prior publications,[19] using Medicare payments to providers and resource intensity weights that reflect the true monetary resources required to deliver each type of health care.
Patient Characteristics
Age, race, and educational status were recorded at the baseline visit. Femoral neck bone mineral density (BMD) was measured in participants at the second (1989 to 1990), fourth (1992 to 1994), sixth (1996 to 1998), eighth (2002 to 2004) and ninth (2006 to 2008) study visits, with quality control methods described in previous publications.[23, 24] Self-reported health status (recorded as a single question survey item), impairment of instrumental activities of daily living (IADLs), smoking status, whether the participant had a fall during the previous 12 months, and whether or not the participant walked for exercise were ascertained at each study visit. Grip strength,[25] the ability and time to complete 5 chair stands,[26] and 6 meter walk speed at usual pace were measured at each study visit. Depressive symptoms were measured using the 15-item Geriatric Depression Scale (GDS)[27] score at the second, fourth, sixth, eighth, and ninth study visits, and categorized as none or minimal (GDS score of 0 or 1), mild (2, 3, 4, or 5), or moderate to severe (GDS scale score of ≥ 6). Cognitive function was assessed using a modification of the Mini-Mental State Examination (MMSE)[28] at all visits except the 2nd and 3rd, and scored from 0 to 26. Height (measured with a Harpenden stadiometer) and weight were assessed at each study visit.
Participant comorbidity burden at the time of the hip fracture was ascertained by the Elixhauser method that takes into account 31 specific medical conditions (summary score range 0–31) using Medicare inpatient and outpatient claims data for the year prior to the hip fracture.[29] We ascertained if participants were already residing in a nursing facility at the time of their hip fracture using a previously validated algorithm[30] with outpatient Medicare claims.
Hospital Characteristics
We used Centers for Medicare and Medicaid Provider of Service (CMS POS) files and Medicare Cost Reports files to ascertain characteristics of the hospitals in which patients’ hip fractures were surgically treated that might influence utilization and cost of care after hip fractures. These characteristics, included as covariates in our analytic models, were hospital size (defined as the number of Medicare certified acute care beds), the number of prospective payment (PPS) exempt rehabilitation beds in the hospital, and hospital ownership of a SNF and/or a home health care agency.[31]
Local Supply of Health Care Providers
Since the presence of SNFs close to the woman’s residence may increase the likelihood of SNF utilization for post-acute care following any hospitalization[31] (and therefore health care costs), the addresses of all SNFs within the patient’s county of residence and adjacent counties were identified in CMS POS files. Because the local supply of hospital beds has been associated with length of stay and intensity of inpatient utilization,[32] and the supply of primary care providers has also been inversely associated with health care costs,[33] we also adjusted for these factors in our analytic models. We used CMS POS files and the Area Health Resource File to estimate the numbers of Medicare certified acute hospital beds and primary care providers per capita within the patient’s county of residence, and to also to estimate the numbers of orthopedic surgeons, occupational therapists, and physical therapists per capita within the patient’s county of residence.
Statistical Analysis
Predictor variables that represented continuous phenomena were modeled as continuous variables unless their distribution was highly skewed or their association with total health care costs was non-linear. In that case, they were modeled as ordinal variables. The most recent SOF visit proximally preceding the hip fracture occurred a median 1.6 years (IQR 0.8 to 2.7) before the hip fracture. If the participant did not attend that particular study visit, predictor values were imputed for that participant at that visit with imputation models using data for all predictors from prior visits for that individual and all visits for all 10,336 SOF study participants. The missing values were imputed 50 times with the multiple chained regression equations method using the ice command of Stata 13.1.
We performed both single-level models and multilevel models with random intercepts for both hospitals in which the hip fractures were surgically treated and counties of residence. However, likelihood ratio tests comparing the multilevel models to single-level models showed no difference between them, hence we included only analyses from single-level generalized linear models with a gamma variance function and identity link. Models were run using the mi estimate command of Stata, in order to compute robust parameter coefficients and standard errors. Initial models were run to assess the association of each individual patient predictor with costs after hip fractures, adjusted only for study enrollment site and the year in which the hip fracture occurred. Those predictor variables with a p-value of association < 0.1 with total health care costs were then included in full multivariable models; variables with a multi-variable adjusted p-value of association > 0.2 with costs were then eliminated in a stepwise fashion.
