Abstract

The synthesis of complex oligosaccharides is often hindered by a lack of knowledge on the reactivity and selectivity of their constituent building blocks. We investigated the reactivity and selectivity of 2-azidofucosyl (FucN3) donors, valuable synthons in the synthesis of 2-acetamido-2-deoxyfucose (FucNAc) containing oligosaccharides. Six FucN3 donors, bearing benzyl, benzoyl, or tert-butyldimethylsilyl protecting groups at the C3-O and C4-O positions, were synthesized, and their reactivity was assessed in a series of glycosylations using acceptors of varying nucleophilicity and size. It was found that more reactive nucleophiles and electron-withdrawing benzoyl groups on the donor favor the formation of β-glycosides, while poorly reactive nucleophiles and electron-donating protecting groups on the donor favor α-glycosidic bond formation. Low-temperature NMR activation studies of Bn- and Bz-protected donors revealed the formation of covalent FucN3 triflates and oxosulfonium triflates. From these results, a mechanistic explanation is offered in which more reactive acceptors preferentially react via an SN2-like pathway, while less reactive acceptors react via an SN1-like pathway. The knowledge obtained in this reactivity study was then applied in the construction of α-FucN3 linkages relevant to bacterial saccharides. Finally, a modular synthesis of the Staphylococcus aureus type 5 capsular polysaccharide repeating unit, a trisaccharide consisting of two FucNAc units, is described.
Introduction
The rare sugar 2-acetamido-2-deoxyfucose (FucNAc) is a constituent monosaccharide of several bacterial capsular polysaccharides (CPS).1,2 Both d- and l-enantiomers are found in Nature, and they can be linked through either α- or β-glycosidic linkages (see Chart 1). For example, the repeating trisaccharide of the type 5 CPS of Staphylococcus aureus features both a β-d-FucNAc residue and an α-l-FucNAc moiety, while the type 8 CPS of S. aureus has d- and l-FucNAc constituents that are both 1,2-cis-linked.3 The S. aureus strain M is built up from trisaccharide repeats,4,5 which are composed of two galactosaminuronic acid (GalNAcA) residues and an α-d-FucNAc monosaccharide. Various O-antigens of Escherichia coli contain FucNAc residues as exemplified by the structures in Chart 1.6,7
Chart 1. Structures of the Repeating Units of FucNAc-Containing Polysaccharides.
Well-defined fragments of bacterial polysaccharides have been used extensively in the development of (semi)-synthetic vaccines, as part of diagnostic tools, to unravel binding and interactions with carbohydrate binding receptors and as probes for bacterial CPS-biomachinery enzymes.8 Organic synthesis can deliver these fragments as well-defined single molecules, devoid of any bacterial impurity and functionalized at predetermined sites with, for example, a conjugation handle for further manipulation.9,10 The synthesis of complex oligosaccharides, such as those depicted in Chart 1, however, can be an arduous task, requiring a significant time and labor investment. This is largely due to the complexity associated with the stereoselective construction of glycosidic linkages.11−14 Few studies have been directed at the incorporation of fucosamine residues in oligosaccharides, and there is no general method to install the challenging α-fucosamine linkage. There have been reports on the assembly of the trisaccharide repeating units of S. aureus type 5 and 8,15−18 but the syntheses reported were developed to target a single trisaccharide providing little insight into the reactivity and selectivity of fucosamine building blocks in a broader context, thus making it difficult to transpose the outcome of these studies to other relevant oligosaccharide targets or synthetic approaches.
To facilitate the effective assembly of fucosamine-containing bacterial oligosaccharides, we here report an in-depth study of the reactivity and selectivity of a variety of fucosazide building blocks with the goal to understand and control the stereoselectivity of these donors. We investigated reactive intermediates formed upon activation of fucosazide donor synthons and we have formulated a mechanistic rationale to account for the stereoselectivity observed in fucosaminylation reactions. We applied the generated insight in the construction of several relevant 1,2-cis-fucosamine linkages as well as a modular synthesis of the S. aureus type 5 trisaccharide.
Results and Discussion
To achieve the stereoselective introduction of 1,2-cis glycosamine linkages, the C2 amino functionality of a donor glycoside is generally masked as the nonparticipating azide.19 To generate a series of fucosazide (FucN3) donors, we decided to target phenylseleno fucosazides because selenoglycosides20,21 are generally very potent glycosyl donors and phenylseleno fucosazides can be effectively generated from readily available fucal precursors.22 To map the reactivity and selectivity of fucosazide donors, we investigated a set of donors having different protecting groups. Whereas the glycosylating properties of fucosazide donors have received relatively little attention, there is a large body of data available on the stereoselective introduction of fucosyl linkages.23−34
It appears that the α-fucosyl linkage can be installed with relative ease. For the stereoselective construction of this linkage, fucosyl building blocks, bearing acyl protecting groups at C3 and/or C4, are commonly used, and it is often assumed that these groups are capable of “remote participation”.23 Of note, tri-O-benzyl-protected fucosyl donors have also been employed, and these have also been reported to provide the desired 1,2-cis fucosyl linkages with good selectivity.25,26 No mechanistic rationale has been forwarded to account for this striking selectivity.
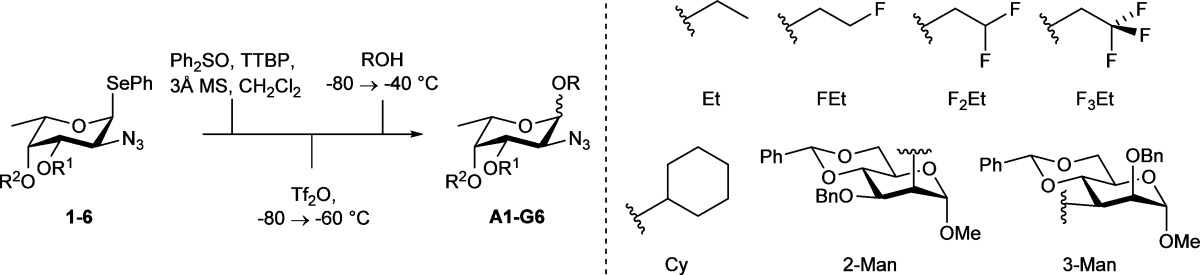
For our study, we generated six l-FucN3 donors (1–6, Chart 2) from l-fucal, featuring benzyl, benzoyl, or tert-butyldimethylsilyl groups. We probed these donor fucosides in a series of glycosylation reactions using a preactivation protocol in which the donor glycosides were activated with the diphenyl sulfoxide (Ph2SO)–triflic anhydride (Tf2O) reagent couple.35−37 This reagent combination provides a very powerful electrophile for activation of thio- and selenoglycosides, and it allows for the detection of reactive intermediates by low-temperature NMR spectroscopy to provide insight into the glycosylation mechanism of the preactivated donor glycosides.38
Chart 2. Structures of l-FucN3 Donors 1–6 and Model Acceptors.
The synthesis of l-FucN3 donors 1–6 is depicted in Scheme 1. Homogeneous azidoselenylation22 of easily accessible l-fucal17 installed the azide and the anomeric phenylseleno moiety in one step in the desired α-fucosyl configuration, accompanied by minor amounts of inseparable isomers. Deacetylation of the crude product mixture allowed separation, yielding diol 9 in 58% yield over two steps. Donors 1, 2, and 5 could be accessed in one step each from diol 9 by benzylation (BnBr, NaH in DMF, 85% yield), benzoylation (BzCl and a catalytic amount of DMAP in a mixture of CH2Cl2 and pyridine, 90% yield), and silylation (TBSOTf and a catalytic amount of DMAP in pyridine at elevated temperature, 85% yield), respectively. In the last case, standard silylation conditions employing TBSCl as the silylating agent and either imidazole in DMF, or DMAP and pyridine, failed to give the disilylated product. Donor 3, bearing C3-O-benzyl and C4-O-benzoyl protection, was procured by Bu2SnO-mediated, regioselective benzylation on the C3-O position followed by benzoylation of the remaining C4-O position using similar conditions as described for 2 to give 3 in 47% yield over two steps. A more elaborate protection sequence was required to access C4-O-benzyl donors 4 and 6, owing to the less reactive nature of the C4 position. Thus, regioselective, Bu2SnO-mediated p-methoxybenzylation of the C3-O position, benzylation of the remaining free C4 alcohol, followed by acid-mediated cleavage of the C3-O-PMB ether, using HCl in a mixture of CH2Cl2 and hexafluoroisopropanol (HFIP),39 gave key intermediate 10 in 47% yield over three steps. The use of oxidative conditions to remove the PMB group was avoided, owing to the potentially oxidation-sensitive phenylseleno moiety. With 10 in hand, donors 4 and 6 were obtained after benzoylation and silylation using conditions described above, in 96% and 92% yield, respectively.
Scheme 1. Synthesis of l-FucN3 Donors 1–6.
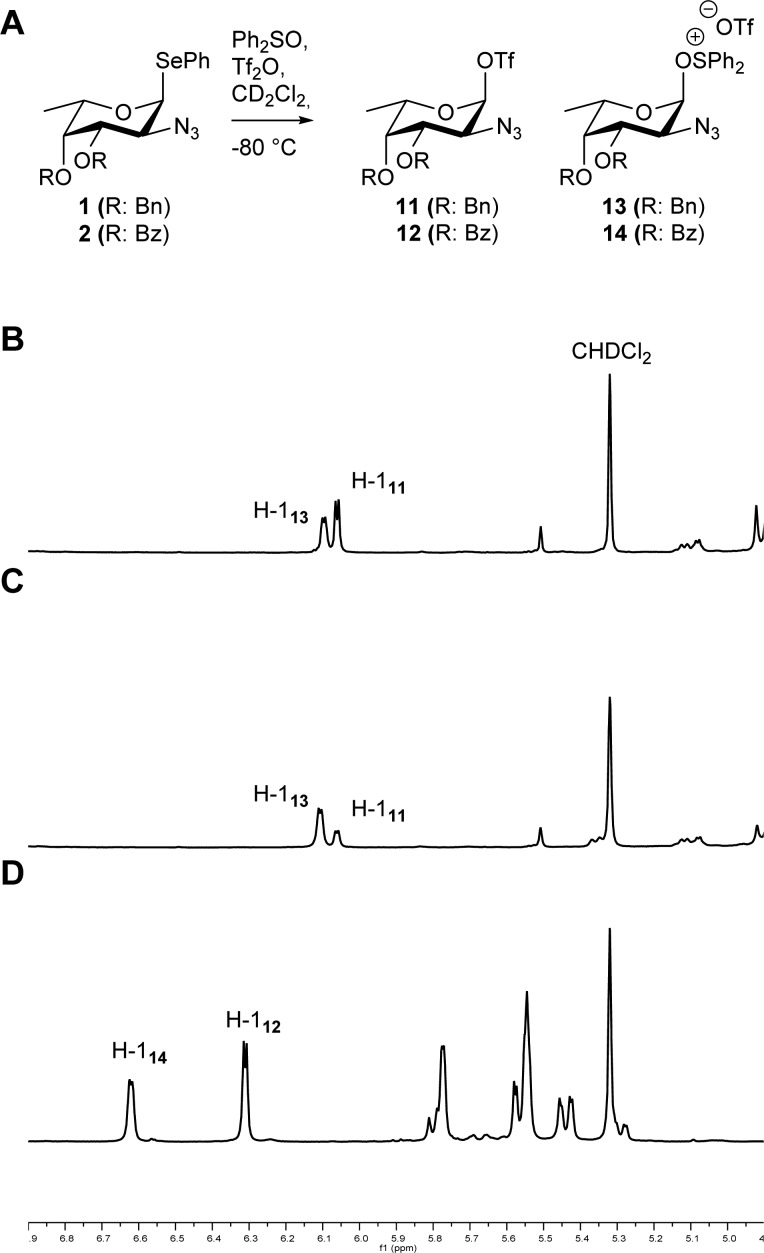
We started our investigation with the detection of the reactive intermediates, generated upon activation of two different donor synthons: di-O-benzyl- and di-O-benzoyl fucosazides 1 and 2, respectively. Thus, a mixture of 1 and Ph2SO (1.3 equiv) in CD2Cl2 was treated with Tf2O (1.3 equiv) at −80 °C (Figure 1A). After a 1H NMR spectrum (Figure 1B) was recorded, two new anomeric signals appeared (δ 6.06 and 6.10 ppm), which were assigned as α-triflate 11 (J = 3.2 Hz) and α-oxosulfonium triflate 13 (J = 3.2 Hz), respectively, based on their chemical shift.38 While the formation of the anomeric triflate was anticipated, oxosulfonium triflate formation under these conditions is quite surprising. The oxosulfonium species likely arises from reaction of the anomeric triflate with Ph2SO present in the reaction mixture.40,41 Because the amount of oxosulfonium fucosazide 13 is higher than what could be expected based on the excess of Ph2SO (0.3 equiv), it appears that the selenodonor 1 does not require a full equivalent of Ph2SO for complete activation. To account for complete activation of donor 1, we assume that the electrophile, generated upon reaction of the anomeric phenylselenol group with the diphenylsulfonium bis-triflate activator (PhSe–SPh2OTf), is reactive enough to activate the nucleophilic phenylselenium moiety. Addition of more Ph2SO to the reaction mixture resulted in an increase of the signal at δ 6.10 ppm (Figure 1C), reinforcing the presence of oxosulfonium triflate 11. In order to assess the stability of the two reactive intermediates, the NMR probe was gradually warmed with increments of 10 °C. Both triflate 11 and oxosulfonium triflate 13 started to decompose at −20 °C. The activation of dibenzoyl donor 2 proceeded in a similar manner to provide α-triflate 12 and oxosulfonium triflate 14 (Figure 1D). These reactive intermediates proved to be more stable than their dibenzyl counterparts, with decomposition setting in around 0 °C.
Figure 1.
Generation of reactive species from donors 1 and 2 (A). Partial 1H NMR spectra (400 MHz, 193 K) of reactive species from 1 using 1.3 and 2.0 equiv of Ph2SO (B and C, respectively) and 2 (1.3 equiv of Ph2SO, D).
Next, we investigated the behavior of donor fucosazides 1–6 in a series of glycosylation reactions. To this end, we applied a unified glycosylation protocol to all condensation reactions, involving preactivation of the donor glycoside at low temperature (in the presence of the non-nucleophilic base 2,4,6-tri-tert-butylpyrimidine (TTBP)42), then acceptor addition, subsequently warming the reaction mixture slowly to −40 °C, and finally quenching the reaction at this temperature. We used the set of model acceptors depicted in Chart 2 to map the selectivity of the fucosazide donors 1–6. To study the dependency of acceptor nucleophilicity on the outcome of the glycosylation reactions a set of partially fluorinated ethanols was used.43 In addition, three secondary alcohol acceptors were used: cyclohexanol, mannoside 7, having an axial C2-OH, and mannoside 8, with an equatorial C3-OH.44,45
Glycosylation of donors 1–6 with the series of ethanols (Table 1, rows A–D) revealed a clear dependency of the stereochemical outcome of the glycosylations on the nucleophilicity of the acceptor alcohols. All donors showed the same trend: with decreasing nucleophilicity (increasing amount of fluorine atoms in the acceptors) α-selectivity increased. While the more reactive donors (1, 5, and 6) reacted in a nonselective manner with the most nucleophilic acceptor, ethanol (row A), the less reactive, benzoyl-bearing fucosazide donors reacted with moderate β-selectivity. With the reactive secondary alcohol, cyclohexanol (row E), a similar picture emerged: less reactive donors provided more β-product than the reactive fucosaminylating agents. The condensations of the secondary carbohydrate acceptors 7 and 8 (rows F and G) all proceeded with good to excellent α-selectivity, again with the more reactive donors providing better α-selectivity than their less reactive counterparts. Across the board, donors 1, 5, and 6 outperformed the benzoylated donors 2–4 in terms of yield of the glycosylation reactions.
Table 1. Glycosylations of l-FucN3 Donors 1–6 with Model Acceptors.
| A, Et (%) | B, FEt (%) | C, F2Et (%) | D, F3Et (%) | E, Cy (%) | F, 2-Man (%) | G, 3-Man (%) | |
|---|---|---|---|---|---|---|---|
| 1 (R1, R2: Bn) | 88 (1:1) | 72 (1:1) | 81 (2:1) | 80 (19:1) | 75 (2:1) | 68 (19:1) | 72 (19:1) |
| 2 (R1, R2: Bz) | 59 (1:3) | 34 (1:2) | 74 (3:2) | 50 (10:1) | 38 (1:9) | 38 (4:1) | 64 (19:1) |
| 3 (R1: Bn; R2: Bz) | 61 (1:3) | 56 (1:1) | 76 (3:1) | 77 (7:1) | 75 (1:4) | 58 (10:1) | 54 (19:1) |
| 4 (R1: Bz; R2: Bn) | 58 (1:3) | 60 (2:3) | 80 (1:1) | 45 (19:1) | 71 (1:4) | 68 (4:1) | 64 (10:1) |
| 5 (R1, R2: TBS) | 63 (2:5) | 81 (2:3) | 75 (5:2) | 84 (19:1) | 84 (1:3) | 67 (19:1) | 73 (19:1) |
| 6 (R1: TBS; R2: Bn) | 81 (1:1) | 80 (1:1) | 87 (2:1) | 90 (19:1) | 80 (1:2) | 74 (19:1) | 64 (9:1) |
Isolated yields reported; α/β ratios in parentheses.
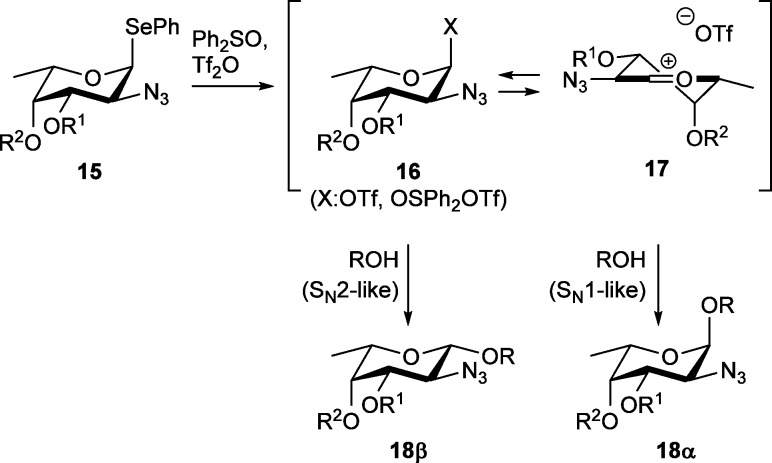
The observed β-selectivity in the condensation reactions of the benzoylated fucosazide donors with ethanol and cyclohexanol strongly argue against a remote participation scenario for these donors.23 The selectivity in these reactions is better explained with the α-anomeric triflates or oxosulfonium triflates 16 as glycosylating species (Scheme 2). The presence of benzoyl groups on the fucosazide donors stabilizes these intermediates, as judged from the higher decomposition temperature found in the variable temperature NMR measurements. Strong nucleophiles can substitute the covalent α-triflates/oxosulfonium triflates with inversion of configuration to provide the β-linked products (18β). Weaker nucleophiles, such as di- and trifluoroethanol and the carbohydrate alcohols (also featuring two or three electron withdrawing atoms at a β-position with respect to the alcohol function), are unable to directly displace a covalently bound leaving group and require a more electrophilic glycosylating agent to react. The covalent triflate/oxosufonium species can serve as a reservoir for a more reactive oxocarbenium ion 17 with a loosely associated triflate counterion.46,47 It is now well established that the geometry of an oxocarbenium ion can be decisive for the stereochemical course of a glycosylation reaction.48−53 The fucosazide oxocarbenium ions that can form from the covalent triflates/oxosulfonium triflates can adopt a 3H4-like conformation (as in 17) in which the substituents at C2 and C4 are positioned properly to allow for stabilization of the electron depleted anomeric center, while the groups at C3 and C5 are positioned in sterically favorable pseudo-equatorial positions.54 This oxocarbenium ion is preferentially attacked on the diastereotopic face that leads to the product via an energetically favorable chairlike transition state, leading to the 1,2-cis product 18α. This reaction trajectory is sterically relatively unhindered, and it can account for the selective formation of the 1,2-cis-products as observed here. The fact that more electron-rich donors provide higher α-selectivity strongly supports this rationale.55 It also provides an adequate explanation for glycosylations of highly reactive per-benzylated fucosyl donors previously reported in literature.25,26
Scheme 2. Mechanistic Explanation for Observed Stereoselectivities.