Our secondary goal was to estimate the associations of the individual predictor variables with each of three major components of health care costs after hip fracture (acute hospital costs, outpatient care costs, and Medicare paid SNF costs). Each of these separate generalized linear models had the same predictor variables as the final multivariable model for total health care costs after hip fracture. Twenty five percent of participants did not have any Medicare paid SNF claims during the year after their hip fracture. Hence, we estimated the associations of predictor variables with any Medicare paid SNF utilization (any SNF costs versus none) using logistic regression, and then the associations of predictor variables with total Medicare paid SNF costs for the subset of participants with non-zero costs using a generalized linear model.
Hip fracture is associated with a 20% or more cumulative mortality within several months of the fracture,[34] and health care costs tend to be very high in the year preceding death.[35] Therefore, all of the above analyses were repeated after excluding participants who did not survive for 1 year after hip fracture.
Finally, in order to explore whether certain key comorbid conditions were important predictors of total health care costs after hip fracture, we adjusted for the presence or absence of five key comorbid conditions (diabetes mellitus, cardiovascular disease (CVD), dementia depression, and chronic obstructive pulmonary disease [COPD]) in place of the overall comorbidity score.
RESULTS
The 738 women with hip fracture in our cohort had a mean age of 83.7 years (range 70 to 99 years) and were overwhelmingly Caucasian (Table 1). Over 50% of participants had three or more comorbid conditions, and 65% had at least one IADL impairment before experiencing their hip fracture. Five hundred sixty five (77%) survived the entire first year after their hip fracture, and among those who did not survive, 173 (23%) died a mean 4.1 months after hip fracture.
Table 1.
Characteristics of 738 Hip Fracture Patients and Associations with Total Health Care Costs after Hip Fracture, Base Modela
| Parameter | Mean (SD) or Frequency (%) | Incremental Costb (95% CI)c |
|---|---|---|
| Age, years (per 5 year increase)d | 83.7 (5.5) | −$1,816 (−3,395 to −236) |
| Race/Ethnicity | ||
| Caucasian | 726 (98.4%) | Reference |
| African American | 12 (1.6%) | −$7,778 (−18,706 to 3,149) |
| Education | ||
| Less than high school | 231 (24.8%) | Reference |
| High school | 343 (36.9%) | −$2,363 (−6,558 to 1,832) |
| Less than 4 years college | 176 (18.9%) | $3,689 (−2,280 to 9,657) |
| 4 or more years college | 180 (19.3%) | −$2,046 (−6,609 to 2,516) |
| Number of comorbiditiesd | ||
| None | 174 (18.7%) | Reference |
| 1 or 2 | 259 (27.8%) | $2,171 (−3,014 to 7,356) |
| 3 or 4 | 215 (23.1%) | $5,281 (−205 to 10,767) |
| 5 or 6 | 139 (15.0%) | $13,485 (6,971 to 19,999) |
| ≥7 | 143 (15.4%) | $14,185 (7,741 to 20,630) |
| Self-rated healthd | ||
| Good or excellent | 669 (71.9%) | Reference |
| Fair, poor, or very poor | 261 (28.1%) | $5,648 (1,551 to 9,746) |
| Walks for exercised | ||
| No | 540 (73.2%) | Reference |
| Yes | 198 (26.8%) | −$3,003 (−6,663 to 656) |
| IADLs impairedd | ||
| None | 326 (35.0%) | Reference |
| 1 | 239 (60.7%) | $5,914 (796 to 11,032) |
| 2 or 3 | 232 (25.0%) | $6,100 (1,473 to 10,727) |
| ≥4 | 133 (14.3%) | $5,028 (−367 to 10,422) |
| Self-Reported Falls Prior 12 months | ||
| None | Reference | |
| One or more | $2,071 (−1267 to 5410) | |
| Body mass indexd, kg/m2 | ||
| <20 | 97 (13.1%) | $1,083 (−4,428 to 6,595) |
| 20 to 24.9 | 312 (42.3%) | Reference |
| 25 to 29.9 | 238 (32.2%) | $3,146 (−1,333 to 7,625) |
| ≥30 | 91 (12.3%) | $11,669 (4,862 to 18,476) |
| Femoral neck BMD, g/cm2 | 0.548 (0.098) | $1,533 (−529 to 3,596)e |
| Mini-Mental State Exam score (0–30) | ||
| 26 | 127(17.3%) | Reference |
| 25 | 149 (20.1%) | $3,139 (−3,106 to 9,384) |
| 23 or 24 | 245 (33.2%) | $1,413 (−3,905 to 6,733) |
| ≤22 | 217 (29.4%) | $−1,951 (−7,897 to 3,994) |
| GDS-15 scored | ||
| 0 or 1 | 232 (31.5%) | Reference |
| 2 to 5 | 368 (49.8%) | $3,925 (−78 to 7,929) |
| ≥6 | 138 (18.7%) | $5,764 (383 to 11,145) |