The reactivity study described above has revealed a clear dependence of the stereochemical course of the glycosylations on both the reactivity of the donor glycoside and the reactivity of the acceptor alcohol. The best 1,2-cis selectivity is obtained with reactive fucosazide donors, bearing benzyl or silyl ether protecting groups and relatively weak nucleophiles, such as secondary carbohydrate alcohols.
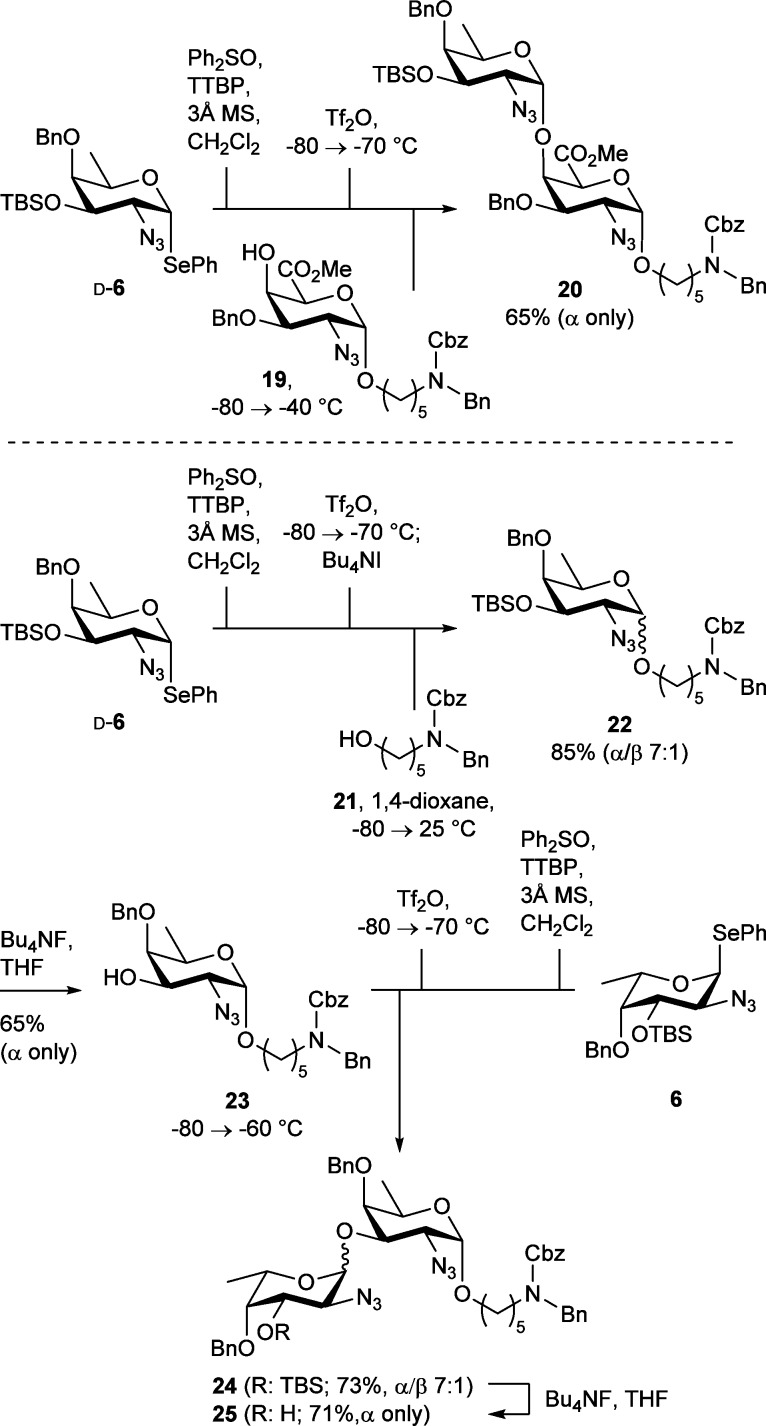
Building on this knowledge, we set out to investigate the construction of a series of relevant glycosidic linkages, present in capsular polysaccharides of S. aureus. The repeating unit of the S. aureus strain M CPS (Figure 1) contains an α-d-FucNAc unit linked to two α-linked N-acetylgalactosaminuronic acid (GalNAcA) residues.4,5 We anticipated that the glycosylation between a galacturonic acid C4-OH acceptor, generally considered to be a weak nucleophile, and a reactive fucosamine donor, would likely lead to a highly α-selective glycosylation. Indeed, when d-fucosazide donor d-6 was coupled to acceptor 19, the α-linked disaccharide 20 was obtained as the sole product in 65% yield (Scheme 3).
Scheme 3. Synthesis of Protected S. aureus Strain M and Type 8 CPS Disaccharides 20 and 25.
The repeating unit of S. aureus type 8 CPS contains two α-linked N-acetyl fucosamine units (Figure 1).3 To investigate the construction of the α-linkage between the two fucosamine residues, we first generated an α-d-fucosazide acceptor bearing a spacer at its reducing end. Because the reactivity study described above indicated that nucleophilic primary alcohols react in a non- or β-selective manner with fucosazide donors we turned our attention to the use of tetrabutylammonium iodide (Bu4NI) as a stereochemistry-directing additive56 in the condensation of aminopentanol 21(57) and fucosazide donor d-6. Bennett and co-workers have previously reported a Ph2SO/Tf2O-based activation protocol (in the presence of the electrophilic scavenger N-methylmaleimide (NMM)), utilizing an excess of Bu4NI to generate an intermediate anomeric iodide as a reactive species.58 As first conceived by Lemieux and co-workers,59 an equilibrium is established between the α- and β-iodides, with the latter species being less stable but much more reactive. Nucleophiles can displace the β-iodide in an SN2-like fashion, leading to the selective formation of the α-product. When d-6 was glycosylated with 21 using a slight modification of Bennett’s protocol, product 22 was obtained in 85% yield with good α-selectivity. Removal of the TBS group facilitated separation of the anomers, giving pure 23 in 65% yield. In the next glycosylation event, the reactive l-fucosazide donor 6 was paired with d-fucosazide acceptor 23 in a Ph2SO/Tf2O-mediated preactivation glycosylation event to provide disaccharide 24 in 73% yield and high stereoselectivity. Removal of the TBS group under the agency of Bu4NF allowed chromatographic separation of the two anomers, yielding the α-linked disaccharide 25 in 71% yield.
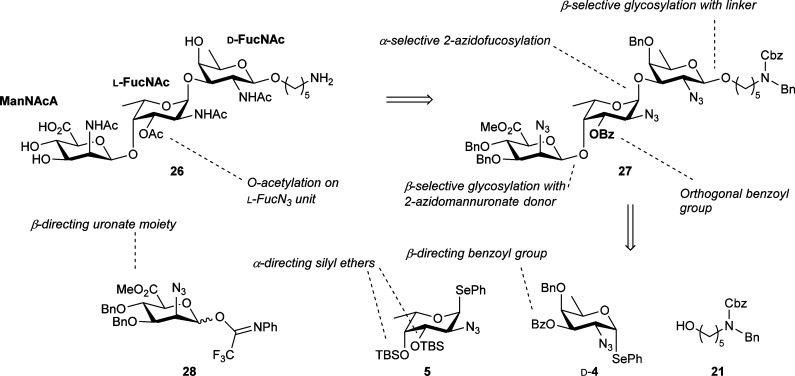
As a final endeavor, we set out to synthesize the repeating unit of the S. aureus type 5 CPS repeating unit (Scheme 4). Target trisaccharide 26 consists of a rare N-acetylmannosaminuronic acid (ManNAcA), a central α-linked l-FucNAc residue, and a terminal β-d-FucNAc connected to an aminopentanol spacer for future conjugation purposes. The central l-FucNAc contains a 3-O acetate group. The synthesis of this repeating unit has previously been reported by the groups of Adamo,16 Boons,17 and very recently, Demchenko.15 Adamo and co-workers relied on a strategy starting from the nonreducing end and using glucosyl and rhamnosyl synthons to form the ManNAcA and FucNAc units, respectively. The final glycosylation between the l-FucN3-containing disaccharide and a d-FucNAc unit proceeded with modest stereoselectivity. Demchenko and co-workers used a similar approach with glucosyl and fucosyl synthons. Boons and co-workers built the trisaccharide repeating unit, starting from the reducing end, using FucN3 building blocks. The installation of the glycosidic linkage between the two FucN3 units proved problematic, proceeding in low yield or with relatively poor stereoselectivity. The ManNAcA unit was installed using a nonoxidized 2-azidomannosyl (ManN3) donor.60
Scheme 4. Retrosynthetic Analysis for the S. aureus Type 5 CPS Trisaccharide 26.
Our strategy is presented in Scheme 4. In order to differentiate the C3′-O position from the other alcohols in the trisaccharide, this position was protected as an ester in fully protected intermediate 27, while the others were masked as benzyl ethers. Based on the reactivity/selectivity study described above, we reasoned that the β-fucosamine linkage could be constructed using a disarmed fucosazide donor, such as d-4. For the pivotal α-glycosidic linkage between the l-FucN3 and d-FucN3 moieties, the use of reactive 3,4-di-O-TBS donor 5 was anticipated because of the highly α-selective glycosylations of this donor (Table 1). We thus aimed to use a FucN3 donor for the installation of both the 1,2-cis and 1,2-trans fucosamine linkages. This will shorten the sequence of protecting group manipulations at the trisaccharide stage. For the introduction of the mannosaminuronic unit, we selected 2-azidomannuronate donor 28 because of the excellent β-selectivity observed with this class of donors as we have disclosed previously.61,62 The use of a “pre-oxidized” mannosaminuronic acid synthon circumvents the necessity of a late-stage oxidation step in the assembly sequence.
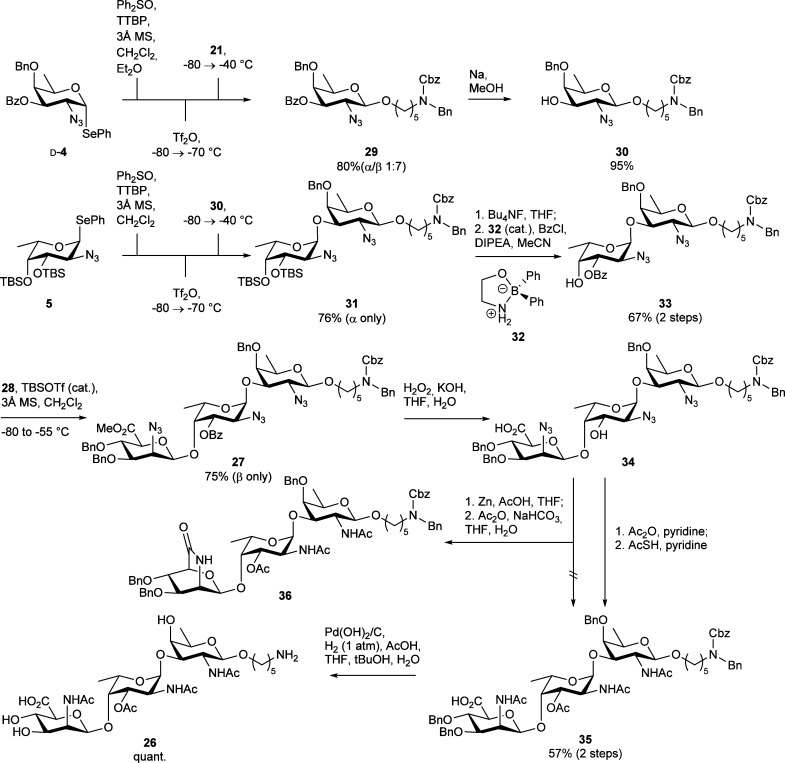
To effect the β-selective glycosylation between donor d-4 and spacer 21 in the absence of a participating group on the C2-position of the donor, several modifications of our standard glycosylation conditions were tested (data not shown). It was found that the use of ether as a cosolvent effectively increased the β-selectivity of the glycosylation. This is somewhat surprising given the fact that ether is commonly used to promote the formation of α-glycosidic linkages.56,63,64 It can, however, be rationalized with the mechanistic scheme depicted in Scheme 1. The low polarity of ether (in comparison to dichloromethane) stabilizes the covalent anomeric triflate/oxosulfonium triflate because it disfavors charge separation as in oxocarbenium ion pairs. If the incoming alcohol acceptor is nucleophilic enough, it can displace the covalent reactive species in an SN2-manner leading to the stereoselective formation of the β-FucN3 bond. The use of an 1:1 mixture of CH2Cl2 and Et2O in the glycosylation between aminopentanol 19 and disarmed FucN3 donor d-4 led to a spacer containing d-fucosamine building block 29 in 80% yield and 1:7 α/β-selectivity (Scheme 5). Removal of the benzoyl group using Zemplén conditions afforded the d-FucN3 acceptor 30 in 95% yield.
Scheme 5. Synthesis of Trisaccharide 26.
Next, the pivotal glycosylation between l-FucN3 donor 5 and d-FucN3 acceptor 30 was performed. Using the standard preactivation glycosylation protocol provided disaccharide 31 as a single anomer in 76% yield. Removal of both TBS ethers was followed by regioselective benzoylation of the C3-O′ position, using Taylor’s diphenylborinate catalyst 32,65 to give disaccharide acceptor 33 in 67% yield over two steps. The final glycosylation between mannosaminuronic acid donor 28 and dimer 33 proved challenging. Mannuronic acids are relatively reactive,66 and it was difficult to pair the reactive ManN3A donor with the weakly nucleophilic FucN3 alcohol. It was found that the use of an excess donor and almost an equimolar amount of Lewis acid promotor was most effective, allowing for the generation of trisaccharide 27 in 75% yield with complete stereoselectivity.67
With trisaccharide 27 in hand, its optimal deprotection sequence was investigated. First, the methyl mannuronate and the benzoyl ester on the central l-FucN3 unit were removed to protect the mannuronic acid moiety for potential lactamization upon exposure of the C2-amino group.62 Next the azides in 34 were reduced using Zn in AcOH and THF.68 It was found, however, that the subsequent O- and N-acetylation reaction resulted in a complex mixture of products, with lactam 36 as the major product. We therefore moved to an alternative reaction sequence, in which we first acetylated the free C3′–OH. Next both azides were transformed into the corresponding acetamido functionalities using thioacetic acid (AcSH).69 This step likely proceeds via a one-step process and circumvents formation of the free amine. Intermediate 35 was obtained in 57% yield over these two steps. The synthesis of the S. aureus type 5 trisaccharide 26 was finalized by hydrogenation of 35 using Pearlman’s catalyst (Pd(OH)2 on carbon) to remove all benzyl groups and the benzyloxycarbonyl carbamate.
Conclusion
In conclusion, we have mapped the reactivity and selectivity of a panel of phenylseleno fucosazide donors. Low-temperature NMR studies on activated donors revealed the formation of the covalent α-glycosyl triflates and oxosulfonium triflates, the stability of which depended on the protecting group pattern of the donor glycosides. Using a series of glycosylations involving a set of partially fluorinated ethanols, we were able to pinpoint how the stereoselectivity of the glycosylations of the different donors depends on the nucleophilicity of the acceptor alcohols. A mechanistic rationale was established that accounts for the stereoselectivity in glycosylations featuring fucosazide donors. Disarmed donors bearing acyl-protecting groups can selectively provide β-linked products when paired with reactive nucleophiles in an SN2-like glycosylation reaction. Armed donors, having benzyl or silyl ether groups, on the other hand, are well suited for the installation of the challenging 1,2-cis fucosamine linkages, and this is rationalized with a 3H4-oxocarbenium ion like reactive intermediate that is selectively attacked on its α-face. It is likely that reactions using reactive fucosyl donors proceed via similar pathways, providing a rationale for the high stereoselectivity obtained with these donors. It is anticipated that the use of the family of partially fluorinated ethanols to map reactivity–selectivity relationships for other donor types will provide valuable insight into glycosylation mechanism of these donors and significantly increase our insight how effective stereoselective glycosylation reactions can be achieved. The insight into the reactivity–selectivity of fucosazide donors generated here has paved the way for the construction of a variety of relevant glycosidic linkages and the modular assembly of the S. aureus type 5 repeating unit.
Experimental Section
All reactions were carried out in oven-dried glassware (85 °C). Prior to reactions, traces of water and solvent were removed by coevaporation with toluene where appropriate. Reactions sensitive to air or moisture were carried out under an atmosphere of argon (balloon). Solvents for reactions were of reagent grade and stored over 4 Å molecular sieves (3 Å for CH2Cl2, MeOH, and MeCN), except pyridine and DMF. NEt3 was stored over KOH pellets. Tf2O used in glycosylations was dried over P2O5 (∼3 h), followed by distillation, and stored in a Schlenk flask at −20 °C. All other chemicals were used as received. Reaction progress was monitored using aluminum-supported silica gel TLC plates (with fluorescent indicator); visualization was carried out by irradiation with UV light (λ: 254 nm), followed by spraying with 20% H2SO4 in EtOH (w/v) or Hanessian’s stain ((NH4)6Mo7O24·4H2O, 25 g/L; (NH4)4Ce(SO4)4·2H2O, 10 g/L; in 10% aq H2SO4). Column chromatography was carried out using silica gel (0.040–0.063 mm). Size-exclusion chromatography was carried out using Sephadex LH-20. NMR spectra were recorded on 400/100 MHz (for 1H and 13C, respectively) or 500/125 MHz spectrometers. Chemical shifts (δ) are reported in ppm relative to Me4Si (δ: 0.00 ppm) or residual solvent signals. NMR spectra were recorded at ambient temperature, and samples were prepared in CDCl3 unless noted otherwise. 13C-APT spectra are 1H decoupled. The structural assignment was achieved using HH–COSY and HSQC 2D experiments. Coupling constants of anomeric carbon atoms (JH1,C1) were determined using HMBC-GATED experiments. Infrared spectra were recorded with an FTIR instrument with wavenumbers (ν) reported in cm–1. LC–MS analyses were performed on an HPLC system equipped with a C-18 column (50 × 4.6 mm) connected to an ion-trap mass spectrometer with ESI+. Eluents used were MeCN and H2O with addition of TFA (0.1%). Runtimes were 13 min with a flow rate of 1 mL/min. HRMS spectra were recorded on a LTQ-Orbitrap instrument equipped with ESI+ (source voltage 3.5 kV, sheath gas flow 10, capillary temperature 275 °C) with resolution R 60.000 at m/z 400 (mass range: 150–4000) and dioctyl phthalate (m/z 391.28428) as a “lock mass”.