| Grip strength, kg | 15.2 (4.85) | −$1,526 (−3,474 to 422)e |
| Chair stand speedd, stands/sec | 3.11 (1.28) | $2,237 (−28 to 4,502)e |
| Walk speedd, m/s | ||
| ≥1.0 | 112 (15.1%) | Reference |
| 0.8 to 0.99 | 220 (29.8%) | $4,520 (−1,031 to 10,072) |
| 0.6 to 0.79 | 202 (27.4%) | $6,546 (908 to 12,184) |
| <0.6 | 204 (27.6%) | $7,472 (1,781 to 13,163) |
| Permanent nursing home resident before hip fracture | ||
| No | 551 (74.7%) | Reference |
| Yes | 187 (25.3%) | $−3,025 (−6,794 to 744) |
Adjusted for study site and year of hip fracture
2010 U.S. Dollars
Associations with p-value <0.05 are in bold
Selected for multivariable models based on p-value <0.1
per SD increase
The median (inter-quartile range [IQR]) total costs of care for the year after and year before hip fracture were, respectively, $35,536 ($24,830 to $50,903) and $4,465 ($1,120 to $14,066). Major components of total health care costs during the year after hip fracture were acute hospital stays (median $12,236, IQR 10,248 to 18,725), Medicare paid SNF stays (median $9,587, IQR 0 to 24,416), and outpatient costs (median $5,807, IQR 3,843 to 8,625). Only 13.8% of the cohort had an IRF stay during the year after their hip fracture, and 52% incurred home health care costs that on average were modest in magnitude (Table 2).
Table 2.
Standardized Costs of Health Care Year After and Year Before Hip Fracture
| Parameter | Costs After Hip Fracture, Median (IQR) | Costs Before Hip Fracture, Median (IQR) |
|---|---|---|
| Total | $35,536 (24,830 to 50,903) | $4,465 (1,120 to 14,066) |
| Acute hospital costs | $12,236 (10,248 to 18,725) | $0 (0 to 5,766)d |
| SNF costs | $9,587 (0 to 20,416)a | $0 (0 to 0)e |
| IRF costs | $0 (0 to 0)b | $0 (0 to 0)f |
| Outpatient costs | $5,807 (3,843 to 8,625) | $2,500 (1,066 to 5,037) |
| Home health care | $442 (0 to 4,310)c | $0 (0 to 0)g |
553 (74.9%) had positive part A paid SNF costs after hip fracture (mean $13,718 across all 738 individuals)
102 (13.8%) had positive IRF costs after hip fracture (mean $1,790 across all 738 individuals)
384 (52.0%) has positive Home Health Care costs after hip fracture (mean $2,692 across all 738 individuals)
286 (38.3%) had non-zero acute hospital costs year before hip fracture (mean $4,422 across all 738 individuals)
96 (13.0%) had non-zero SNF costs year before hip fracture (mean $1,606 across all 738 individuals)
10 (1.4%) had non-zero IRF costs year before hip fracture (mean $204 across all 738 individuals)
163 (22.1%) had non-zero Home Health Care costs year before hip fracture (mean $942 across all 738 individuals)
With adjustment only for study enrollment site and the year during which the hip fracture occurred, pre-fracture characteristics associated with higher total health care costs after hip fracture included poorer self-rated health, 5 or more comorbid conditions, younger age, IADL impairment, obesity (BMI ≥ 30 kg/m2), depressive symptoms, and slower walk speed (Table 1). Pre-fracture global cognitive function, BMD, self-reported walking for exercise, grip strength, chair stand speed, current smoking, and pre-fracture nursing home residence were not significantly associated with total health care costs after hip fracture.
After further multi-variable adjustment for pre-fracture comorbidity, health care costs the year before hip fracture, size of the hospital in which the hip fracture was surgically treated, and per capita supply of primary care physicians, women with walk speed <0.6 meters/sec and those with walk speed 0.6 to <0.8 meters/sec both had a 1.14-fold higher total health care costs after hip fracture compared to those with walk speed ≥ 1.0 meter/sec (Table 3). Obese women with BMI ≥ 30 kg/m2 compared to normal weight women with BMI 20 to 24.9 kg/m2 had a 1.25-fold increase in total health care costs the year after hip fracture. Compared to those with no pre-fracture comorbid conditions, those with 5 or 6 or ≥ 7 more comorbid conditions had 1.21-fold higher total health care costs after hip fracture. Age, self-reported health, depressive symptoms, and IADL impairment were no longer associated with total health care costs after hip fracture in the multivariable model.