Phenyl 2-Azido-2-deoxy-1-seleno-α-l-fucopyranoside (9)
A solution of 3,4-di-O-acetyl-l-fucal17 (12.5 g, 58.4 mmol, 1.0 equiv) and (PhSe)2 (18.2 g, 58.4 mmol, 1.0 equiv) in CH2Cl2 (300 mL, 0.2 M) was degassed by sonication (30 min) before being cooled to −30 °C. PhI(OAc)2 (18.8 g, 58.4 mmol, 1.0 equiv) and TMSN3 (15 mL, 116.8 mmol, 2.0 equiv) were added. The mixture was stirred for 1 h at −30 °C and subsequently at −20 °C overnight. To the mixture was added cyclohexene (∼15 mL), and the mixture was allowed to warm to room temperature. The bright orange solution was concentrated in vacuo, and the brown residual oil was subjected to column chromatography (PE/EtOAc, 1:0 → 9:1 v/v) to separate the lipophilic impurities from the carbohydrate fraction. The latter was concentrated and suspended in MeOH (190 mL, 0.3 M), after which NaOMe (0.31 g, 5.8 mmol, 0.1 equiv) was added. The mixture was stirred overnight, after which TLC analysis (PE/EtOAc, 1:1 v/v) showed complete conversion of the starting material. The reaction mixture was neutralized by addition of ion-exchange resin (Amberlite IR-120, H+ form). The resin was filtered and the filtrate concentrated in vacuo. The solid thus obtained was crystallized from toluene to obtain the title compound as an amorphous solid (11.1 g, 33.8 mmol, 58%). 1H NMR (400 MHz, acetone-d6) δ: 7.62–7.57 (m, 2H, CHarom); 7.32–7.28 (m, 3H, CHarom); 5.96 (d, 1H, J = 5.2 Hz, H-1); 4.29 (q, 1H, J = 6.4 Hz, H-5); 4.40 (dd, 1H, J = 5.2 Hz, 10.4 Hz, H-2); 3.82–3.79 (m, 2H, H-3, H-4); 1.17 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz, acetone-d6); 135.4 (CHarom); 130.1 (Cq,arom); 129.8, 128.3 (CHarom); 86.7 (C-1); 72.4, 72.2 (C-3, C-4); 70.2 (C-5); 62.6 (C-2); 16.5 (C-6). IR (neat) ν: 3279, 2100, 1578, 1252, 1094, 1059. HRMS: [M – N2 + H]+ calcd for C12H16NO3Se 302.02899, found 302.02914. Mp: 138–140 °C.
Phenyl 2-Azido-3,4-di-O-benzyl-2-deoxy-1-seleno-α-l-fucopyranoside (1)
To a stirred solution of 9 (0.66 g, 2.0 mmol, 1.0 equiv) in DMF (8 mL, 0.25 M) were added BnBr (0.71 mL, 6.0 mmol, 3.0 equiv) and Bu4NI (0.15 g, 0.4 mmol, 0.2 equiv). The mixture was cooled in an ice bath, and NaH (60% w/w in oil, 0.32 g, 8.0 mmol, 4.0 equiv) was added. The mixture was stirred until TLC analysis (PE/EtOAc, 9:1 v/v) indicated complete consumption of the starting material (≤3 h). Excess NaH was quenched by slow addition of cold water until gas evolution ceased. The mixture was diluted with water and Et2O, and the aqueous phase was washed twice with Et2O. The combined ethereal phases were washed with brine (1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (PE/Et2O 1:0 → 9:1) to furnish the title compound as an oil which solidified on standing, in 85% yield (0.87 g, 1.7 mmol). 1H NMR (400 MHz) δ: 7.57–7.47 (m, 2H, CHarom); 7.45–7.22 (m, 13H, CHarom); 5.93 (d, 1H, J = 5.2 Hz, H-1); 4.92 (d, 1H, J = 11.2 Hz, PhCHH); 4.80–4.73 (m, 2H, PhCH2); 4.61 (d, 1H, J = 11.6 Hz, PhCHH); 4.35 (dd, 1H, J = 5.2 Hz, 9.8 Hz, H-2); 4.22 (q, 1H, J = 6.4 Hz, H-5); 3.75–3.72 (m, 2H, H-3, H-4); 1.13 (q, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz) δ: 138.1, 137.4 (Cq,arom); 134.3, 129.0, 128.6, 128.3, 128.1, 128.0, 127.8, 127.7, 127.6 (CHarom); 85.5 (C-1); 80.6, 75.7 (C-3, C-4); 75.0, 72.5 (PhCH2); 69.4 (C-5); 60.9 (C-2); 16.5 (C-6). IR (neat) ν: 2882, 2112, 1474, 1298, 1101, 1063, 1047. HRMS: [M – N2 + H]+ calcd for C26H28NO3Se 482.1229, found 482.1229.
Phenyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-1-seleno-α-l-fucopyranoside (2)
To a stirred solution of 9 (0.66 g, 2.0 mmol, 1.0 equiv) in CH2Cl2/pyridine (3:1 v/v, 8 mL, 0.2 M) was slowly added BzCl (0.7 mL, 6.0 mmol, 3.0 equiv), followed by DMAP (0.05 g, 0.4 mmol, 0.2 equiv). The mixture was stirred until TLC analysis (PE/EtOAc, 4:1 v/v) indicated complete conversion of the starting material (∼3 h). The reaction was quenched with MeOH, and the mixture was diluted with CH2Cl2, washed (1 M aq HCl, 2×; satd aq NaHCO3, 1×; brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was subjected to column chromatography (PE/EtOAc, 1:0 → 4:1) to furnish the title compound in 90% yield (0.96 g, 1.79 mmol). 1H NMR (400 MHz) δ: 7.25–8.15 (m, 15H, CHarom), 6.12 (d, 1H, J = 5.2 Hz, H-1), 5.76 (d, 1H, J = 2.8 Hz, H-4), 5.51 (dd, 1H, J = 3.2 Hz, 10.8 Hz, H-3), 4.53 (dd, 1H, J = 5.6, 10.8 Hz, H-2), 4.73 (q, 1H, J = 6.4 Hz, H-5), 1.19 (d, 3H, J = 6.4 Hz, H-6); 13C-APT NMR (100 MHz) δ: 165.7, 165.4 (COBz), 134.9–127.2 (CHarom), 84.6 (C-1), 72.4 (C-3), 70.8 (C-4), 68.0 (C-5), 59.6 (C-2), 16.0 (C-6). IR (thin film) ν: 3061, 2984, 2108, 1724, 1450, 1273, 1257, 1109, 1080, 1067, 1024. HRMS: [M – N2 + H]+ calcd for C26H24NO5Se 510.0814, found 510.0819.
Phenyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-1-seleno-α-l-fucopyranoside (3)
Compound 9 (0.66 g, 2.0 mmol, 1.0 equiv) was suspended in toluene (7 mL, 0.3 M). Bu2SnO (0.50 g, 2.0 mmol, 1.0 equiv) was added, and the mixture was heated to 140 °C for 3 h, during which time a clear reaction mixture was obtained. The mixture was concentrated in vacuo and coevaporated once with dry toluene. The mixture was dissolved in DMF (9 mL, 0.2 M), BnBr (0.26 mL, 2.2 mmol, 1.1 equiv), and CsF (0.33 g, 2.2 mmol, 1.1 equiv), and the mixture was stirred overnight, after which TLC analysis indicated conversion of the starting material (PE/EtOAc, 7:3 v/v). The reaction was diluted with H2O and extracted (Et2O, 3×), and the combined ethereal phases were washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was passed over a small column (PE/EtOAc, 1:0 → 4:1 v/v) to obtain the 3-O-benzylated intermediate (0.42 mmol, 1 mmol, 50%). 1H NMR (400 MHz) δ: 7.59–7.56 (m, 2H, CHarom); 7.42–7.24 (m, 8H, CHarom); 5.89 (d, 1H, J = 5.2 Hz, H-1); 4.76 (d, 1H, J = 11.2 Hz, PhCHH); 4.69 (d, 1H, J = 11.2 Hz, PhCHH); 4.30 (q, 1H, J = 6.8 Hz, H-5); 4.17 (dd, 1H, J = 5.2 Hz, 10.4 Hz, H-2); 3.88 (s, 1H, H-4); 3.70 (dd, 1H, J = 3.2 Hz, 10.4 Hz, H-3); 2.36 (s, 1H, 3-OH); 1.26 (d, 3H, J = 6.8 Hz, H-6). 13C-APT NMR (100 MHz, CDCl3) δ: 137.1 (Cq,arom), 134.5, 129.2, 128.9, 128.5 (CHarom), 128.2 (Cq,arom), 127.9 (CHarom), 85.3 (C-1), 79.3 (C-3), 72.3 (CH2 Bn), 68.7, 68.6 (C-4, C-5); 60.3 (C-2); 16.2 (C-6). The intermediate was dissolved in CH2Cl2/pyridine (4:1 v/v, 5 mL, 0.2 M), and BzCl (0.14 mL, 1.2 mmol, 1.2 equiv) and DMAP (12 mg, 0.1 mmol, 0.1 equiv) were added at 0 °C. After TLC analysis (PE/EtOAc, 9:1 v/v) indicated complete conversion of the starting material (∼1 h), the mixture was quenched by the addition of water. The mixture was diluted with CH2Cl2 washed (1 M aq HCl, 2×; satd aq NaHCO3 1×; H2O 1×; brine 1×), dried over MgSO4, filtrated, and concentrated under reduced pressure. Purification by column chromatography (PE/EtOAc, 17:3 v/v) afforded the title compound (0.49 g; 0.93 mmol; 47% over two steps). 1H NMR (400 MHz) δ: 8.09–8.04 (m, 4H, CHarom), 7.64–7.20 (m, 11H, CHarom), 6.00 (d, 1H, J = 5.2 Hz, H-1), 5.71 (d, 1H, J = 2.8 Hz, H-4), 4.85 (d, 1H, J = 10.8 Hz, PhCHH), 4.57 (d, 1H, J = 10.8 Hz, PhCHH), 4.52 (q, 1H, J = 6.4 Hz, H-5), 4.26 (dd, 1H, J = 5.2 Hz, J = 10.4 Hz, H-2), 3.90 (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H-3), 1.16 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz) δ: 166.0 (COBz), 136.9 (Cq,arom), 134.7–127.7 (CHarom), 85.1 (C-1), 77.5 (C-3), 71.6 (PhCH2), 69.4 (C-4), 68.1 (C-5), 60.5 (C-2), 16.2 (C-6). IR (thin film) ν: 3061, 2984, 2897, 2108, 1719, 1452, 1263, 1109, 1078, 1062, 1024. HRMS: [M – N2 + H]+ calcd for C26H26NO4Se 496.1022, found 496.1023.
Phenyl 2-Azido-3-O-benzyl-2-deoxy-1-seleno-α-l-fucopyranoside (10)
In a three-necked flask equipped with a Dean–Stark trap, a suspension of phenyl 2-azido-2-deoxy-1-seleno-α-l-fucopyranoside (4.27 g, 13 mmol, 1.0 equiv) and Bu2SnO (3.40 g, 13.7 mmol, 1.05 equiv) in toluene (65 mL, 0.2 M) was heated to 140 °C for 1 h. The resultant clear, brown solution was cooled to 60 °C, and Bu4NBr (4.42 g, 13.7 mmol, 1.05 equiv), CsF (2.08 g, 13.7 mmol, 1.05 equiv), and PMBCl (1.9 mL, 13.7 mmol, 1.05 equiv) were added. The mixture was heated to 120 °C for ∼2 h, after which TLC analysis (PE/EtOAc, 3:2 v/v) indicated complete conversion of the starting diol. The mixture was cooled to room temperature, KF (10% in H2O, w/v) was added, and the resulting misture was stirred vigorously for ∼15 min. The aqueous phase was extracted (EtOAc, 2×), and the combined organic fractions were washed (brine 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (PE/EtOAc, 1:0 → 4:1) furnished the 3-O-PMB-protected intermediate as a yellow oil in 81% yield (4.71 g, 10.5 mmol). 1H NMR (400 MHz) δ: 7.58–7.56 (m, 2H, CHarom); 7.34–7.24 (m, 5H, CHarom); 6.93–6.87 (m, 2H, CHarom); 5.87 (d, 1H, J = 5.2 Hz, H-1); 4.68 (d, 1H, J = 10.8 Hz, PhCHH); 4.62 (d, 1H, J = 10.8 Hz, PhCHH); 4.28 (q, 1H, J = 6.4 Hz, H-5); 4.14 (dd, 1H, J = 5.2 Hz, 10.2 Hz, H-2); 3.83 (d, 1H, J = 2.4 Hz, H-4); 3.81 (s, 3H, OCH3); 3.68 (dd, 1H, J = 3.2 Hz, 10.4 Hz, H-3); 1.25 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz) δ: 159.6 (Cq,arom); 134.4, 134.3,129.7, 129.0 (CHarom); 129.0, 128.6 (Cq,arom); 127.7, 114.0 (CHarom); 85.2 (C-1); 78.8 (C-3); 71.7 (PhCH2); 68.5, 68.4 (C-4, C-5); 60.0 (C-2); 55.2 (OCH3); 16.0 (C-6). IR (thin film) ν: 3441, 2897, 2106, 1612, 1512, 1246, 1088, 1063, 1031. HRMS: [M + H]+ calcd for C20H24N3O4Se 450.0927, found 450.0923. A solution of the intermediate building block (1.56 g, 3.48 mmol, 1.0 equiv) and BnBr (0.83 mL, 6.96 mmol, 2.0 equiv) in DMF (12 mL, 0.3 M) was cooled to 0 °C. NaH (60% dispersion in oil, 0.21 g, 5.22 mmol, 1.5 equiv) was added, and the mixture was allowed to reach room temperature. After ∼3 h, TLC analysis (PE/EtOAc, 9:1 v/v) indicated complete conversion of the starting material, and the reaction was quenched by slow addition of water. After gas evolution had ceased, the mixture was partitioned between water and Et2O. The aqueous phase was extracted (Et2O, 2×), and the combined ethereal phases were washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (PE/Et2O, 1:0 → 9:1) delivered the fully protected intermediate as a colorless oil (1.68 g, 3.12 mmol, 90%). 1H NMR (400 MHz) δ: 7.57–7.54 (m, 2H, CHarom); 7.36–7.23 (m, 10H, CHarom); 6.92 (d, 2H, J = 8.8 Hz, CHarom); 5.91 (d, 1H, J = 5.2 Hz, H-1); 4.94 (d, 1H, J = 11.6 Hz, PhCHH); 4.72–4.66 (m, 2H, PhCH2); 4.60 (d, 1H, J = 11.6 Hz, PhCHH); 4.32 (dd, 1H, J = 5.2 Hz, 10.2 Hz, H-2); 4.21 (q, 1H, J = 6.4 Hz, H-5); 3.82 (s, 3H, OCH3); 3.73–3.68 (m, 2H, H-3, H-4). 13C-APT NMR (100 MHz) δ: 159.5, 138.2 (Cq,arom); 134.3, 129.5, 129.0 (CHarom); 128.7 (Cq,arom); 128.3, 128.1, 127.7, 127.6, 114.0 (CHarom); 85.6 (C-1); 80.3, 75.8 (C-3, C-4); 74.9, 72.2 (PhCH2); 69.4 (C-5); 60.8 (C-2), 55.3 (OCH3); 16.5 (C-6). IR (thin film) ν: 2868, 2104, 1612, 1512, 1246, 1099, 1063, 1034. HRMS: [M + H]+ calcd for C27H30N3O4Se 540.1396, found 540.1394. To a stirred solution of the fully protected 2-azidofucoside (1.56 g, 2.9 mmol, 1.0 equiv) and Et3SiH (0.73 mL, 8.7 mmol, 3.0 equiv) in CH2Cl2 (15 mL, 0.2 M) was added a solution of HCl (0.25 mL of an 37% solution, w/v in water) in HFIP (15 mL).39 After 1 min, the mixture was poured into a solution of NaHCO3 (satd aq). After separation of the layers, the aqueous phase was extracted (CH2Cl2, 1×), and the combined organic phases were washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. After column chromatography (toluene/EtOAc, 1:0 → 9:1), the C3-OH intermediate was obtained as an oil in 64% yield (0.78 g, 1.9 mmol). 1H NMR (400 MHz) δ: 7.58–7.56 (m, 2H, CHarom); 7.38–7.25 (m, 8H, CHarom); 5.91 (d, 1H, J = 5.2 Hz, H-1); 4.81 (d, 1H, J = 11.6 Hz, PhCHH); 4.72 (d, 1H, J = 11.6 Hz, PhCHH); 4.33 (q, 1H, J = 6.4 Hz, H-5); 4.02 (dd, 1H, J = 5.2 Hz, 10.2 Hz, H-2); 3.85–3.79 (m, 1H, H-3); 3.69 (d, 1H, J = 2.8 Hz, H-4); 2.26 (d, 1H, J = 8.8 Hz, 3-OH); 1.25 (d, 3H, J = 6.8 Hz, H-6). 13C-APT NMR (100 MHz) δ: 137.7 (Cq,arom); 134.3, 129.1, 128.7, 128.2, 128.1, 127.7 (CHarom); 85.2 (C-1); 79.3 (C-4); 71.9 (C-3); 69.3 (C-5); 62.5 (C-2); 16.6 (C-6). IR (thin film) ν: 3468, 2882, 2106, 1263, 1094, 1057, 1022. HRMS: [M – N2 + H]+ calcd for C19H22NO3Se 392.0759, found 392.0759.
Phenyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-1-seleno-α-l-fucopyranoside (4)
To a stirred solution of 10 (0.21 g, 0.5 mmol, 1.0 equiv) in CH2Cl2/pyridine (1.6 mL, 0.3 M, 1:1 v/v) were added BzCl (0.12 mL, 1.0 mmol, 2 equiv) and DMAP (6 mg, 0.05 mmol, 0.1 equiv) at 0 °C. After TLC analysis indicated complete conversion of the starting material (typically, the reaction mixture was left overnight), the reaction was quenched by addition of MeOH. The mixture was diluted with CH2Cl2, washed with CuSO4·5H2O (in H2O, 10% w/v, 2×), water (1×), and brine (1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (PE/Et2O, 1:0 → 9:1) furnished the title compound in 96% yield (0.25 g, 0.48 mmol). 1H NMR (400 MHz) δ: 8.09 (d, 2H, J = 7.6 Hz, CHarom); 7.63–7.58 (m, 3H, CHarom); 7.48 (t, 2H, J = 7.6 Hz, CHarom); 7.41–7.23 (m, 8H, CHarom); 6.01 (d, 1H, J = 5.2 Hz, H-1); 5.29 (dd, 1H, J = 2.8 Hz, 11.0 Hz, H-3); 4.67 (d, 1H, J = 11.2 Hz, PhCHH); 4.58 (dd, 1H, J = 5.2 Hz, 11.2 Hz, H-2); 4.53 (d, 1H, J = 11.6 Hz, PhCHH); 4.43 (q, 1H, J = 6.4 Hz, H-5); 4.01 (d, 1H, J = 2.0 Hz, H-4); 1.17 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz) δ: 165.7 (COBz); 137.4 (Cq,arom); 134.5, 133.7, 129.9, 129.1 (CHarom); 129.0 (Cq,arom); 128.6 (CHarom); 128.4 (Cq,arom); 128.3, 128.1, 127.9, 127.8 (CHarom); 84.9 (C-1); 76.6 (C-4); 75.6 (PhCH2); 75.1 (C-3); 69.1 (C-5); 59.6 (C-2); 16.3 (C-6). IR (thin film) ν: 2936, 2108, 1722, 1267, 1107, 1086, 1070. HRMS: [M + Na]+ calcd for C26H25N3O4SeNa 546.0903, found 546.0902.
Phenyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-1-seleno-α-l-fucopyranoside (5)
A 100 mL, three-necked flask was equipped with a septum, a gas inlet, and a Liebig condenser fitted with a drying tube. Under a flow of N2 gas, the flask was charged with a solution of phenyl 2-azido-2-deoxy-1-seleno-α-l-fucopyranoside (1.31 g, 4.0 mmol, 1.0 equiv) in pyridine (20 mL, 0.2 M). At 0 °C, DMAP (98 mg, 0.8 mmol, 0.2 equiv) was added followed by TBSOTf (3.7 mL, 16.0 mmol, 4.0 equiv, in a dropwise fashion). The mixture was heated to 70 °C and stirred for 16 h, after which TLC analysis (PE/Et2O, 19:1 v/v) showed complete conversion of the starting material. The reaction mixture was cooled to rt, quenched with MeOH, and diluted with EtOAc. The mixture was washed with 10% aq CuSO4 solution (2×), H2O, and brine, dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (PE/Et2O, 1:0 → 49:1 v/v) furnished the title compound as a light-yellow oil in 85% yield (3.4 mmol, 1.90 g). 1H NMR (CD2Cl2, 193 K) δ: 7.53 (d, 2H, J = 7.6 Hz, CHarom); 7.27–7.25 (m, 3H, CHarom); 5.89 (d, 1H, J = 5.2 Hz, H-1); 4.21 (q, 1H, J = 6.4 Hz, H-5); 4.06 (dd, 1H, J = 4.8 Hz, 10.0 Hz, H-2); 3.70–3.67 (m, 2H, H-3, H-4); 1.06 (d, 3H, J = 6.0 Hz, H-6); 0.90, 0.82 (s, 9H, CH3,tBu); 0.14, 0.11, 0.09, 0.03 (s, 3H, CH3,Me). 13C-APT NMR (CD2Cl2, 193 K) δ: 134.3, 128.7 (CHarom); 128.0 (Cq,arom); 127.4 (CHarom); 85.3 (C-1); 73.6, 72.9 (C-3, C-4); 69.5 (C-2); 61.6 (C-5); 25.5, 25.3 (CH3,tBu); 18.1, 17.8 (Cq,tBu); 16.6 (C-6); −4.3, −4.7, −5.3, −5.3 (CH3,Me). IR (thin film) ν: 2953, 2930, 2856, 2106, 1472, 1252, 1115, 1067, 1022. HRMS: [M – N2 + H]+ calcd C24H44NO3SeSi2 530.2020, found 530.2017.
Phenyl 2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-1-seleno-α-l-fucopyranoside (6)
A 50 mL, three-necked flask was equipped with a septum, a gas inlet, and a Liebig condenser fitted with a drying tube. Under a flow of N2 gas, the flask was charged with a solution of 10 (0.63 g, 1.5 mmol, 1.0 equiv) in pyridine (7.5 mL, 0.2 M). At 0 °C, DMAP (4 mg, 0.3 mmol, 0.2 equiv) was added followed by TBSOTf (0.69 mL, 3.0 mmol, 2.0 equiv, in a dropwise fashion). The mixture was heated to 70 °C and stirred for 16 h, after which TLC analysis (PE/Et2O, 19:1 v/v) showed complete conversion of the starting material. The reaction mixture was cooled to rt, quenched with MeOH, and diluted with EtOAc. The mixture was washed with 10% aq CuSO4 solution (2×), H2O and brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by column chromatography (PE/Et2O, 1:0 → 19:1 v/v) furnished the title compound as a light yellow oil in 92% yield (0.73 g, 1.38 mmol). 1H NMR (400 MHz) δ: 7.57–7.55 (m, 2H, CHarom); 7.39–7.26 (m, 8H, CHarom); 5.96 (d, 1H, J = 4.8 Hz, H-1); 5.06 (d, 1H, J = 11.2 Hz, PhCHH); 4.59 (d, 1H, J = 11.2 Hz, PhCHH); 4.27 (q, 1H, J = 6.4 Hz, H-5); 4.22 (dd, 1H, J = 5.2 Hz, 10.0 Hz, H-2); 3.88 (dd, 1H, J = 2.4 Hz, 10.0 Hz, H-3); 3.53 (bs, 1H, H-4); 1.15 (d, 3H, J = 6.4 Hz, H-6); 0.99 (s, 9H, (CH3)3CSi); 0.25, 0.22 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.5 (Cq,arom); 134.3, 129.0, 128.3, 127.8, 127.7, 127.6 (CHarom); 85.6 (C-1); 80.1 (C-4); 75.6 (PhCH2); 74.2 (C-3); 69.4 (C-5); 62.9 (C-2); 26.0 ((CH3)3CSi); 16.5 (C-6). IR (thin film) ν: 2953, 2930, 2886, 2857, 2106, 1472, 1260, 1111, 1080, 1062, 1042, 1022. HRMS: [M + H]+ calcd for C25H36N3O3SeSi 534.1686, found 534.1688.
General Procedure for Generation of Glycosyl Triflates and Oxosulfonium Triflates
A mixture of glycosyl donor (0.038 mmol, 1.0 equiv) and Ph2SO (10 mg, 0.049 mmol, 1.3 equiv; 15 mg, 0.076 mmol, 2.0 equiv; or 31 mg, 0.152 mmol, 4.0 equiv) was dried by coevaporation with toluene (3×) followed by three vacuum/argon purges. The mixture was dissolved in CD2Cl2 (0.75 mL, 0.05 M) and transferred to a dry NMR tube, which was subsequently capped with a septum. The tube was placed in the probe of a NMR magnet and cooled to −80 °C, after which a 1H NMR spectrum was recorded. The tube was removed from the magnet and placed in a acetone/N2 (l) bath (temperature ≤−80 °C). Tf2O (8 μL, 0.049 mmol, 1.3 equiv) was added with a microliter syringe, and after rapid mixing and recooling, the tube was placed back in the NMR instrument. A 1H NMR spectrum was recorded, which revealed the formation of reactive intermediate(s). After further characterization (13C-APT NMR, HH–COSY, and HSQC) the temperature of the sample was increased by increments of 10 °C until decomposition of the intermediate(s) was observed.
General Procedure for Glycosylations of 2-Azido-2-deoxy Donors 1–6 with Model Acceptors
To a mixture of donor (0.1 mmol, 1.0 equiv), Ph2SO (26 mg, 0.13 mmol, 1.3 equiv), and TTBP (62 mg, 0.25 mmol, 2.5 equiv) in dry CH2Cl2 (2 mL, 0.05 M) were added flame-dried 3 Å molecular sieves. The mixture was subsequently stirred for 30 min before being cooled to −80 °C. At this temperature, Tf2O (22 μL, 0.13 mmol, 1.3 equiv) was added via syringe, and the temperature was raised to −60 °C over the course of ∼30 min. After the temperature was recooled to −80 °C, the acceptor (0.2 mmol, 2.0 equiv, 0.4 mL of a 0.5 M stock solution in CH2Cl2) was added at −80 °C, and the reaction mixture was allowed to warm to −40 °C, after which the reaction was quenched by addition of NEt3 (0.1 mL) and subsequently diluted with CH2Cl2. The mixture was filtered through a small bed of Celite, the residue was washed with CH2Cl2, and the filtrate was washed once with brine, dried over MgSO4, filtered, and concentrated in vacuo. Purification by ordinary column chromatography and/or size-exclusion chromatography afforded the corresponding O-glycoside(s).
Ethyl 2-Azido-3,4-di-O-benzyl-2-deoxy-α/β-l-fucopyranoside (A1)
The title compounds (α/β 1:1) were obtained after column chromatography (hexane/Et2O, 1:0 → 9:1) in 88% yield (35 mg, 0.088 mmol). 1H NMR (400 MHz) δ: 7.43–7.25 (m, 18H, CHarom); 4.95–4.92 (m, 1.8H, PhCHHα, PhCHHβ); 4.90 (d, 0.8H, J = 4.0 Hz, H-1α); 4.74–4.60 (m, 5.4H, PhCH2α, PhCH2β); 4.18 (d, 1H, J = 8.0 Hz, H-1β); 3.99–3.78 (m, 4.2H, H-2α, H-2β, H-3α, H-5α, CHHCH3α); 3.75–3.67 (m, 1.8H, H-4β, CHHCH3β); 3.60–3.50 (m, 2.8H, H-4β, CHHCH3α, CHHCH3β); 3.41 (q, 1H, J = 6.4 Hz, H-5β); 3.30 (dd, 1H, J = 2.8 Hz, 10.4 Hz, H-3β); 1.28–1.16 (m, 10.8H, H-6α, H-6β, CH2CH3α, CH2CH3β). 13C-APT NMR (100 MHz) δ: 138.3, 137.7 (Cq,arom); 128.5, 128.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.7, 127.6 (CHarom); 102.0 (C-1β); 97.9 (C-1α); 81.1 (C-3β); 78.0 (C-3α); 76.3 (C-4α); 74.9 (C-4β); 74.9, 74.6, 72.6, 72.4 (PhCH2); 70.5 (C-5β); 66.5 (C-5α); 65.3 (CH2CH3α); 63.7 (CH2CH3β); 63.0 (C-2β); 59.6 (C-2α); 16.9, 16.7 (C-6); 15.0, 15.0 (CH2CH3). IR (thin film) ν: 2893, 2106, 1454, 1356, 1099, 1063. HRMS: [M + NH4]+ calcd for C22H31N4O4 415.2340, found 415.2339.
Ethyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranoside (A2)
The title compounds (α/β 1:4) were obtained after column chromatography (hexane/EtOAc, 1:0 → 4:1 v/v) in 39% yield (25 mg, 0.059 mmol). 1H NMR (400 MHz) δ: 8.09–8.03 (m, 10H, CHarom); 7.89–7.86 (m, 9H, CHarom); 7.64–7.59 (m, 6H, CHarom); 7.53–7.46 (m, 17H, CHarom); 7.35–7.31 (m, 10H, CHarom); 5.78 (dd, 1H, J = 3.2 Hz, 10.8 Hz, H-3α); 5.71 (dd, 1H, J = 1.2 Hz, 3.2 Hz, H-4α); 5.59 (dd, 4H, J = 0.8 Hz, 3.2 Hz, H-4β); 5.17 (dd, 4H, J = 3.6 Hz, 10.8 Hz, H-3β); 5.13 (d, 1H, J = 3.6 Hz, H-1α); 4.51 (d, 4H, J = 8.0 Hz, H-1β); 4.37 (q, 1H, J = 6.4 Hz, H-5α); 4.10 (dq, 4H, J = 7.2 Hz, 9.6 Hz, CHHCH3β); 3.98–3.90 (m, 8H, H-2β, H-5β); 3.88–3.84 (m, 2H, H-2α, CHHCH3α); 3.76–3.63 (m, 6H, CHHCH3α, CHHCH3β); 1.37–1.21 (m, 30H, H-6α, H-6β, CH2CH3α, CH2CH3β). 13C-APT NMR (100 MHz) δ: 165.8, 165.8, 165.4 (COBz); 133.4, 133.4, 133.3, 133.2, 129.9, 129.7 (CHarom); 129.2, 129.1 (Cq,arom); 128.5, 128.5, 128.3, 128.3 (CHarom); 102.2 (C-1β); 98.1 (C-1α); 72.0 (C-3β); 71.4 (C-4α); 70.3 (C-4β); 69.5 (C-5β); 69.3 (C-3α); 66.2 (CH2CH3β); 65.1 (C-5α); 64.3 (CH2CH3α); 61.2 (C-2β); 57.9 (C-2α); 16.3 (C-6β); 16.1 (C-6α); 15.1 (CH2CH3β); 15.0 (CH2CH3α). IR (thin film) ν: 1980, 2927, 2110, 1724, 1450, 1261, 1175, 1109, 1094, 1067, 1026. HRMS: [M + Na]+ calcd for C22H23N3O6Na 448.1479, found 448.1478.
Ethyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α/β-l-fucopyranoside (A3)
The title compounds (α/β 1:3) were obtained after column chromatography (hexane/Et2O, 1:0 → 4:1 v/v) in 61% yield (25 mg, 0.061 mmol). 1H NMR (400 MHz) δ: 8.15–8.07 (m, 8H, CHarom); 7.60–7.56 (m, 4H, CHarom); 7.48–7.44 (m, 8H, CHarom); 7.36–7.24 (m, 20H, CHarom); 5.68 (d, 1H, J = 2.4 Hz, H-4α); 5.54 (dd, 3H, J = 0.8 Hz, 3.2 Hz, H-4β); 4.98 (d, 1H, J = 3.6 Hz, H-1α); 4.83 (d, 1H, J = 10.8 Hz, PhCHHα); 4.79 (d, 3H, J = 11.6 Hz, PhCHHβ); 4.56–4.53 (m, 4H, PhCHHα, PhCHHβ); 4.28 (d, 4H, J = 8.0 Hz, H-1β); 4.18 (q, 1H, J = 6.8 Hz, H-5α); 4.11 (dd, 1H, J = 3.2 Hz, 10.4 Hz, H-3α); 4.06–3.99 (m, 3H, CHHCH3β); 3.78–3.60 (m, 11H, H-2α, H-2β, H-5β, CHHCH3α, CHHCH3β); 3.45 (dd, 3H, J = 3.2 Hz, 10.4 Hz, H-3β); 1.59–1.26 (m, 21H, H-6β, CH2CH3α, CH2CH3β); 1.22 (d, 3H, J = 6.8 Hz, H-6α). 13C-APT NMR (100 MHz) δ: 166.2 (COBz); 137.2, 137.1 (Cq,arom); 133.3, 133.2, 130.0, 129.8 (CHarom); 129.4 (Cq,arom); 128.5, 128.4, 128.4, 128.4, 128.2, 128.4, 1f27.9, 127.8 (CHarom); 102.0 (C-1β); 97.9 (C-1α); 77.6 (C-3β); 74.4 (C-3α); 71.5 (PhCH2β); 71.5 (PhCH2α); 70.0 (C-4α); 69.5 (C-5β); 68.9 (C-4β); 65.9 (CH2CH3β); 65.1 (C-5α); 64.0 (CH2CH3α); 62.6 (C-2β); 59.3 (C-2α); 16.5 (C-6β); 16.3 (C-6α); 15.1 (CH2CH3β); 15.0 (CH2CH3α). IR (thin film) ν: 2980, 2870, 2108, 1721, 1452, 1265, 1175, 1111, 1065, 1026. HRMS: [M + H]+ calcd for C22H26N3O5 412.1867, found 412.1870.
Ethyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranoside (A4)
The title compounds (α/β 2:5) were obtained after column chromatography (hexane/Et2O 1:0 → 9:1) in 58% yield (24 mg, 0.058 mmol). 1H NMR (400 MHz) δ: 8.10–8.06 (m, 14H, CHarom); 7.62–7.58 (m, 7H, CHarom); 7.49–7.45 (m, 14H, CHarom); 7.26–7.19 (m, 35H, CHarom); 5.57 (dd, 2H, J = 3.0 Hz, 11.0 Hz, H-3α); 5.00 (d, 2H, J = 3.6 Hz, H-1α); 4.95 (dd, 5H, J = 3.0 Hz, 11.0 Hz, H-3β); 4.72–4.68 (m, 7H, PhCHHα, PhCHHβ); 4.57–4.52 (m, 7H, PhCHHα, PhCHHβ); 4.37 (d, 5H, J = 8.0 Hz, H-1β); 4.11 (q, 2H, J = 6.8 Hz, H-5α); 4.05–3.94 (m, 14H, H-2α, H-2β, H-4α, CHHCH3β); 3.82–3.74 (m, 7H, H-4β, CHHCH3α); 3.68–3.56 (m, 12H, H-5β, CHHCH3α, CHHCH3β); 1.31–1.20 (m, 42H, H-6α, H-6β, CH2CH3α, CH2CH3β). 13C-APT NMR (100 MHz) δ: 165.9 (CO Bz); 137.6, 137.5 (Cq,arom); 133.5, 133.5, 129.9 (CHarom); 129.2 (Cq,arom); 128.5, 128.3, 128.2, 128.1, 127.8, 127.8, 127.6 (CHarom); 101.9 (C-1β); 98.0 (C-1α); 77.4 (C-4α); 76.0 (C-4β); 75.5 (PhCH2α); 75.4 (PhCH2β); 75.0 (C-3β); 72.3 (C-3α); 70.5 (C-5β); 66.2 (C-5α); 65.6 (CH2CH3β); 63.9 (CH2CH3α); 61.2 (C-2β); 58.0 (C-2α); 16.6, 16.4, 15.0, 15.0 (CH2CH3, C-6α, C-6β). IR (thin film) ν: 2978, 2932, 2108, 1721, 1452, 1265, 1175, 1094, 1069, 1026. HRMS: [M + NH4]+ calcd for C22H29N4O5 429.2133, found 429.2134.
Ethyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (A5)
The title compounds (α/β 2:5) were obtained after column chromatography (hexane/Et2O, 1:0 → 19:1) along with a minor amount of inseparable, hydrolyzed donor in 63% yield (28 mg, 0.063 mmol). 1H NMR (400 MHz) δ: 4.91 (d, 2H, J = 3.6 Hz, H-1α); 4.19 (d, 5H, J = 8.0 Hz, H-1β); 4.01–3.95 (m, 7H, H-3α, OCHHCH3β); 3.88 (q, 2H, J = 6.4 Hz, H-5α); 3.73–3.70 (m, 6H, H-2α, H-4α, OCHHCH3α); 3.62–3.52 (m, 17H, H-2β, H-4β, OCHHCH3α; OCHHCH3β); 3.45 (q, 5H, J = 6.4 Hz, H-5β); 3.35 (dd, 5H, J = 2.4 Hz, 10.4 Hz, H-3β); 1.29–1.18 (m, 42H, H-6α, H-6β, CH2CH3α, CH2CH3β); 0.96–0.93 (m, 126H, (CH3)3CSiα, (CH3)3CSiβ); 0.19–0.09 (m, 84H, CH3Siα, CH3Siβ). 13C-APT NMR (100 MHz) δ: 102.5 (C-1β); 97.7 (C-1α); 75.2 (C-4α); 74.5 (C-3β); 74.0 (C-4β); 71.3 (C-3α); 71.2 (C-5β); 67.7 (C-5α); 65.5 (OCH2CH3β); 63.8 (C-2β); 63.4 (OCH2CH3α); 61.1 (C-2α); 26.3, 26.2, 26.1 ((CH3)3CSi); 18.6, 18.5 (CqSi); 17.6, 17.3 (OCH2CH3); 15.1, 15.0 (C-6α, C-6β); −3.5, −3.6, −4.2, −4.4 (CH3Si). IR (thin film) ν: 2928, 2857, 2112, 1252, 1117, 1069. HRMS: [M + NH4]+ calcd for C20H47N4O4Si2 463.3130, found 463.3129.