Table 3.
Multivariable-Adjusted Incremental Change of Total Health Care Costs after Hip Fracture with Change of Predictor Variable Level Compared to Reference Category
| Predictor | Change of Total Health Care Costs (95% CI)a,b
|
|
|---|---|---|
| Model 1 (n = 738) | Model 2c (n = 565) | |
| Total health care costs before hip fracture | ||
| Quintile 1 | Reference | Reference |
| Quintile 2 | $937 (−3,328 to 5,203) | $351 (−3,921 to 4,625) |
| Quintile 3 | −$298 (−5,039 to 4,442) | −$1,011 (−5,849 to 3,826) |
| Quintile 4 | $4,803 (−770 to 10,377) | $3,468 (−2,218 to 9,153) |
| Quintile 5 | $8,234 (1,938 to 1,530) | $8,964 (1,922 to 16,006) |
| Comorbidities | ||
| None | Reference | Reference |
| 1 or 2 | $848 (−4,268 to 5,964) | $1,523 (−3,440 to 6,486) |
| 3 or 4 | $2,262 (−3,497 to 8,022) | $2,920 (−2,608 to 8,449) |
| 5 or 6 | $7,859 (769 to 14,949) | $10,210 (2,667 to 17,752) |
| 7 or more | $7,936 (346 to 15,526) | $13,763 (5,311 to 22,214) |
| Walk speed, m/s | ||
| ≥1.0 | Reference | Reference |
| 0.8 to 0.99 | $3,891 (−1057 to 8839) | $2,902 (−1921 to 7724) |
| 0.6 to 0.79 | $5,142 (104 to 10,180) | $5,637 (482 to 10,792) |
| <0.6 | $5,256 (156 to 10,356) | $7,523 (1,809 to 13,236) |
| Body mass index, kg/m2 | ||
| <20 | $75 (−5,149 to 5,300) | −$1,473 (−7,107 to 4,160) |
| 20 to 24.9 | Reference | Reference |
| 25 to 29.9 | $2,003 (−2,237 to 6,242) | $751 (−3,533 to 5,034) |
| ≥30 | $9,601 (3,134 to 16,069) | $7,233 (506 to 13,959) |
Adjusted for study enrollment site, year of hip fracture, size of hospital in which hip fracture was treated, and primary care providers per 10,000 inhabitants in county of residence at the time of hip fracture
Model predicted costs for reference level, and incremental costs for other levels compared to reference level; statistically significant values at p-value <0.05 are in bold
Model 2 restricted to those who survived at least 12 months post hip fracture
When indicator variables for diabetes mellitus, CVD, depression, dementia and COPD were included in the multivariable models in place of the overall comorbidity score, only pre-existing CVD was significantly associated with total health care costs after hip fracture (incremental cost compared to those with compared to those without CVD $5,168, 95% C.I. 1026 to 9310). Notably, the associations of walk speed and BMI with total health care costs after hip fracture were unchanged (data not shown).
The effects of the individual patient characteristics differed for the major components of post-fracture health care costs. The multivariable-adjusted association between slow walk speed and total health care costs was primarily due to its association with Medicare paid SNF costs the year after hip fracture (Table 4); associations of walk speed with acute hospital costs (Table 5) or outpatient costs (Table 5) the year after hip fracture appeared smaller in magnitude and did not reach significance. These results were unchanged in analyses limited to the women who survived for at least 1 year after hip fracture. In contrast, obesity was associated with increases in all major components (adjusted acute hospital care costs, outpatient costs (Table 5), and Medicare paid SNF costs (Table 4) of total health care costs\ for the year after hip fracture. Among the subset of women who survived for at least 1 year after hip fracture, patterns were similar but the association of obesity with increased costs reached the level of significance only for outpatient costs. Finally, the association of pre-fracture comorbidity with higher total health costs appeared to be primarily driven by increases in both acute hospital and Medicare paid SNF costs, though the association was significant only for acute hospital costs among the subset of women who survived at least 1 year after hip fracture.
Table 4.