Ethyl 2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (A6)
The title products (α/β 1:1) were obtained after column chromatography (hexane/Et2O, 1:0 → 19:1 v/v) in 81% yield (34 mg, 0.081 mmol). 1H NMR (400 MHz) δ: 7.39–7.26 (m, 10H, CHarom); 5.05–5.02 (m, 2H, PhCHHα, PhCHHβ); 4.91 (d, 1H, J = 3.6 Hz, H-1α); 4.61–4.56 (m, 2H, PhCHHα, PhCHHβ); 4.19 (d, 1H, J = 8.0 Hz, H-1β); 4.12 (dd, 1H, J = 2.8 Hz, 10.0 Hz, H-3α); 3.98–3.93 (m, 2H, H-5α, OCHHCH3); 3.74–3.50 (m, 8H, H-2α, H-2β, H-3β, H-4α, H-5β, OCHHCH3, 2× OCHHCH3); 3.37 (d, 1H, J = 2.4 Hz, H-4β); 1.27–1.19 (m, 12H, H-6α, H-6β, OCH2CH3α, OCH2CH3β); 0.98, 0.96 (s, 9H, (CH3)3CSi); 0.24 (s, 3H, CH3Si); 0.18 (m, 6H, CH3Si); 0.15 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.6, 138.6 (Cq,arom); 128.3, 128.1, 128.1, 127.9, 127.6, 127.5 (CHarom); 102.1 (C-1β); 97.8 (C-1α); 80.9 (C-4α), 79.2 (C-4β); 75.6, 75.3 (PhCH2); 74.9 (C-3β or C-5β); 71.5 (C-3α); 70.3 (C-3β or C-5β); 66.5 (C-5α); 65.4 (OCH2CH3); 64.6 (C-2β); 63.6 (OCH2CH3); 61.5 (C-2α); 25.9, 25.9 ((CH3)3CSi); 18.2, 18.1 (CqSi); 16.8, 16.7 (C-6α, C-6β); 15.1, 15.0 (OCH2CH3α, OCH2CH3β); −4.0, −4.3, −4.7, −5.0 (CH3Si). IR (thin film) ν: 2930, 2891, 2857, 2108, 1254, 1171, 1117, 1067, 1047. HRMS: [M + NH4]+ calcd for C21H39N4O4Si 439.2735, found 439.2732.
2-Fluoroethyl 2-Azido-3,4-di-O-benzyl-2-deoxy-α/β-l-fucopyranoside (B1)
The title products (α/β 1:1) were obtained after column chromatography (hexane/EtOAc, 1:0 → 4:1) in 72% yield (30 mg, 0.072 mmol). 1H NMR (400 MHz) δ: 7.66–7.63 (m, 2H, CHarom); 7.46–7.25 (m, 18H, CHarom); 4.95–4.91 (m, 3H, H-1α, 2× PhCHH); 4.74–4.50 (m, CH2Fα, CH2Fβ, 2× PhCHH; 4x PhCHH); 4.26 (d, 1H, J = 8.0 Hz, H-1β); 4.02–3.73 (m, 9H, H-2α, H-2β, H-3α; H-4α, H-5α, CH2CH2Fα, CH2CH2Fβ); 3.54 (d, 1H, J = 2.4 Hz, H-4β); 3.42 (q, 1H, J = 6.4 Hz, H-5β); 3.31 (dd, 1H, J = 2.8 Hz, 10.4 Hz, H-3β); 1.20–1.17 (m, 6H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 138.2, 137.6 (Cq,arom); 131.0, 129.9, 128.5, 128.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.7, 127.7, 124.7 (CHarom); 102.3 (C-1β); 98.4 (C-1α); 82.7 (d, J = 168 Hz, CH2F); 82.5 (d, J = 168 Hz, CH2F); 80.9 (C-3β); 77.8 (C-3α); 76.1 (C-4α); 74.9 (PhCH2); 74.8 (C-4β); 72.7, 72.4 (PhCH2); 70.6 (C-5β); 68.3 (d, 20 Hz, CH2CH2F) 67.1 (d, 20 Hz, CH2CH2F); 66.7 (C-5α); 62.9 (C-2β); 59.5 (C-2α); 16.8, 16.7 (C-6α, C-6β). IR (thin film) ν: 2876, 2108, 1726, 1358, 1109, 1062, 1045. HRMS: [M + NH4]+ calcd for C22H30FN4O4 433.2246, found 433.2242.
2-Fluoroethyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranoside (B2)
The title compounds (α/β 1:2) were obtained after column chromatography (hexane/EtOAc, 1:0 → 4:1) in 34% yield (15 mg, 0.034 mmol). 1H NMR (400 MHz) δ: 8.08–8.03 (m, 6H, CHarom); 7.89–7.86 (m, 6H, CHarom); 7.63–7.60 (m, 3H, CHarom); 7.53–7.46 (m, 9H, CHarom); 7.35–7.31 (m, 6H, CHarom); 5.78 (dd, 1H, J = 3.2 Hz, 11.2 Hz, H-3α); 5.72 (d, 1H, J = 3.2 Hz, H-4α); 5.59 (d, 2H, J = 3.2 Hz, H-4β); 5.19–5.16 (m, 3H, H-1α, H-3β); 4.77–4.60 (m, 6H, CH2Fα, CH2Fβ, H-1β); 4.58 (d, 2H, J = 8.4 Hz, H-1β); 4.42 (q, 1H, J = 6.8 Hz, H-5α); 4.25–3.89 (m, 11H, H-2α, H-2β, H-5β, CH2CH2Fα, CH2CH2Fβ); 1.31 (d, 6H, J = 6.4 Hz, H-6β); 1.25 (d, 3H, J = 6.4 Hz, H-6α). 13C-APT NMR (100 MHz) δ: 165.8, 165.4 (COBz); 133.5, 133.4, 133.3, 133.3, 129.9, 129.8 (CHarom); 129.3, 129.2, 129.0 (Cq,arom); 128.6, 128.3 (CHarom); 102.5 (C-1β); 98.6 (C-1α); 82.6 (d, J = 169 Hz, CH2CH2Fβ); 82.4 (d, J = 170 Hz, CH2CH2Fα); 72.0 (C-3β); 71.3 (C-4α); 70.2 (C-4β); 69.7 (C-5β); 69.2 (C-3α); 69.1 (d, J = 21 Hz, CH2CH2Fβ); 67.5 (d, J = 20 Hz, CH2CH2Fα); 65.4 (C-5α); 61.3 (C-2β); 58.0 (C-2α); 16.3 (C-6β); 16.1 (C-6α). IR (thin film) ν: 2984, 2924, 2110, 1721, 1450, 1260, 1169, 1107, 1094, 1067, 1026. HRMS: [M + Na]+ calcd for C22H22FN3O6Na 466.1385, found 466.1384.
2-Fluoroethyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α/β-l-fucopyranoside (B3)
The products (α/β 1:1) were obtained after column chromatography (hexane/EtOAc, 1:0 → 9:1) and size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) in 56% yield (24 mg, 0.056 mmol), accompanied by a small amount of inseparable, hydrolyzed donor. 1H NMR (400 MHz) δ: 8.14–8.07 (m, 4H, CHarom); 7.60–7.56 (m, 2H, CHarom); 7.49–4.44 (m, 4H, CHarom); 7.35–7.25 (m, 10H, CHarom); 5.70 (d, 1H, J = 2.8 Hz, H-4α); 5.55 (d, 1H, J = 3.2 Hz, H-4β); 5.02 (d, 1H, J = 3.6 Hz, H-1α); 4.85–4.53 (m, 8H, CH2Fα, CH2Fβ, PhCH2α, PhCH2β); 4.36 (d, 1H, J = 8.0 Hz, H-1β); 4.24 (q, 1H, J = 6.4 Hz, H-5α); 4.15–3.71 (m, 8H, H-2α, H-2β, H-3α, H-5β, CH2CH2Fα, CH2CH2Fβ); 3.47 (dd, 1H, J = 3.2 Hz, 10.2 Hz, H-3β); 1.28–1.21 (m, 6H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 166.2, 166.1 (COBz); 137.1, 137.0 (Cq,arom); 133.4, 133.3, 130.2, 130.0, 129.8, 129.6 (CHarom); 129.4 (Cq,arom); 128.5, 128.4, 128.2, 128.0, 127.9, 127.8 (CHarom); 102.3 (C-1β); 98.4 (C-1α); 82.7 (d, J = 169 Hz, CH2Fβ); 82.5 (d, J = 170 Hz, CH2Fα); 77.5 (C-3β); 74.3 (C-3α); 71.6 (PhCH2β); 71.5 (PhCH2α); 69.8 (C-4α); 69.6 (C-5β); 68.8 (d, J = 20 Hz, CH2CH2Fβ); 68.8 (C-4β); 67.4 (d, J = 20 Hz, CH2CH2Fα); 65.4 (C-5α); 62.6 (C-2β); 59.2 (C-2α); 16.5, 16.3 (C-6α, C-6β). IR (thin film) ν: 2926, 2110, 1721, 1452, 1267, 1169, 1111, 1067, 1026. HRMS: [M + H – N2]+ calcd for C22H25FNO5 402.1711, found 402.1711.
2-Fluoroethyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranoside (B4)
The products (α/β 2:3) were obtained after column chromatography (hexane/EtOAc, 1:0 → 9:1) and size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) in 60% yield (26 mg, 0.060 mmol). 1H NMR (400 MHz) δ: 8.10–8.06 (m, 10H, CHarom); 7.62–7.58 (m, 5H, CHarom); 7.49–7.45 (m, 10H, CHarom); 7.26–7.20 (m, 25H, CHarom); 5.58 (dd, 2H, J = 2.8 Hz, 11.2 Hz, H-3α); 5.05 (d, 2H, J = 3.6 Hz, H-1α); 4.95 (dd, 3H, J = 3.2 Hz, 10.8 Hz, H-3β); 4.73–4.52 (m, 20H, CHCH2F, PhCH2); 4.43 (d, 3H, J = 8.0 Hz, H-1β); 4.17–3.78 (m, 22H, H-2α, H-2β, H-4α, H-4β, H-5α, CH2CH2F); 3.67 (q, 3H, J = 6.4 Hz, H-5β); 1.26–1.21 (m, 15H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 165.8 (COBz); 137.5, 137.4 (Cq,arom); 133.6, 133.5, 129.9 (CHarom); 129.3, 129.1 (Cq,arom); 128.6, 128.3, 128.3, 128.1, 127.9, 127.9 (CHarom); 102.3 (C-1β); 98.5 (C-1α); 82.6 (d, J = 168 Hz, CH2CH2Fβ); 82.4 (d, J = 169 Hz, CH2CH2Fα); 77.2 (C-4α); 75.9 (C-4β); 75.6 (PhCH2α); 75.4 (PhCH2β); 74.9 (C-3β); 72.2 (C-3α); 70.6 (C-5β); 68.6 (d, J = 21 Hz, CH2CH2Fβ); 67.3 (d, J = 20 Hz, CH2CH2Fα); 66.5 (C-5α); 61.2 (C-2β); 58.0 (C-2α); 16.6 (C-6β); 16.4 (C-6α). IR (thin film) ν: 2934, 2110, 1721, 1452, 1267, 1171, 1096, 1069, 1026. HRMS: [M + NH4]+ calcd for C22H28FN4O5 447.2038, found 447.2038.
2-Fluoroethyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (B5)
The products (α/β 2:3) were obtained after column chromatography (hexane/Et2O, 1:0 → 9:1) in 82% yield (38 mg, 0.082 mmol). 1H NMR (400 MHz) δ: 4.94 (d, 2H, J = 3.6 Hz, H-1α); 4.66–4.51 (m, 10H, CH2Fα, CH2Fβ); 4.26 (d, 3H, J = 8.0 Hz, H-1β); 4.15–4.01 (m, 5H, H-3α, CHHCH2Fβ); 3.94–3.68 (m, 13H, H-2α, H-4α, H-5α, CH2CH2Fα, CHHCH2Fβ); 3.59–3.55 (m, 6H, H-2β, H-4β); 3.47 (q, 3H, J = 6.4 Hz, H-5β); 3.36 (dd, 3H, J = 2.4 Hz, 10.2 Hz, H-3β); 1.23 (d, 9H, J = 6.4 Hz, H-6β); 1.19 (d, 6H, J = 6.4 Hz, H-6α), 0.96–0.93 (m, 90H, (CH3)3CSi); 0.19–0.08 (m, 60H, CH3Si). 13C-APT NMR (100 MHz) δ: 102.8 (C-1β); 98.3 (C-1α); 82.8 (d, J = 168 Hz, CH2CH2Fβ); 82.6 (d, J = 168 Hz, CH2CH2Fα); 75.1 (C-4α); 74.4 (C-3β); 73.9 (C-4β); 71.3 (C-5β); 71.2 (C-3α); 68.3 (d, J = 20 Hz, CH2CH2Fβ); 66.9 (d, J = 20 Hz, CH2CH2Fα); 63.8 (C-2β); 61.1 (C-2α); 26.3, 26.1, 26.1 ((CH3)3CSi); 18.6, 18.6, 18.5 (CqSi); 17.5 (C-6β), 17.3 (C-6α); −3.5, −3.5, −3.6, −3.7, −4.3, −4.5, −4.5, −4.7 (CH3Si). IR (thin film) ν: 2930, 2857, 2108, 1252, 1177, 1119, 1069, 1045, 1028. HRMS: [M + NH4]+ calcd for C20H46FN4O4Si2 481.3036, found 481.3034.
2-Fluoroethyl 2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (B6)
The title products (α/β 1:1) were isolated after column chromatography (hexane/Et2O, 1:0 → 9:1) in 80% yield (35 mg, 0.080 mmol). 1H NMR (400 MHz) δ: 7.39–7.26 (m, 10H, CHarom); 5.05–5.02 (m, 2H, 2× PhCHH); 4.94 (d, 1H, J = 3.6 Hz, H-1α); 4.67–5.51 (m, 6H, 2× PhCHH, CH2Fα. CH2Fβ); 4.26 (d, 1H, J = 8.0 Hz, H-1β); 4.13 (dd, 1H, J = 2.8 Hz, 10.4 Hz, H-3α); 4.00–3.76 (m, 7H, H-2α, H-2β; H-5α; CH2CH2Fα, CH2CH2Fβ); 3.52–3.48 (m, 3H, H-3β, H-4α, H-5β); 3.38 (d, 1H, J = 2.8 Hz, H-4β); 1.21–1.18 (m, 6H, H-6α, H-6β); 0.98, 0.97 (s, 9H, (CH3)3CSi); 0.24 (s, 3H, CH3Si); 0.20 (s, 6H, 2× CH3Si); 0.16 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.5, 138.5 (Cq,arom); 128.3, 128.2, 128.1, 127.9, 127.6, 127.6 (CHarom); 102.5 (C-1β); 98.4 (C-1α); 82.7 (d, J = 168 Hz, CH2F); 82.6 (d, J = 168 Hz, CH2F); 80.7 (C-4α); 79.0 (C-4β); 75.6, 75.4 (PhCH2); 74.8 (C-5β); 71.4 (C-3α); 70.5 (C-3β); 68.4 (d, J = 20 Hz, CH2CH2F); 67.1 (d, J = 20 Hz, CH2CH2F); 66.8 (C-5α); 64.6 (C-2β); 61.4 (C-2α); 25.9, 25.9 ((CH3)3CSi); 18.1, 18.0 (CqSi); 16.7, 16.6 (C-6α, C-6β); −4.0, −4.3, −4.8, −5.1 (CH3Si). IR (thin film) ν: 2930, 2886, 2857, 2108, 1254, 1169, 1119, 1065, 1045. HRMS: [M + NH4]+ calcd for C21H38FN4O4Si 457.2643, found 457.2636.
2,2-Difluoroethyl 2-Azido-3,4-di-O-benzyl-2-deoxy-α/β-l-fucopyranoside (C1)
The products (α/β 3:2) were obtained after column chromatography (toluene/EtOAc, 1:0 → 9:1 v/v) in 81% yield (35 mg, 0.081 mmol). 1H NMR (400 MHz) δ: 7.43–7.25 (m, 50H, CHarom); 6.08–5.78 (m, 5H, CHF2α, CHF2β); 4.95–4.91 (m, 7H, H-1α, PhCHHα, PhCHHβ); 4.74–4.59 (m, 14H, PhCH2); 4.24 (d, 2H, J = 8.0 Hz, H-1β); 4.01–3.73 (m, 24H, H-2α, H-2β, H-3α, H-4α, H-5α, CH2CHF2α, CH2CHF2β); 3.54 (d, 2H, J = 2.4 Hz, H-4β); 3.43 (q, 2H, J = 6.4 Hz, H-5β); 3.31 (dd, 2H, J = 2.8 Hz, 10.0 Hz, H-3β); 1.20–1.17 (m, 15H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 138.1, 138.0, 137.5, 137.5 (Cq,arom); 128.6, 128.5, 128.3, 128.3, 128.2, 128.0, 127.8, 127.8, 127.8, 127.6 (CHarom); 114.3 (CHF2β); 113.9 (CHF2α); 102.4 (C-1β); 99.0 (C-1α); 80.8 (C-3β); 77.6 (C-3α or C-5α); 75.9 (C-4α); 75.0 (PhCH2α); 74.7 (PhCH2β); 74.6 (C-4β); 72.8 (PhCH2β); 72.4 (PhCH2α); 70.9 (C-5β); 68.3 (t, J = 29 Hz, CH2CHF2β); 67.2 (C-3α or C-5α); 67.2 (t, J = 29 Hz, CH2CHF2β); 62.8 (C-2β); 59.4 (C-2α); 16.7, 16.6 (C-6α, C-6β). IR (thin film) ν: 2926, 2110, 1738, 1454, 1360, 1109, 1069. HRMS: [M + NH4]+ calcd for C22H29F2N4O4 451.2151, found 451.2150.
2,2-Difluoroethyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranoside (C2)
The title compounds (α/β 3:2) were obtained after column chromatography (toluene/EtOAc, 1:0 → 9:1 v/v) in 74% yield (34 mg, 0.074 mmol). 8.07–8.02 (m, 5.4H, CHarom); 7.89–7.86 (m, 5.4H, CHarom); 7.64–7.60 (m, 2.7H, CHarom); 7.53–7.46 (m, 8.1H, CHarom); 7.35–7.31 (m, 5.4H, CHarom); 6.17–5.87 (m, 2.7H, CHF2α, CHF2β); 5.76–5.73 (m, 3.4H, H-3α, H-4α); 5.60 (d, 1H, J = 3.2 Hz, H-4β); 5.19–5.16 (m, 2.7H, H-1α, H-3β); 4.56 (d, 1H, J = 8.0 Hz, H-1β); 4.38 (q, 1H, J = 6.4 Hz, H-5α); 4.17–3.86 (m, 9.1H, H-2α, H-2β, H-5β, CH2CH2Fα, CH2CH2Fβ); 1.32–1.23 (m, 8.1H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 165.7, 165.7, 165.4 (COBz); 133.5, 133.5, 133.4, 133.3, 129.9, 129.8 (CHarom); 129.2, 129.1, 129.0 (Cq,arom); 129.0, 128.6, 128.4, 128.3 (CHarom); 114.0 (t, J = 240 Hz, CHF2β); 113.7 (t, J = 240 Hz, CHF2α); 102.6 (C-1β); 99.1 (C-1α); 71.9 (C-3β); 71.1 (C-3α or C-4α); 70.0, 69.9 (C-4β, C-5β); 69.0 (C-3α or C-4α); 68.8 (t, J = 30 Hz, CH2CHF2β); 67.4 (t, J = 30 Hz, CH2CH2Fα); 65.8 (C-5α); 61.2 (C-2β); 57.9 (C-2α); 16.2 (C-6β), 16.0 (C-6α). IR (thin film) ν: 2926, 2110, 1726, 1450, 1261, 1163, 1107, 1094, 1067. HRMS: [M + Na]+ calcd for C22H21F2N3O6Na 484.1291, found 484.1289.
2,2-Difluoroethyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α/β-l-fucopyranoside (C3)
The products (α/β 3:1) were obtained after column chromatography (toluene/EtOAc, 1:0 → 19:1) in 76% yield (34 mg, 0.076 mmol), accompanied by a small amount of inseparable, hydrolyzed donor. 1H NMR (400 MHz) δ: 8.12–8.07 (m, 8H, CHarom); 7.60–7.58 (m, 4H, CHarom); 7.49–7.44 (m, 10H, CHarom); 7.35–7.25 (m, 18H, CHarom); 6.12–5.82 (m, 4H, CHF2α, CHF2β); 5.70 (d, 3H, J = 2.8 Hz, H-4α); 5.45 (d, 1H, J = 2.8 Hz, H-4β); 5.01 (d, 3H, J = 3.6 Hz, H-1α); 4.83 (d, 3H, J = 10.8 Hz, PhCHHα); 4.79 (d, 1H, J = 11.6 Hz, PhCHHβ); 4.56–4.53 (d, 4H, PhCHHα, PhCHHβ); 4.33 (d, 1H, J = 8.0 Hz, H-1β); 4.19 (q, 3H, J = 6.4 Hz, H-5α); 4.08 (dd, 3H, J = 3.2 Hz, 10.8 Hz, H-3α); 4.03–3.69 (m, 13H, H-2α, H-2β, H-5β, CH2CHF2α, CH2CHF2β); 3.47 (dd, 1H, J = 3.6 Hz, 10.8 Hz, H-3β); 1.31–1.22 (m, 12H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 166.1, 166.0 (COBz); 137.0, 136.9 (Cq,arom); 133.4, 133.3, 130.0, 129.9, 129.5, 129.4, 129.2 (CHarom); 128.6, 128.5 (Cq,arom); 128.4, 128.4, 128.3, 128.2, 128.0, 127.9, 127.6 (CHarom); 114.2 (t, J = 240 Hz, CHF2β); 113.8 (t, J = 240 Hz, CHF2α); 102.4 (C-1β); 99.0 (C-1α); 77.5 (C-3β); 74.1 (C-3α); 71.6 (PhCH2β); 71.5 (PhCH2α); 69.8 (C-5β); 69.6 (C-4α); 68.6 (C-4β); 64.6 (t, J = 30 Hz, CH2CHF2β); 67.4 (t, J = 28 Hz, CH2CHF2α); 65.8 (C-5α); 62.5 (C-2β); 59.1 (C-2α); 16.4, 16.3 (C-6α, C-6β). IR (thin film) ν: 2924, 2110, 1721, 1452, 1265, 1167, 1109, 1067, 1053, 1026. HRMS: [M + H–N2]+ calcd for C22H24F2NO5 420.1617, found 420.1617.
2,2-Difluoroethyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranoside (C4)
The title compounds (α/β 1:1) were obtained after column chromatography (toluene/EtOAc, 1:0 → 9:1) in 80% yield (36 mg, 0.080 mmol). 1H NMR (400 MHz) δ: 8.10–8.06 (m, 4H, CHarom); 7.63–7.59 (m, 2H, CHarom); 7.49–7.46 (m, 4H, CHarom); 7.26–7.20 (m, 10H, CHarom); 6.11–5.82 (m, 2H, CHF2α, CHF2β); 5.54 (dd, 1H, J = 3.2 Hz, 11.2 Hz, H-3α); 5.04 (d, 1H, J = 3.6 Hz, H-1α); 4.95 (dd, 1H, J = 2.8 Hz, 10.8 Hz, H-3β); 4.72–4.69 (d, 2H, J = 11.6 Hz, 2× PhCHH); 4.57–4.52 (m, 2H, 2× PhCHH); 4.42 (d, 1H, J = 8.8 Hz, H-1β); 4.14–4.3.78 (m, 9H, H-2α, H-2β, H-4α, H-4β, H-5α, CH2CHF2α, CH2CHF2β); 3.68 (q, 1H, J = 6.4 Hz, H-5β); 1.30–1.20 (m, 6H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 165.8 (COBz); 137.4, 137.3 (Cq,arom); 133.6, 133.6, 130.2, 129.9 (CHarom); 129.3 (Cq,arom); 129.0, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.9 (CHarom); 114.1 (t, J = 240 Hz, CHF2); 113.8 (t, J = 240 Hz, CHF2); 102.4 (C-1β); 99.0 (C-1α); 77.0, 75.7 (C-4α, C-4β); 75.6, 75.5 (PhCH2); 74.7 (C-3β); 71.9 (C-3α); 70.9 (C-5β); 68.7–67.3 (m, 2C, CH2CHF2α, CH2CHF2β); 66.9 (C-5α); 61.1 (C-2β); 57.9 (C-2α); 16.5, 16.3 (C-6α, C-6β). IR (thin film) ν: 2924, 2110, 1721, 1452, 1265, 1169, 1096, 1069, 1026. HRMS: [M + NH4]+ calcd for C22H27F2N4O5 465.1944, found 465.1943.
2,2-Difluoroethyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (C5)
The title products (α/β 5:2) were obtained after column chromatography (hexane/Et2O, 1:0 → 19:1 v/v) in 75% yield (36 mg, 0.075 mmol). 1H NMR (400 MHz) δ: 6.09–5.79 (m, 7H, CHF2α, CHF2β) 4.93 (d, 5H, J = 3.2 Hz, H-1α); 4.26 (d, 2H, J = 8.0 Hz, H-1β); 4.01–3.73 (m, 27H, H-2α, H-3α, H-5α, OCH2CHF2α, OCH2CHF2β); 3.71 (d, 5H, J = 2.0 Hz, H-4α); 3.58–3.53 (m, 4H, H-2β, H-4β); 3.47 (q, 2H, J = 6.4 Hz, H-5β); 3.36 (dd, 2H, J = 2.4 Hz, 10.4 Hz, H-3β); 1.23 (d, 6H, J = 6.4 Hz, H-6β); 1.19 (d, 15H, J = 6.4 Hz, H-6α); 0.96–0.89 (m, 126H, ((CH3)3CSi); 0.18–0.09 (m, 84H, CH3Si). 13C-APT NMR (100 MHz) δ: 114.4 (t, J = 240 Hz, CHF2β); 114.1 (t, J = 240 Hz, CHF2α); 102.8 (C-1β); 98.9 (C-1α); 75.0 (C-4α); 74.3 (C-3β); 73.8 (C-4β); 71.5 (C-5β); 71.1 (C-3α); 68.5 (C-5α); 68.2 (t, J = 29 Hz, CH2CHF2β); 67.1 (t, J = 29 Hz, CH2CHF2α); 63.8 (C-2β); 61.0 (C-2α); 26.3, 26.1 ((CH3)3CSi); 18.6, 18.5 (CqSi); 17.4, (C-6β); 17.3 (C-6α); −3.5, −3.5, −3.8, −4.4, −4.5, −4.7 (CH3Si). IR (thin film) ν: 2930, 2859, 2108, 1252, 1177, 1113, 1069, 1043, 1028. HRMS: [M + H–N2]+ calcd for C20H42F2NO4Si2 454.2615, found 454.2613.
2,2-Difluoroethyl 2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (C6)
The title products (α/β 2:1) were obtained after chromatography (hexane/Et2O, 1:0 → 9:1 v/v) in 87% yield (40 mg, 0.087 mmol). 1H NMR (400 MHz) δ: 7.39–7.25 (m, 7.5H, CHarom); 6.08–5.81 (m, 1.5H, CHF2α, CHF2β); 5.05–5.02 (d, 1.5H, J = 11.2 Hz, PhCHHα, PhCHHβ); 4.93 (d, 1H, J = 3.6 Hz, H-1α); 4.62–4.56 (m, 1.5H, PhCHHα, PhCHHβ); 4.25 (d, 0.5H, J = 8.0 Hz, H-1β); 4.08 (dd, 1H, J = 2.8 Hz, 10.4 Hz, H-3α); 3.94 (q, 1H, J = 6.4 Hz, H-5α); 3.82–3.73 (m, 4H, H-2α, CH2CHF2α, CH2CHF2β); 3.66 (dd, 0.5H, J = 8.0 Hz, 10.4 Hz, H-2β); 3.52–3.50 (m, 2H, H-3β, H-4α, H-5β); 3.39 (d, 0.5H, J = 2.4 Hz, H-4β), 1.26–1.18 (m, 4.5H, H-6α, H-6β); 0.98 (s, 9H, (CH3)3CSi); 0.97 (s, 4.5H, (CH3)3CSi); 0.24 (s, 3H, CH3Si); 0.20 (s, 4.5H, CH3Siα, CH3Siβ); 0.16 (s, 1.5H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.4, 138.4 (Cq,arom); 128.3, 128.2, 128.1, 127.9, 127.7, 127.7 (CHarom); 114.3 (t, J = 240 Hz, CHF2β); 114.0 (t, J = 240 Hz, CHF2α); 102.6 (C-1β); 99.0 (C-1α); 80.5 (C-4α); 78.9 (C-4β); 75.7, 75.5 (PhCH2); 74.7 (C-3β or C-5β); 71.3 (C-3α); 70.7 (C-3β or C-5β); 68.4 (t, J = 27 Hz, CH2CHF2β); 67.3 (t, J = 29 Hz, CH2CHF2α); 67.3 (C-5α); 64.5 (C-2β); 61.3 (C-2α); 25.9, 25.8 ((CH3)3CSi); 18.1, 18.0 (CqSi); 16.7, 16.6 (C-6, C-6′); −4.1, −4.3, −4.8, −5.1 (CH3Si). IR (thin film) ν: 2930, 2110, 1260, 1169, 1115, 1070, 1047. HRMS: [M + NH4]+ calcd for C21H37F2N4O4Si 475.2546, found 475.2547.
2,2,2-Trifluoroethyl 2-Azido-3,4-di-O-benzyl-2-deoxy-α-l-fucopyranoside (D1)
The title compound was obtained after column chromatography (hexane/Et2O 1:0 → 9:1 v/v) in 80% yield (36 mg, 0.080 mmol, 80%). 1H NMR (400 MHz) δ: 7.44–7.25 (m, 10H, CHarom); 4.96–4.92 (m, 2H, PhCHH, H-1); 4.75 (s, 2H, PhCH2); 4.60 (d, 1H, J = 11.6 Hz, PhCHH); 3.99–3.88 (m, 5H, H-2, H-3, H-5, CH2CF3); 3.54 (d, 1H, J = 2.4 Hz, H-4); 1.18 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz) δ: 138.0, 137.5 (Cq,arom); 128.6, 128.3, 128.3, 128.0, 127.8, 127.8 (CHarom); 123.6 (q, J = 277 Hz, CF3); 99.0 (C-1); 77.4 (C-3 or C-5); 75.9 (C-4); 75.0, 72.5 (PhCH2); 67.5 (C-3 or C-5); 64.9 (q, J = 35 Hz, CH2CF3); 59.2 (C-2); 16.7 (C-6). 13C-GATED NMR (100 MHz) δ: 99.0 (d, J = 170 Hz, C-1). IR (thin film) ν: 2927, 2108, 1454, 1356, 1279, 1163, 1082, 1051. HRMS: [M + Na]+ calcd for C22H24F3N3O4Na 474.1611, found 474.1609.
2,2,2-Trifluoroethyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranoside (D2)
The title compounds (α/β 10:1) were isolated after column chromatography (hexane/EtOAc. 1:0 → 4:1) in 50% yield (24 mg, 0.050 mmol). NMR data are reported for the α-product only. 1H NMR (400 MHz) δ: 8.04–8.02 (m, 2H, CHarom); 7.89–7.86 (m, 2H, CHarom); 7.66–7.61 (m, 1H, CHarom); 7.54–7.45 (m, 3H, CHarom); 7.36–7.32 (m, 2H, CHarom); 5.77–7.73 (m, 2H, H-3, H-4); 5.20 (d, 1H, J = 3.6 Hz, H-1); 4.37 (q, 1H, J = 6.4 Hz, H-5); 4.08 (q, 2H, J = 8.4 Hz, CH2CF3); 3.95 (dd, 1H, J = 3.2 Hz, 11.4 Hz, H-2); 1.26 (d, 3H, J = 6.8 Hz, H-6). 13C-APT NMR (100 MHz) δ: 165.6, 165.3 (COBz); 133.5, 133.4, 129.9, 129.8 (CHarom); 129.2, 129.0 (Cq,arom); 128.6, 128.3 (CHarom); 123.4 (q, J = 276 Hz, CF3); 99.2 (C-1); 71.0 (C-3); 68.8 (C-4); 65.8 (C-5); 65.3 (q, J = 35 Hz, CH2CF3); 57.7 (C-2); 16.0 (C-6). IR (thin film) ν: 2928, 2110, 1724, 1452, 1273, 1261, 1157, 1109, 1094, 1069, 1026. HRMS: [M + Na]+ calcd for C22H20F3N3O6Na 502.1196, found 502.1195.
2,2,2-Trifluoroethyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α-l-fucopyranoside (D3)
The title compound was obtained after column chromatography (hexane/Et2O 1:0 → 9:1 v/v) in 45% yield (21 mg, 0.045 mmol, α/β ≥ 19:1). NMR data are reported for the α-isomer only. 1H NMR (400 MHz) δ: 8.08 (d, 2H, J = 7.2 Hz, CHarom); 7.59 (t, 1H, J = 7.6 Hz, CHarom); 7.46 (t, 2H, J = 8.0 Hz, CHarom); 7.34–7.24 (m, 5H, CHarom); 5.72 (d, 1H, J = 2.8 Hz, H-4); 5.05 (d, 1H, J = 3.6 Hz, H-1); 4.85 (d, 1H, J = 10.4 Hz, PhCHH); 4.55 (d, 1H, J = 10.8 Hz, PhCHH); 4.19 (q, 1H, J = 6.4 Hz, H-5); 4.11 (dd, 1H, J = 2.8 Hz, 10.4 Hz, H-3); 4.01 (q, 2H, J = 8.4 Hz, CH2CF3); 3.80 (dd, 1H, J = 3.6 Hz, 10.4 Hz, H-2); 1.24 (d, 3H, J = 6.8 Hz, H-6). 13C-APT NMR (100 MHz) δ: 166.0 (COBz); 137.0 (Cq,arom); 133.4, 129.8 (CHarom); 129.5 (Cq,arom); 128.5, 128.4, 128.3, 127.9 (CHarom); 123.5 (q, J = 277 Hz, CF3); 99.1 (C-1); 74.1 (C-3); 71.6 (PhCH2); 69.6 (C-4); 66.2 (C-5); 65.3 (q, J = 35 Hz, CH2CF3); 58.9 (C-2); 16.3 (C-6). IR (thin film) ν: 2924, 2110, 1721, 1452, 1267, 1157, 1111, 1084, 1055, 1026. HRMS: [M + H]+ calcd for C22H23F3N3O5 466.1584, found 466.1581.
2,2,2-Trifluoroethyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranoside (D4)
The title compounds (α/β 7:1) were obtained after column chromatography (hexane/Et2O 1:0 → 4:1 v/v) in 77% yield (36 mg, 0.077 mmol). 1H NMR (400 MHz) δ: 8.10–8.06 (m, 2.3H, CHarom); 7.63–7.59 (m, 1.2H, CHarom); 7.49–7.46 (m, 2.5H, CHarom); 7.29–7.21 (m, 6H, CHarom); 5.54 (dd, 1H, J = 2.8 Hz, 11.2 Hz, H-3α); 5.07 (d, 1H, J = 3.6 Hz, H-1α); 4.95 (dd, 0.15H, J = 2.8 Hz, 11.2 Hz, H-3β); 4.72–4.69 (m, 1.15H, PhCHHα, PhCHHβ); 4.57–4.52 (m, 1.15H, PhCHHα, PhCHHβ); 4.48 (d, 0.15H, J = 8.0 Hz, H-1β); 4.11 (q, 1H, J = 6.8 Hz, H-5α); 4.07–3.95 (m, 4.45H, H-2α, H-4α, CH2CF3α, H-2β, CH2CF3β); 3.82 (d, 0.15H, J = 2.8 Hz, H-4β); 3.68 (q, 0.15H, J = 6.8 Hz, H-5β); 1.26–1.22 (m, 3.45H, H-6α, H-6β). 13C-APT NMR (100 MHz) δ: 165.8 (CO Bz); 137.3 (Cq,arom); 133.6, 129.9 (CHarom); 129.1 (Cq,arom); 128.6, 128.4, 128.3, 128.2, 128.0 (CHarom); 123.5 (q, J = 277 Hz, CF3α); 102.0 (C-1β); 99.1 (C-1α); 76.9 (C-4α); 75.6 (PhCH2α); 75.6 (C-4β); 75.5 (PhCH2β); 74.6 (C-3β); 71.8 (C-3α); 71.0 (C-5β); 67.2 (C-5α); 65.0 (q, J = 35 Hz, CH2CF3α); 61.1 (C-2β); 57.6 (C-2α); 16.4 (C-6β); 16.3 (C-6α). IR (thin film) ν: 2924, 2110, 1721, 1452, 1267, 1155, 1105, 1096, 1070, 1045, 1026. HRMS: [M + NH4]+ calcd for C22H26F3N4O5 483.1850, found 483.1849.
2,2,2-Trifluoroethyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (D5)
The title compounds (α/β 19:1) were isolated after column chromatography (hexane/Et2O, 1:0 → 49:1) in 84% yield (42 mg, 0.084 mmol). NMR data are reported for the α-isomer only. 1H NMR (400 MHz) δ: 4.97 (d, 1H, J = 3.2 Hz, H-1); 4.01 (dd, 1H, J = 2.0 Hz, 10.4 Hz, H-3); 3.98–3.88 (m, 3H, H-5, CH2CF3); 3.79 (dd, 1H, J = 3.6 Hz, 10.4 Hz, H-2); 3.72 (d, 1H, J = 1.2 Hz, H-4); 1.20 (d, 3H, J = 6.4 Hz, H-6); 0.96, 0.94 (s, 9H, (CH3)3CSi); 0.19 (s, 3H, CH3Si); 0.16–0.15 (m, 6H, CH3Si); 0.07 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 123.7 (q, J = 276 Hz, CF3); 98.8 (C-1); 74.9 (C-4); 70.9 (C-3); 68.8 (C-5); 64.7 (q, J = 35 Hz, CH2CF3); 60.8 (C-2); 26.2, 26.1 ((CH3)3CSi); 18.6, 18.5 (CqSi); 17.2 (C-6); −3.5, −3.8, −4.5, −4.8 (CH3Si). IR: 2932, 2859, 2108, 1279, 1256, 1177, 1045. HRMS: [M + H–N2]+ calcd for C20H41F3NO4Si2 472.2521, found 472.2518.