Associations of Predictors with Use of SNF During First Year Post Hip Fracture and SNF Costs
| Predictor | Odds Ratio (95% CI) | Incremental Cost (95% CI)a,b
|
|
|---|---|---|---|
| SNF usec (n=738) | Model 1d (n=533) | Model 2d,e (n=420) | |
| Total health care costs before hip fracture | |||
| Quintile 1 | Reference | Reference | Reference |
| Quintile 2 | 0.92 (0.49 to 1.73) | $−762 (−4,150 to 2,625) | $−619 (−4,017 to 2,780) |
| Quintile 3 | 1.08 (0.52 to 2.24) | $−3147 (−6,772 to 479) | $−3,438 (−7,115 to 238) |
| Quintile 4 | 1.17 (0.59 to 2.31) | $−721 (−4,847 to 3,406) | $−352 (−4,736 to 4,033) |
| Highest Quintile | 1.12 (0.54 to 2.33) | $−394 (−5,049 to 4,260) | $−1,174 (−6,075 to 3,727) |
| Comorbidities | |||
| None | Reference | Reference | Reference |
| 1 or 2 | 0.86 (0.43 to 2.12) | $−2,476 (−6,612 to 1,661) | $−2,211 (−6,627 to 2,206) |
| 3 or 4 | 1.24 (0.53 to 2.92) | $−1,467 (−6,019 to 3,084) | $−1,404 (−6,193 to 3,386) |
| 5 or 6 | 2.67 (1.09 to 6.57) | $905 (−4,520 to 6,331) | $4,044 (−1,788 to 9,876) |
| 7 or more | 1.08 (0.42 to 2.80) | $4,329 (−1,622 to 10,101) | $5,371 (−883 to 11,626) |
| Walk speed, m/s | |||
| ≥1.0 | Reference | Reference | Reference |
| 0.8 to 0.99 | 1.04 (0.56 to 1.92) | $3,035 (−507 to 6,577) | $2,167 (−1,602 to 5,936) |
| 0.6 to 0.79 | 0.99 (0.47 to 2.10) | $2,049 (−2,050 to 6,147) | $1,992 (−2,375 to 6,359) |
| <0.6 | 1.05 (0.50 to 2.21) | $4,646 (690 to 8,601) | $5,371 (947 to 9975) |
| Body mass index, kg/m2 | |||
| <20 | 1.23 (0.60 to 2.58) | $−504 (−4,272 to 3,264) | $−1,164 (−5,298 to 2,971) |
| 20 to 24.9 | Reference | Reference | Reference |
| 25 to 29.9 | 1.08 (0.60 to 1.94) | $1,113 (−2251 to 4,477) | $80 (−3,288 to 3,449) |
| ≥30 | 1.61 (0.74 to 3.48) | $4,802 (441 to 9,165) | $3,889 (−938 to 8,715) |
Model predicted costs for reference level, and incremental costs for other levels compared to reference level; statistically significant values at p-value <0.05 are in bold
Adjusted for study enrollment site, year of hip fracture, size of hospital in which hip fracture was treated, and primary care providers per 10000 inhabitants in county of residence at the time of hip fracture
Defined as any non-zero SNF costs for the year after hip fracture vs. no SNF costs
Regression limited to those with non-zero part A paid SNF costs post hip fracture
Model 2 restricted to those who survived at least 12 months post hip fracture
Table 5.
Multivariable-Adjusted Incremental Change of Acute Hospital and Outpatient Costs after Hip Fracture with Change of Predictor Variable Level Compared to Reference Category
| Predictor | Change of Acute Hospital Costs (95% CI)a,b | Change of Outpatient Costs (95% CI) | ||
|---|---|---|---|---|
|
| ||||
| Model 1 (n = 738) | Model 2c (n = 565) | Model 1b (n = 738) | Model 2c (n = 565) | |
| Total health care costs before hip fracture | ||||
| Quintile 1 | Reference | Reference | Reference | Reference |
| Quintile 2 | $−82 (−2,085 to 1,921) | $61 (−2,055 to 2,177) | $861 (−33 to 1,755) | $958 (39 to 1,877) |
| Quintile 3 | $−279 (−2,474 to 1,916) | $−487 (−2,827 to 1,853) | $1,267 (176 to 2,357) | $1,334 (170 to 2,498) |
| Quintile 4 | $878 (−1,599 to 3,354) | $218 (−2,459 to 2,895) | $2,360 (1,168 to 3,551) | $2,423 (1,157 to 3,710) |
| Highest Quintile | $1,852 (−914 to 4,619) | $2,026 (−1,169 to 5,221) | $4,093 (2,650 to 5,536) | $5,191 (3,423 to 6,959) |
| Comorbidities | ||||
| None | Reference | Reference | Reference | Reference |
| 1 or 2 | $694 (−1,703 to 3,091) | $1,256 (−1,138 to 3,650) | $−329 (−1,438 to 780) | $−179 (−1,259 to 902) |
| 3 or 4 | $1,517 (−1,201 to 4,234) | $2,090 (−665 to 4,845) | $−363 (−1,668 to 943) | $−186 (−1,452 to 1,080) |
| 5 or 6 | $2,749 (−330 to 5,828) | $2,667 (−682 to 6,016) | $−444 (−1,940 to 1,052) | $−158 (−1,773 to 1,458) |
| 7 or more | $2,907 (−446 to 6,260) | $5,601 (1,707 to 9,495) | $−36 (−1,726 to 1,653) | $937 (−1,027 to 2,902) |
| Walk speed, m/s | ||||
| ≥1.0 | Reference | Reference | Reference | Reference |
| 0.8 to 0.99 | $666 (−1,578 to 2,890) | $95 (−2,224 to 2,414) | $570 (−509 to 1,649) | $457 (−610 to 1,524) |
| 0.6 to 0.79 | $1,304 (−1,074 to 3,682) | $1,068 (−1,426 to 3,563) | $742 (−381 to 1,864) | $772 (−381 to 1,925) |
| <0.6 | $991 (−1,367 to 3,349) | $1,543 (−1,099 to 4,186) | $483 (−648 to 1,614) | $892 (−359 to 2,142) |
| Body mass index, kg/m2 | ||||
| <20 | $269 (−2,037 to 2,575) | $−583 (−3,264 to 2,098) | $36 (−1,032 to 1,105) | $−29 (−1,244 to 1,184) |
| 20 to 24.9 | Reference | Reference | Reference | Reference |
| 25 to 29.9 | $1,092 (−707 to 2,892) | $859 (−1,082 to 2,799) | $458 (−419 to 1,335) | $360 (−569 to 1,290) |
| ≥30 | $2,949 (173 to 5,724) | $2213 (−687 to 5,114) | $1,849 (471 to 3,228) | $1,608 (178 to 3,088) |
Adjusted for study enrollment site, year of hip fracture, size of hospital in which hip fracture was treated, and primary care providers per 10,000 inhabitants in county of residence at the time of hip fracture
Model predicted costs for reference level, and incremental costs for other levels compared to reference level; statistically significant values at p-value <0.05 are in bold
Model 2 restricted to those who survived at least 12 months post hip fracture
DISCUSSION
Total health care costs after hip fracture are highly variable, and individual characteristics that may predict these costs have not been previously explored. Our results indicate that pre-fracture slow walk speed, pre-fracture obesity (BMI≥ 30 kg/m2), and greater comorbidity burden are independently associated with higher total health care costs after hip fracture, adjusted for health care utilization before the hip fracture. The association of walk speed with total health costs after hip fracture is primarily driven by its association with Medicare paid SNF costs after hip fracture. These findings suggest that those with higher pre-fracture lower extremity physical performance have more reserve to withstand the effects of hip fracture such that they may require less intense rehabilitation afterward. In contrast, obesity is associated more broadly with increases in all of the major components of health care utilization after hip fracture.
One previous study reported an association of self-reported ability to walk ¼ mile with total health care costs,[36] but our investigation is the first to show any association between objectively measured walk speed and total health care costs. In addition, other studies have reported that objectively assessed impaired mobility is associated with a higher risk of inpatient hospitalization[12] and nursing home admission,[13, 14] but our investigation expands on these findings by showing the incremental effect of slow walk speed on total health care costs. Slow walk speed is associated with a higher incidence of hip fracture,[10] and is amenable to interventions designed to improve physical mobility and activity in older individuals.[37, 38] Improving mobility may reduce health care costs not only by reducing risk of incident hip fracture but also by reducing cost of care following hip fracture should it occur. Future research studies to evaluate whether or not interventions to improve mobility reduce hip fracture incidence and health care costs after hip fracture clearly are warranted. If interventions that improve mobility result in some health care cost savings that at least partially offset the intervention cost, then routine periodic assessment of lower extremity performance (such as walk speed) in clinical practice settings as some have advocated[11] may be reasonable, particularly in frail older persons at higher hip fracture risk.
Although only an estimated 12.3% of our hip fracture cases were obese, the effect of obesity on health care costs attributable to hip fracture was particularly striking; costs were 25% higher in obese individuals compared to those with normal body weight. Higher body weight is associated with a lower incidence of hip fracture up to a BMI of 30 kg/m2, but further increases in BMI are associated with little or no further reductions in fracture incidence.[39, 40] The effects of interventions to reduce obesity on hip fracture costs are therefore likely to be complex. Hip fracture incidence increases with weight loss irrespective of body weight or intention to lose weight[41] but this may be offset by lower costs of hip fracture should they occur.