2,2,2-Trifluoroethyl 2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α-l-fucopyranoside (D6)
The title product was obtained after column chromatography (hexane/Et2O, 1:0 → 9:1 v/v) in 90% yield (43 mg, 0.090 mmol). 1H NMR (400 MHz) δ: 7.39–7.25 (m, 5H, CHarom); 5.04 (d, 1H, J = 11.2 Hz, PhCHH); 4.96 (d, 1H, J = 3.6 Hz, H-1); 4.57 (d, 1H, J = 11.2 Hz, PhCHH); 4.10 (dd, 1H, J = 2.4 Hz, 10.2 Hz, H-3); 3.97–3.91 (m, 3H, H-5, CH2CF3); 3.81 (dd, 1H, J = 3.6 Hz, 10.0 Hz, H-2); 3.53 (d, 1H, J = 2.0 Hz, H-4); 1.20 (d, 3H, J = 6.4 Hz, H-6); 0.98 (d, 9H, (CH3)3CSi); 0.24, 0.20 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.4 (Cq,arom); 128.3, 128.1, 127.9 (CHarom); 123.6 (q, J = 277 Hz, CF3); 99.0 (C-1); 8.4 (C-4); 75.7 (PhCH2); 71.1 (C-3); 67.7 (C-5); 64.9 (q, J = 35 Hz, CH2CF3); 61.1 (C-2), 25.9 ((CH3)3CSi); 18.1 (CqSi); 16.5 (C-6); −4.1, −5.1 (CH3Si). IR (thin film) ν: 2930, 2859, 2108, 1279, 1261, 1163, 1121, 1084, 1045. HRMS: [M + NH4]+ calcd for C21H36F3N4O4Si 493.2452, found 493.2452.
Cyclohexyl 2-Azido-3,4-di-O-benzyl-2-deoxy-α/β-l-fucopyranoside (E1)
The title compounds (α/β 1:2) were obtained after column chromatography (hexane/Et2O 1:0 → 9:1 v/v) in 75% yield (34 mg, 0.075 mmol). 1H NMR (400 MHz) δ: 7.44–7.25 (m, 30H, CHarom); 5.02 (d, 1H, J = 3.6 Hz, H-1α); 4.94–4.91 (m, 3H, PhCHHα, PhCHHβ); 4.77–4.60 (m, 12H, PhCH2α, PhCH2β); 4.28 (d, 2H, J = 8.0 Hz, H-1β); 4.02–3.96 (m, 2H, H-3α, H-5α); 3.81–374 (m, 4H, H-2α, H-2β, H-4α); 3.65 (tt, 2H, J = 7.6 Hz, 9.6 Hz, CHCy); 3.58 (tt, 1H, J = 7.6 Hz, 9.6 Hz, CHCy); 3.51 (d, 2H, J = 2.4 Hz, H-4β); 3.38 (q, 2H, J = 6.4 Hz, H-5β); 3.26 (dd, 2H, J = 2.8 Hz, 10.4 Hz, H-3β), 1.90–1.62 (m, 12H, CH2,Cy); 1.50–1.34 (m, 10H, CH2,Cy); 1.25–1.15 (m, 15H, H-6α, H-6β, CH2,Cy). 13C-APT NMR (100 MHz) δ: 138.2, 137.8 (Cq,arom); 128.5, 128.5, 128.3, 128.2, 128.1, 127.9, 127.8, 127.7, 127.4, 127.6 (CHarom); 100.3 (C-1β); 96.6 (C-1α); 80.9 (C-3β); 77.6 (C-3α); 77.2 (CHCyβ); 76.2 (C-4α); 76.1 (CHCyα); 74.8 (C-4β); 74.5, 72.6, 72.2 (PhCH2); 70.4 (C-5β); 66.5 (C-5α); 63.2 (C-2β); 59.4 (C-2α); 33.3, 31.5, 31.4, 25.6, 25.5, 24.1, 23.9, 23.8 (CH2,Cy); 17.0 (C-6β); 16.7 (C-6α). IR (thin film) ν: 2932, 2855, 2106, 1454, 1359, 1107, 1067, 1038. HRMS: [M + NH4]+ calcd for C26H37N4O4 469.2809, found 469.2810.
Cyclohexyl 2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranoside (E2)
The title compounds (α/β 1:9) were obtained after column chromatography (hexane/EtOAc 1:0 → 9:1 v/v) in 38% yield (18 mg, 0.038 mmol). NMR data are reported only for the β-glycoside. 1H NMR (400 MHz) δ: 8.09–8.07 (m, 2H, CHarom); 7.89–7.85 (m, 2H, CHarom); 7.64–7.60 (m, 1H, CHarom); 7.52–7.45 (m, 3H, CHarom); 7.32 (t, 2H, J = 7.6 Hz, CHarom); 5.56 (d, 1H, J = 3.6 Hz, H-4); 5.15 (dd, 1H, J = 3.6 Hz, 10.8 Hz, H-3); 4.61 (d, 1H, J = 8.0 Hz, H-1); 3.95–3.88 (m, 2H, H-2, H-5); 3.79 (tt, 1H, J = 7.6 Hz, 9.6 Hz, CHCy); 2.03–2.01 (m, 2H, CH2,Cy); 1.81–1.79 (m, 2H, CH2,Cy); 1.57–1.43 (m, 3H, CH2,Cy); 1.37–1.22 (m, 8H, H-6, CH2,Cy). 13C-APT NMR (100 MHz) δ: 165.9, 165.4 (COBz); 133.4, 133.3, 129.9, 129.7 (CHarom); 129.3, 129.1 (Cq,arom); 128.5, 128.3 (CHarom); 100.5 (C-1); 78.3 (CHCy); 71.9 (C-3); 70.3 (C-4); 69.4 (C-5); 61.5 (C-2); 33.5, 31.6, 25.5, 24.1, 23.9 (CH2,Cy); 16.4 (C-6). IR (thin film) ν: 2934, 2857, 2110, 1724, 1450, 1281, 1263, 1173, 1107, 1096, 1069, 1026. HRMS: [M + Na]+ calcd for C26H29N3NaO6 502.1949, found 502.1948.
Cyclohexyl 2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α/β-l-fucopyranoside (E3)
The title compounds (α/β 1:4) were obtained after column chromatography (hexane/Et2O 1:0 → 9:1 v/v) in 71% yield (33 mg, 0.071 mmol). 1H NMR (400 MHz) δ: 8.14–8.07 (m, 2.5H, CHarom); 7.60–7.56 (m, 1.25H, CHarom); 7.48–7.44 (m, 2.5H, CHarom); 7.35–7.24 (m, 6.25H, CHarom); 5.70 (d, 0.25H, J = 2.4 Hz, H-4α); 5.52 (dd, 1H, J = 0.8 Hz, 3.2 Hz, H-4β); 5.11 (d, 0.25H, J = 3.6 Hz, H-1α); 4.84 (d, 0.25H, J = 10.4 Hz, PhCHHα); 4.78 (d, 1H, J = 11.6 Hz, PhCHHβ); 4.56–4.52 (m, 1.25H, PhCHH); 4.39 (d, 1H, J = 8.4 Hz, H-1β); 4.26 (q, 0.25H, J = 7.2 Hz, H-5α); 4.13 (dd, 0.25H, J = 3.6 Hz, 10.6 Hz, H-3α); 3.74–3.60 (m, 3.5H, H-2α, H-2β, H-5β, CHCy); 3.41 (dd, 1H, J = 3.2 Hz, 10.2 Hz, H-3β); 1.98–1.77 (m, 5H, CH2,Cy); 1.55–1.43 (m, 4H, CH2,Cy); 1.31–1.20 (m, 7.25H, H-6α, H-6β, CH2,Cy). 13C-APT (100 MHz) δ: 166.30 (COBz); 137.2 (Cq,arom); 133.3, 133.2, 130.1, 129.8 (CHarom); 129.5 (Cq,arom); 128.4, 128.4, 128.2, 128.1, 127.8, 127.8 (CHarom); 100.3 (C-1β); 96.7 (C-1α); 78.0 (CHCy); 77.5 (C-3β); 74.1 (C-3α); 71.5 (PhCH2β); 71.5 (PhCH2α); 70.1 (C-4α); 69.4 (C-5β); 69.0 (C-4β); 65.2 (C-5α); 62.9 (C-2β); 59.2 (C-2α); 33.5, 33.3, 31.6, 31.5, 25.5, 24.1, 23.9, 23.8 (CH2,Cy); 16.6 (C-6β); 16.3 (C-6α). IR (thin film) ν: 2922, 2110, 1720, 1446, 1265, 1107, 1068. HRMS: [M + H]+ calcd for C26H32N3O5 466.2337, found 466.2335.
Cyclohexyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranoside (E4)
The title compounds (α/β 1:4) were obtained after column chromatography (hexane/Et2O 1:0 → 9:1 v/v) in 71% yield (35 mg, 0.075 mmol). 1H NMR (400 MHz) δ: 8.10–8.06 (m, 10H, CHarom); 7.62–7.58 (m, 5H, CHarom); 7.48–7.45 (m, 11H, CHarom); 7.25–7.18 (m, 28H, CHarom); 5.59 (dd, 1H, J = 2.4 Hz, 11.2 Hz, H-3α); 5.13 (d, 1H, J = 3.2 Hz, H-1α); 4.93 (dd, 4H, J = 2.8 Hz, 10.8 Hz, H-3β); 4.71–4.69 (m, 5H, PhCHH); 4.57–4.52 (m, 5H, PhCHH); 4.47 (d, 4H, J = 8.0 Hz, H-1β); 4.19 (q, 1H, J = 6.4 Hz, H-5α); 3.98–3.93 (m, 5H, H-2β, H-4α); 3.88 (dd, 1H, J = 3.4 Hz, 11.2 Hz, H-2α); 3.76–3.70 (m, 8H, H-4β, CHCyβ); 3.66–3.61 (m, 5H, H-5β, CHCyα); 1.93–1.75 (m, 22H, CH2,Cy); 1.52–1.43 (m, 18H, CH2,Cy); 1.32–1.18 (m, 38H, H-6α, H-6β, CH2,Cy). 13C-APT NMR (100 MHz) δ: 165.8 (CO Bz); 137.6, 137.5 (Cq,arom); 133.5, 129.9 (CHarom); 129.2 (Cq,arom); 128.5, 128.3, 128.2, 128.2, 127.8, 127.8 (CHarom); 100.3 (H-1β); 96.7 (H-1α); 77.6 (CHCyβ); 77.4 (H-4α); 76.5 (CHCyα); 75.9 (C-4β); 75.5 (PhCH2α); 75.3 (PhCH2β); 74.8 (C-3β); 72.0 (C-3α); 70.4 (C-5β); 66.2 (C-5α); 61.4 (C-2β); 57.8 (C-2α); 33.4, 33.3, 31.4, 29.7, 25.5, 23.9, 23.8 (CH2,Cy); 16.7 (C-6β); 16.4 (C-6α). IR (thin film) ν: 2932, 2857, 2108, 1452, 1265, 1173, 1096, 1069, 1038, 1026. HRMS: [M + NH4]+ calcd for C26H35N4O5 483.2602, found 483.2602.
Cyclohexyl 2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranoside (E5)
The products (α/β 1:3) were obtained after column chromatography (hexane/Et2O, 1:0 → 9:1) in 80% yield (40 mg, 0.080 mmol). 1H NMR (400 MHz) δ: 5.04 (d, 1H, J = 3.6 Hz, H-1α); 4.28 (d, 3H, J = 7.6 Hz, H-1β); 4.05 (dd, 1H, J = 2.4 Hz, 10.4 Hz, H-3α); 3.94 (q, 1H, J = 6.8 Hz, H-5α); 3.69–3.56 (m, 6H, H-2α, H-4α, OCHCyα, OCHCyβ); 3.55 (d, 3H, J = 2.4 Hz, H-4β); 3.51 (dd, 3H, J = 8.0 Hz, 10.2 Hz, H-2β); 3.42 (q, 3H, J = 6.4 Hz, H-5β); 3.33 (dd, 3H, J = 2.4 Hz, 10.4 Hz, H-3β); 1.97–1.25 (m, 40H, CH2,Cy); 1.22 (d, 9H, J = 6.4 Hz, H-6β); 1.17 (d, 3H, J = 6.4 Hz, H-6α); 0.96–0.90 (m, 72H, (CH3)3CSi); 0.18–0.08 (m, 48H, CH3Si). 13C-APT NMR (100 MHz) δ: 100.6 (C-1β); 96.3 (C-1α); 77.4 (CHCyβ); 75.6 (CHCyα); 74.5 (C-3β); 74.0 (C-4β); 71.2 (C-3α); 71.0 (C-5β); 67.8 (C-5α); 64.2 (C-2β); 60.9 (C-2α); 33.5, 33.3, 31.8, 31.4 (CH2,Cy); 26.2, 26.1 ((CH3)CSi); 25.7, 25.6, 24.1, 24.0, 23.9, 23.7 (CH2,Cy); 18.6, 18.5 (CqSi); 17.7 (C-6β); 17.3 (C-6α); −3.4, −3.6, −4.3, −4.4, −4.5, −4.7 (CH3Si). IR (thin film) ν: 2930, 2857, 2110, 1252, 1115, 1069, 1026. HRMS: [M + Na]+ calcd for C24H49N3O4Si2Na 522.3154, found 522.3151.
Cyclohexyl 2-Azido-4-O-benzyl-3-O-(tert-butyldimethylsilyl)-2-deoxy-α/β-l-fucopyranoside (E6)
The title compounds (α/β 1:2) were obtained after column chromatography (hexane/Et2O, 1:0 → 19:1) in 80% yield (38 mg, 0.080 mmol). 1H NMR (400 MHz) δ: 7.39–7.26 (m, 15H, CHarom); 5.03–5.01 (m, 4H, H-1α, PhCHHα, PhCHHβ); 4.61–4.55 (m, 3H, PhCHHα, PhCHHβ); 4.28 (d, 2H, J = 8.0 Hz, H-1β); 4.14 (broad doublet, J = 8.4 Hz, H-3α); 4.00 (q, 1H, J = 6.4 Hz, H-5α); 3.67–3.43 (m, 11H, H-2α, H-2β, H-3β, H-4α, H-5β, OCHCyα, OCHCyβ); 3.36 (bs, 2H, H-4β); 1.89–1.11 (m, 39H, CH2,Cyα/β, H-6α, H-6β); 0.98 (s, 9H, (CH3)3CSiα, 0.96 (s, 18H, (CH3)3CSiβ); 0.23–0.15 (m, 18H, CH3Siα/β).13C-APT NMR (100 MHz) δ: 138.7 (Cq,arom); 128.2, 128.2, 128.1, 127.9,127.5, 127.5 (CHarom); 100.4 (C-1β); 96.6 (C-1α); 81.0 (C-4α); 79.2 (C-4β); 77.3 (OCHCyβ); 76.0 (OCHCyα); 75.6 (PhCH2α); 75.3 (PhCH2β); 74.9 (C-3β or C-5β); 71.3 (C-3α); 70.2 (C-3β or C-5β); 66.6 (C-5α); 64.9 (C-2β); 61.2 (C-2α); 33.4, 33.3, 31.5 (CH2,Cy); 25.9, 25.6 (CH3)3CSiα/β); 24.1, 23.9, 23.8 (CH2,Cy); 16.9 (C-6β); 16.7 (C-6α); −3.9, −4.3, −4.7, −5.0 (CH3Si). IR (thin film) ν: 2930, 2857, 2108, 1254, 1115, 1067, 1040. HRMS: [M + NH4]+ calcd for C25H45N4O4Si 493.3205, found 493.3202.
Methyl 2-O-(2-Azido-3,4-di-O-benzyl-2-deoxy-α/β-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F1)
The product was obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) in 68% yield (49 mg, 0.068 mmol). NMR data are reported for the α-linked fucoside only. 1H NMR (400 MHz) δ: 7.53–7.50 (m, 2H, CHarom); 7.44–7.23 (m, 18H, CHarom); 5.64 (s, 1H, PhCH); 4.95 (d, 1H, J = 3.6 Hz, H-1′); 4.90 (d, 1H, J = 11.2 Hz, PhCHH); 4.79–4.69 (m, 5H, H-1, PhCH2); 4.59 (d, 1H, J = 11.6 Hz, PhCHH); 4.37 (q, 1H, J = 6.4 Hz, H-5′); 4.25 (dd, 1H, J = 4.8 Hz, 10.2 Hz, H-6); 4.19–4.13 (m, 3H, H-2, H-4, H-3′); 3.98 (dd, 1H, J = 3.6 Hz, 10.0 Hz, H-3); 3.89 (t, 1H, J = 10.4 Hz, H-6); 3.78 (dt, 1H, J = 4.8 Hz, 9.2 Hz, H-5); 3.74 (d, 1H, J = 1.2 Hz, H-4′); 3.70 (dd, 1H, J = 3.2 Hz, 10.8 Hz, H-2′); 3.36 (s, 3H, OCH3); 1.70 (d, 3H, J = 6.8 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 138.2, 137.6, 137.5 (Cq,arom); 128.8, 128.5, 128.3, 128.2, 128.2, 128.1, 127.9, 127.9, 127.7, 127.6, 127.5, 126.1 (CHarom); 101.5 (PhCH); 98.8 (C-1); 97.3 (C-1′); 78.5 (C-4); 76.5 (C-2 or C-3′); 76.0 (C-4′); 75.0 (PhCH2); 74.8 (C-3); 73.5 (C-2 or C-3′); 72.7, 71.9 (PhCH2); 68.8 (C-6); 67.1 (C-5′); 64.1 (C-5); 58.9 (C-2′); 55.0 (OCH3); 16.7 (C-6′). 13C-GATED (100 MHz) δ: 98.8 (C,HJ = 168 Hz, C-1); 97.3 (C,HJ = 170 Hz, C-1′). IR (thin film) ν: 2909, 2108, 1454, 1371, 1101, 1059, 1040, 1003. HRMS: [M + Na]+ calcd for C41H45N3O9Na 746.3048, found 746.3048.
Methyl 2-O-(2-Azido-3,4-di-O-benzoyl-2-deoxy-α/β-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F2)
The title compounds (α/β 4:1) were obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v), followed by column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 38% yield (29 mg, 0.038 mmol). 1H NMR (400 MHz, for the α-isomer) δ: 8.12–8.09 (m, 2H, CHarom); 7.60–7.22 (m, 18H, CHarom); 5.72–5.69 (m, 2H, H-3′, PhCH); 5.03 (d, 1H, J = 3.6 Hz, H-1′); 4.85 (d, 1H, J = 12.0 Hz, PhCHH); 4.77 (d, 1H, J = 1.2 Hz, H-1); 4.71–4.65 (m, 2H, PhCH2); 4.56 (q, 1H, J = 6.4 Hz, H-5′); 4.51 (d, 1H, J = 11.2 Hz, PhCHH); 4.30–4.20 (m, H-4, H-6); 4.17 (dd, 1H, J = 1.6 Hz, 3.2 Hz, H-2); 3.98 (dd, 1H, J = 3.6 Hz, 10.2 Hz, H-3); 3.92–3.87 (m, 2H, H-4′, H-6); 3.85–3.75 (m, 2H, H-2′, H-5); 3.76 (s, 3H, OCH3); 0.98 (d, 3H, J = 6.4 Hz, H-6′). Diagnostic peaks for the β-anomer: 5.62 (s, 0.25H, PhCH); 3.54 (q, 0.25H, J = 6.4 Hz, H-5′), 1.22 (d, 0.75H, J = 6.4 Hz, H-6′). 13C-APT NMR 100 MHz, for the α-isomer) δ: 165.8 (COBz); 137.7, 137.4 (Cq,arom); 135.5, 129.9 (CHarom); 129.2 (Cq,arom); 128.7, 128.5, 128.2, 128.1, 128.1, 127.8, 127.6, 127.5, 127.3, 127.1, 126.0 (CHarom); 101.4 (PhCH); 98.9 (C-1); 97.4 (C-1′); 78.8 (C-4); 77.5 (C-4′); 75.6 (PhCH2); 74.6 (C-3); 74.3 (C-2); 73.0 (PhCH2); 71.2 (C-3′); 68.7 (C-6); 66.7 (C-5′); 64.1 (C-5); 57.6 (C-2′); 54.9 (OCH3); 16.1 (C-6′). IR (thin film) ν: 2936, 2110, 1726, 1452, 1273, 1261, 1101, 1070, 1045, 1026, 1006. HRMS: [M + Na]+ calcd for C41H41N3O11Na 774.2631, found 774.2633.