There has been recent strong interest in identifying those with clusters of comorbid conditions with very high health care costs,[42] who then may be appropriate candidates for care management programs to improve the efficiency of their health care.[43] Our results support such efforts, but also show that measured characteristics such as walk speed and body mass index can be combined with comorbidity to identify high risk older adults. Moreover, pharmacologic fracture prevention therapies may be particularly cost-effective for those individuals with these characteristics who also have osteoporosis.
An important limitation of our study is that pre-fracture characteristics were assessed a median 1.6 years before the occurrence of the hip fracture, and we had to impute predictor variable values for a minority of these hip fracture patients because they did not attend the most recent SOF visit preceding the date of their hip fracture. Thus, our estimated associations of pre-fracture characteristics may be biased toward the null hypothesis of no association, and we may have missed some associations of predictors that may change over time, including factors such as depressive symptoms, cognitive impairment, and IADL impairment. However, our findings of significant associations of walk speed, obesity, and comorbidity with total health care costs after hip fracture are robust to this limitation. In addition, The SOF study did not include men, and other cohort studies would be required to investigate whether or not our findings are also true for older men. Our results are not applicable to the small minority of hip fracture patients who do not have surgical fixation of their fractures. We did not have a contemporaneous non-fracture control group, but that is mitigated since we adjusted for pre-fracture total health care costs; using pre-fracture health care costs as a control yields similar estimates of hip fracture attributable costs as does using a contemporaneous control group.[44] Other limitations of our study are that we did not include costs of long-term nursing home care in our calculations of total health care costs, the dearth of minority beneficiaries, the lack of claims data on participants with hip fracture that were enrolled in Medicare Advantage plans, and data from only four geographic sites in the United States.
In conclusion, slower walk speed, obesity, and a greater comorbidity burden are associated with higher total health care costs after hip fracture, after adjustment for each other and for pre-fracture health care costs, hospital characteristics, and local supply of health care providers. Improvement of mobility among older women at high risk of hip fracture may reduce costs of care, should a hip fracture occur. Further studies of the effect of interventions to improve physical performance capability among elderly persons at high risk of hip fractures are warranted.
Acknowledgments
Research Funding for one or more authors: National Institute of Aging, National Institutes of Health
Funding Source: This study was done primarily under funding from the National Institute for Aging / National Institutes of Health, primarily grant number R01 AG038415-01. The Study of Osteoporotic Fractures is also supported by the National Institute for Aging under the following grant numbers; R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
We are grateful for the assistance of David Van Riper, the Director Spatial Analysis at the Minnesota Population Center and M. Taylor Long of the Spatial Analysis Unit, who calculated the distances from study participants’ residences to skill nursing facilities.
This manuscript is also the result of work supported in part with resources and use of facilities at the Minneapolis VA Health Care System. The views expressed herein do not necessarily represent the views of the Department of Veterans Affairs or the United States Government
Footnotes
Role of Sponsor: The sponsor had no role in the study concept and design, data collection or analysis, or drafting or reviewing the manuscript.
Disclosures:
Dr. Ensrud serves as a consultant on a Data Monitoring Committee for Merck Sharpe & Dohme. Authors John T. Schousboe, Misti L. Paudel, Brent C. Taylor, Allyson M Kats, Beth A. Virnig, Bryan E. Dowd, and Lisa Langsetmo all have nothing to disclose.
This study was done primarily under funding from the National Institute for Aging / National Institutes of Health, primarily grant number R01 AG038415-01. The Study of Osteoporotic Fractures is also supported by the National Institute for Aging under the following grant numbers; R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buntin MB. Access to postacute rehabilitation. Arch Phys Med Rehabil. 2007;88:1488–1493. doi: 10.1016/j.apmr.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Kane RL, Finch MD. The cost effectiveness of post-acute care for elderly Medicare beneficiaries. Inquiry. 2000;37:359–375. [PubMed] [Google Scholar]
- 5.Crotty M, Whitehead C, Miller M, Gray S. Patient and caregiver outcomes 12 months after home-based therapy for hip fracture: a randomized controlled trial. Arch Phys Med Rehabil. 2003;84:1237–1239. doi: 10.1016/s0003-9993(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 6.Gindin J, Walter-Ginzburg A, Geitzen M, Epstein S, Levi S, Landi F, Bernabei R. Predictors of rehabilitation outcomes: A comparison of Israeli and Italian geriatric post-acute care (PAC) facilities using the minimum data set (MDS) Journal of the American Medical Directors Association. 2007;8:233–242. doi: 10.1016/j.jamda.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. Jama. 2004;292:837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 8.Cameron I, Crotty M, Currie C, et al. Geriatric rehabilitation following fractures in older people: a systematic review. Health technology assessment. 2000;4:i–iv. 1–111. [PubMed] [Google Scholar]
- 9.Kane RL, Finch M, Blewett L, Chen Q, Burns R, Moskowitz M. Use of post-hospital care by Medicare patients. J Am Geriatr Soc. 1996;44:242–250. doi: 10.1111/j.1532-5415.1996.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004;52:1479–1486. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. Jama. 2014;311:2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 14.Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. Journal of the American Geriatrics Society. 1999;47:1257–1260. doi: 10.1111/j.1532-5415.1999.tb05209.x. [DOI] [PubMed] [Google Scholar]
- 15.Unutzer J, Patrick DL, Simon G, Grembowski D, Walker E, Rutter C, Katon W. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. Jama. 1990;263:665–668. [PubMed] [Google Scholar]
- 17.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. Jama. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 18.Schousboe JT, Paudel ML, Taylor BC, Virnig BA, Cauley JA, Curtis JR, Ensrud KE. Magnitude and consequences of misclassification of incident hip fractures in large cohort studies: the Study of Osteoporotic Fractures and Medicare claims data. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24:801–810. doi: 10.1007/s00198-012-2210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schousboe JT, Paudel ML, Taylor BC, Mau LW, Virnig BA, Ensrud KE, Dowd BE. Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims. Health services research. 2014;49:929–949. doi: 10.1111/1475-6773.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilgore ML, Morrisey MA, Becker DJ, et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999–2005. J Bone Miner Res. 2009;24:2050–2055. doi: 10.1359/jbmr.090523. [DOI] [PubMed] [Google Scholar]
- 21.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. American heart journal. 2003;145:452–458. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 22.Schousboe JT, Paudel ML, Taylor BC, Kats AM, Virnig BA, Ensrud KE, Dowd BE. Estimating True Resource Costs of Outpatient Care for Medicare Beneficiaries: Standardized Costs versus Medicare Payments and Charges. Health services research. 2016;51:205–219. doi: 10.1111/1475-6773.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 24.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 25.Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. Journal of hand therapy : official journal of the American Society of Hand Therapists. 1993;6:259–262. doi: 10.1016/s0894-1130(12)80326-7. [DOI] [PubMed] [Google Scholar]
- 26.Jette AM, Jette DU, Ng J, Plotkin DJ, Bach MA. Are performance-based measures sufficiently reliable for use in multicenter trials? Musculoskeletal Impairment (MSI) Study Group. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54:M3–6. doi: 10.1093/gerona/54.1.m3. [DOI] [PubMed] [Google Scholar]
- 27.Lyons JS, Strain JJ, Hammer JS, Ackerman AD, Fulop G. Reliability, validity, and temporal stability of the geriatric depression scale in hospitalized elderly. International journal of psychiatry in medicine. 1989;19:203–209. doi: 10.2190/nlg4-mc90-78e6-xv80. [DOI] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Yun H, Kilgore M, Curtis J, et al. Identifying Types of Nursing Facility Stays using Medicare Claims Data: an Algorithm and Validation. Health Serv Outcomes Res Method. 2010;10:100–110. [Google Scholar]
- 31.Buntin MB, Garten AD, Paddock S, Saliba D, Totten M, Escarce JJ. How much is postacute care use affected by its availability? Health Services Research. 2005;40:413–434. doi: 10.1111/j.1475-6773.2005.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wennberg JE, Freeman JL, Culp WJ. Are hospital services rationed in New Haven or over-utilised in Boston? Lancet. 1987;1:1185–1189. doi: 10.1016/s0140-6736(87)92152-0. [DOI] [PubMed] [Google Scholar]
- 33.Friedberg MW, Hussey PS, Schneider EC. Primary care: a critical review of the evidence on quality and costs of health care. Health Affairs. 2010;29:766–772. doi: 10.1377/hlthaff.2010.0025. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32:468–473. doi: 10.1016/s8756-3282(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 35.Riley GF, Lubitz JD. Long–term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. Journal of General Internal Medicine. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson D, Seib C, Rasmussen L. Can physical activity prevent physical and cognitive decline in postmenopausal women? Maturitas. 2014;79:14–33. doi: 10.1016/j.maturitas.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. Jama. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. The American journal of medicine. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 41.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR Study of Osteoporotic Fractures Research G. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. Journal of the American Geriatrics Society. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 42.Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Medical care. 2014;52(Suppl 3):S31–36. doi: 10.1097/MLR.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuben DB. Physicians in supporting roles in chronic disease care: the CareMore model. Journal of the American Geriatrics Society. 2011;59:158–160. doi: 10.1111/j.1532-5415.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 44.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ., 3rd Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–330. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]