Methyl 2-O-(2-Azido-4-O-benzoyl-3-O-benzyl-2-deoxy-α/β-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F3)
The title compound (α/β 10:1) was obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 58% yield (43 mg, 0.058 mmol). NMR data are reported for the α-isomer only. 1H NMR (400 MHz) δ: 8.07–8.05 (m, 2H, CHarom); 7.58–7.25 (m, 18H, CHarom); 5.66 (s, 1H, PhCH); 5.65 (d, 1H, J = 2.4 Hz, H-4′); 5.03 (d, 1H, J = 3.2 Hz, H-1′); 4.88–4.80 (m, 2H, PhCHH 2×, H-1); 4.72 (d, 1H, J = 12.0 Hz, PhCHH); 4.65 (q, 1H, J = 6.4 Hz, H-5′); 4.54 (d, 1H, J = 10.8 Hz, PhCHH); 4.29–4.23 (m, 2H, H-6, H-3′); 4.20–4.15 (m, 2H, H-2, H-4); 4.02 (dd, 1H, J = 3.2 Hz, 10.0 Hz, H-3); 3.90 (t, 1H, J = 10.4 Hz, H-6); 3.80 (dt, 1H, J = 4.8 Hz, 9.6 Hz, H-5); 3.59 (dd, 1H, J = 3.6 Hz, 10.8 Hz, H-2′); 3.39 (s, 3H, OCH3); 1.04 (d, 3H, J = 6.8 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 166.1 (COBz); 138.1, 137.6, 137.1 (Cq,arom); 133.2, 130.0, 129.8 (CHarom); 129.6 (Cq,arom); 128.9, 128.4, 128.4, 128.3, 128.2, 128.2, 127.8, 127.8, 127.7, 127.1, 126.1 (CHarom); 101.5 (PhCH); 98.8 (C-1); 97.3 (C-1′); 78.7 (C-2 or C-4); 74.5 (C-3); 74.2 (C-2 or C-4); 73.2 (C-3′); 73.1 (PhCH2); 71.3 (PhCH2); 69.8 (C-4′); 68.8 (C-6); 65.7 (C-5′); 64.1 (C-5); 58.7 (C-2′); 55.0 (OCH3); 16.2 (C-6′). IR (thin film) ν: 2932, 2108, 1721, 1452, 1373, 1267, 1175, 1101, 1074, 1061, 1045, 1026, 1003. HRMS: [M + Na]+ calcd for C41H43N3O10Na 760.2841, found 760.2839.
Methyl 2-O-(2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F4)
The disaccharides (α/β 4:1) were isolated after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 68% yield (50 mg, 0.068 mmol). 1H NMR (400 MHz, for the α-anomer) δ: 8.11 (d, 2H, J = 7.2 Hz, CHarom); 7.60–7.22 (m, 18H, CHarom); 5.72–5.69 (m, 2H, H-3′, PhCH); 5.03 (d, 1H, J = 3.6 Hz, H-1′); 4.85 (d, 1H, J = 4.85 Hz, PhCHH); 4.70 (d, 1H, J = 1.2 Hz, H-1); 4.70–4.65 (m, 2H, PhCH2); 4.56 (q, 1H, J = 6.4 Hz, H-5′); 4.51 (d, 1H, J = 11.2 Hz, PhCHH); 4.30–4.20 (m, 2H, H-4, H-6); 4.17 (dd, 1H, J = 1.6 Hz, 3.2 Hz, H-2); 3.98 (dd, 1H, J = 3.2 Hz, 10.2 Hz, H-3); 3.92–3.87 (m, 2H, H-4′, H-6); 3.92–3.75 (m, 2H, H-2′, H-5); 3.38 (s, 3H, OCH3); 0.98 (d, 3H, J = 6.4 Hz, H-6′). Diagnostic peaks for the β-anomer: 5.62 (s, 0.25H, PhCH); 3.54 (q, 0.25H, J = 6.4 Hz, H-5′); 3.35 (s, 0.75H, OCH3); 1.22 (d, 0.75H, J = 6.4 Hz, H-6′). 13C-APT NMR (100 MHz, for the α-anomer) δ: 165.8 (COBz); 138.4, 137.7, 137.4 (Cq,arom); 133.5, 129.9 (CHarom); 129.2 (Cq,arom); 128.8, 128.5, 128.2, 128.1, 127.8, 127.6, 127.5, 127.3, 127.1, 126.0 (CHarom); 101.4 (PhCH); 98.9 (C-1); 97.4 (C-1′); 78.8 (C-4); 77.5 (C-4′); 75.6 (PhCH2); 74.6 (C-3); 74.3 (C-2); 73.0 (PhCH2); 71.2 (C-3′); 68.7 (C-6); 66.7 (C-5′); 64.1 (C-5); 57.6 (C-2′); 54.9 (OCH3); 16.1 (C-6′). IR (thin film) ν: 2934, 2909, 2110, 1722, 1452, 1373, 1269, 1103, 1074, 1043, 1028. HRMS: [M + Na]+ calcd for C41H43N3O10Na 760.2841, found 760.2838.
Methyl 2-O-(2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F5)
The title compound was obtained after column chromatography (hexane/Et2O, 1:0 → 9:1) as the sole product in 67% yield (52 mg, 0.067 mmol). 1H NMR (400 MHz) δ: 7.50–7.49 (m, 2H, CHarom); 7.39–7.25 (m, 13H, CHarom); 5.59 (s, 1H, PhCH); 4.95 (d, 1H, J = 3.6 Hz, H-1′); 4.80 (d, 1H, J = 12.4 Hz, PhCHH); 4.74–4.70 (m, 2H, H-1, PhCHH); 4.29–4.25 (m, H-5′, H-6); 4.17 (dd, 1H, J = 2.4 Hz, 10.4 Hz, H-3′); 4.12–4.05 (m, H-2, H-4); 3.96 (dd, 1H, J = 3.2 Hz, 9.8 Hz, H-3); 3.86–3.76 (m, 2H, H-5, H-6); 3.71 (dd, 1H, J = 3.2 Hz, 10.4 Hz, H-2′); 3.61 (d, 1H, J = 1.6 Hz, H-4′); 3.36 (s, 3H, OCH3); 1.01–0.99 (m, 12H, H-6′, (CH3)3CSi); 0.91 (s, 9H, (CH3)3CSi); 0.22, 0.16, 0.15, 0.14 (4x s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.4, 137.6 (Cq,arom); 128.8, 128.3, 128.1, 127.5, 127.4, 126.1 (CHarom); 101.5 (PhCH); 99.1 (C-1); 97.3 (C-1′); 79.0 (C-4); 75.3 (C-4′); 74.5 (C-3); 73.6 (C-2); 72.3 (PhCH2); 70.9 (C-3′); 69.0 (C-6); 68.4 (C-5′); 64.0 (C-5); 60.9 (C-2′); 55.0 (OCH3); 26.2, 26.1 ((CH3)3CSi); 18.6, 18.6 (CqSi); 17.2 (C-6′); −3.4, −3.4, −4.6, −4.7 (CH3Si). IR (thin film) ν: 2930, 2857, 2108, 1254, 1177, 1103, 1061, 1042, 1028, 1004. HRMS: [M + Na]+ calcd for C39H61N3O9Si2Na 794.3839, found 794.3839.
Methyl 2-O-(2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α-l-fucopyranosyl)-3-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (F6)
The title disaccharide was isolated after column chromatography (hexane/Et2O, 1:0 → 4:1) as the sole product in 74% yield (55 mg, 0.074 mmol). 1H NMR (400 MHz) δ: 7.51–7.50 (m, 2H, CHarom); 7.40–7.24 (m, 13H, CHarom); 5.62 (s, 1H, PhCH); 5.00 (d, 1H, J = 11.2 Hz, PhCHH); 4.95 (d, 1H, J = 3.2 Hz, H-1′); 4.81–4.70 (m, 3H, H-1, PhCH2); 4.53 (d, 1H, J = 11.2 Hz, PhCHH); 4.37 (q, 1H, J = 6.4 Hz, H-5′); 4.29–4.24 (m, 2H, H-3′, H-6); 4.14–4.09 (m, 2H, H-2, H-4); 3.97 (dd, 1H, J = 3.2 Hz, 10.0 Hz, H-3); 3.86 (t, 1H, J = 10.4 Hz, H-6); 3.78 (dt, 1H, J = 4.4 Hz, 9.6 Hz, H-5); 3.64 (dd, 1H, J = 3.2 Hz, 10.4 Hz, H-2′); 3.44 (d, 1H, J = 2.4 Hz, H-4′); 3.36 (s, 3H, OCH3); 1.04 (d, 3H, J = 6.4 Hz, H-6′); 0.99 (s, 9H, (CH3)3CSi); 0.26 (s, 3H, CH3Si); 0.21 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.6, 138.3, 137.6 (Cq,arom); 128.8, 128.3, 128.2, 128.1, 127.8, 127.6, 127.5, 127.0, 126.1 (CHarom); 101.5 (PhCH); 98.9 (C-1); 97.4 (C-1′); 81.0 (C-4′); 78.8 (C-4); 75.6 (PhCH2); 74.6 (C-3); 73.5 (C-2); 72.5 (PhCH2); 70.7 (C-3′); 68.9 (C-6); 67.1 (C-5′); 64.1 (C-5); 61.0 (C-2′); 55.0 (OCH3); 25.8 ((CH3)3CSi); 18.1 (CqSi); 16.6 (C-6′); −3.6, −5.0 (CH3Si). IR (thin film) ν: 2928, 2857, 2106, 1454, 1371, 1258, 1171, 1101, 1040, 1004. HRMS: [M + Na]+ calcd for C40H53N3O9SiNa 770.3443, found 770.3441.
Methyl 3-O-(2-Azido-3,4-di-O-benzyl-2-deoxy-α-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G1)
The title compound was obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 9:1 v/v) in 72% yield (52 mg, 0.072 mmol). 1H NMR (400 MHz) δ: 7.39–7.22 (m, 20H, CHarom); 4.95–4.91 (m, 2H, H-1′, PhCHH); 4.86 (d, 1H, J = 11.6 Hz, PhCHH); 4.72 (d, 1H, J = 12.0 Hz, PhCHH); 4.69–4.63 (m, 3H, H-1, PhCH2); 4.53 (d, 1H, J = 11.6 Hz, PhCHH); 4.23 (dd, 1H, J = 4.0 Hz, 9.6 Hz, H-6); 4.16–4.09 (m, 3H, H-3, H-4, H-5′); 4.00 (m, 2H, H-2′, H-3′); 3.88–3.77 (m, 3H, H-2, H-5, H-6); 3.56 (s, 1H, H-4′); 3.31 (s, 3H, OCH3); 0.88 (d, 3H, J = 6.4 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 138.3, 138.1, 137.7, 137.6 (Cq,arom); 128.9. 128.6. 128.4, 128.4, 128.2, 128.1, 127.8, 127.8, 127.6, 127.4, 126.2, 125.9 (CHarom); 101.8 (PhCH); 100.3 (C-1); 95.7 (C-1′); 78.3 (C-3′); 77.1 (C-3 or C-4); 76.3 (C-4′); 75.4 (C-2); 74.8, 73.8 (PhCH2); 73.4 (C-2); 72.1 (PhCH2); 68.9 (C-6); 66.6 (C-5′); 64.2 (C-5); 59.9 (C-2′); 54.8 (OCH3); 16.3 (C-6′). IR (thin film) ν: 2930, 2108, 1454, 1098, 1057, 1026. HRMS: [M + Na]+ calcd for C41H45N3O9Na 746.3048, found 746.3041.
Methyl 3-O-(2-Azido-3,4-di-O-benzoyl-2-deoxy-α-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G2)
The title compound was obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 64% yield (48 mg, 0.064 mmol). 1H NMR (400 MHz) δ: 8.00–7.98 (m, 2H, CHarom); 7.90–7.87 (m, 2H, CHarom); 7.60–7.24 (m, 16H, CHarom); 5.79 (dd, 1H, J = 3.2 Hz, 10.0 Hz, H-3′); 5.64 (s, 1H, PhCH); 5.55 (d, 1H, J = 2.0 Hz, H-4′); 5.14 (d, 1H, J = 3.6 Hz, H-1′); 4.96 (d, 1H, J = 12.0 Hz, PhCHH); 4.78 (d, 1H, J = 12.0 Hz, PhCHH); 4.70 (d, 1H, J = 1.6 Hz, H-1); 4.57 (q, 1H, J = 6.4 Hz, H-5′); 4.27 (dd, 1H, J = 4.4 Hz, 9.6 Hz, H-6); 4.22–4.18 (m, 2H, H-3, H-4); 4.10 (dd, 1H, J = 3.6 Hz, 11.0 Hz, H-2′); 3.93–3.88 (m, 3H, H-2, H-5, H-6); 3.35 (s, 3H, OCH3); 0.76 (d, 3H, J = 6.4 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 165.7, 165.3 (COBz); 138.1, 137.4 (Cq,arom); 133.3, 133.2, 129.7 (CHarom); 129.5, 129.2 (Cq,arom); 129.1, 128.5, 128.4, 128.3, 128.2, 128.0, 127.7, 126.3 (CHarom); 102.1 (PhCH); 100.4 (C-1); 96.3 (C-1′) 77.1 (C-3 or C-4); 75.8 (C-2); 74.8 (C-3 or C-4); 73.9 (PhCH2); 71.4 (C-4′); 70.1 (C-3′); 68.8 (C-6); 65.2 (C-5′); 64.4 (C-5); 58.8 (C-2′); 54.9 (OCH3); 15.4 (C-6′). IR (thin film) ν: 2932, 2108, 1724, 1452, 1275, 1261, 1098, 1065, 1026, 1005. HRMS: [M + Na]+ calcd for C41H41N3O11Na 774.2633, found 774.2629.
Methyl 3-O-(2-Azido-4-O-benozyl-3-O-benzyl-2-deoxy-α-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G3)
The title compound was obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 54% yield (40 mg, 0.054 mmol). 1H NMR (400 MHz) δ: 8.08–8.06 (m, 2H, CHarom); 7.61–7.59 (m, 1H, CHarom); 7.47–7.19 (m, 17H, CHarom); 5.59 (s, 1H, PhCH); 5.52 (d, 1H, J = 2.8 Hz, H-4′); 5.02 (d, 1H, J = 3.6 Hz, H-1′); 4.94 (d, 1H, J = 11.6 Hz, PhCHH); 4.81–4.75 (m, 2H, PhCH2); 4.68 (d, 1H, J = 1.2 Hz, H-1); 4.50 (d, 1H, J = 10.8 Hz, PhCHH); 4.41 (q, 1H, J = 6.4 Hz, H-5′); 4.25 (dd, 1H, J = 3.6 Hz, 9.4 Hz, H-6); 4.17–4.12 (m, H-3, H-4, H-3′); 3.91–3.80 (m, 4H, H-2, H-5, H-6, H-2′); 3.33 (s, 3H, OCH3); 0.83 (d, 3H, J = 6.4 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 166.1 (COBz); 138.1, 137.6, 137.1 (Cq,arom); 133.2, 129.8 (CHarom); 129.7 (Cq,arom); 129.2, 128.4, 128.3, 128.3, 128.1, 127.8, 127.8, 26.2 (CHarom); 102.1 (PhCH); 100.4 (C-1); 96.0 (C-1′); 77.1, 75.6, 75.0, 74.2 (C-2, C-3, C-4, C-3′); 73.8, 71.4 (PhCH2); 70.0 (C-4′); 68.9 (C-6); 65.2 (C-5′); 64.3 (C-5); 59.6 (C-2′); 54.9 (OCH3); 15.8 (C-6′). IR (thin film) ν: 2930, 2110, 1721, 1454, 1269, 1110, 1099, 1059, 1026, 1003. HRMS: [M + Na]+ calcd for C41H43N3O10Na 760.2841, found 760.2839.
Methyl 3-O-(2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-α/β-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G4)
The products (α/β 10:1) were obtained after size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) and column chromatography (toluene/acetone, 1:0 → 49:1 v/v) in 64% yield (47 mg, 0.064 mmol). 1H NMR (400 MHz) δ: 8.08–8.06 (m, 2H, CHarom); 7.61–7.17 (m, 18H, CHarom); 5.61–5.57 (m, 2H, H-3′, PhCH); 5.03 (d, 1H, J = 3.6 Hz, H-1′); 4.93 (d, 1H, J = 12.0 Hz, PhCHH); 4.73 (d, 1H, J = 12.0 Hz, PhCHH); 4.66 (d, 1H, J = 1.6 Hz, H-1); 4.58 (d, 1H, J = 11.2 Hz, PhCHH); 4.46 (d, 1H, J = 11.2 Hz, PhCHH); 4.32 (q, 1H, J = 6.4 Hz, H-5′); 4.25 (dd, 1H, J = 4.0 Hz, 9.8 Hz, H-6); 4.21–4.13 (m, 3H, H-3, H-4, H-2′); 3.88 (t, 1H, J = 10.0 Hz, H-6); 3.83–3.78 (m, 3H, H-2, H-5, H-4′); 3.32 (s, 3H, OCH3); 0.82 (d, 3H, J = 6.4 Hz, H-6′). 13C-APT NMR (100 MHz) δ: 165.8 (COBz); 138.1, 137.6 (Cq,arom); 133.5, 129.8 (CHarom); 129.3 (Cq,arom); 128.9, 128.7, 128.5, 128.3, 128.2, 128.1, 128.0, 127.8, 127.7 (CHarom); 102.0 (PhCH); 100.4 (C-1); 96.2 (C-1′); 77.5 77.1, 75.7, 74.2 (C-2, C-3, C-4, C-4′); 75.6, 73.8 (PhCH2); 73.0 (C-3′); 68.8 (C-6); 66.4 (C-5′); 64.3 (C-5); 58.8 (C-2′); 54.8 (OCH3; 15.8 (C-6′). IR (thin film) ν: 2932, 2108, 1722, 1452, 129, 1098, 1067, 1026. [M + Na]+ calcd for C41H43N3O10 760.2841, found 760.2836.
Methyl 3-O-(2-Azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G5)
The title product was obtained after column chromatography (hexane/Et2O, 1:0 → 4:1) in 73% yield (56 mg, 0.073 mmol). 1H NMR (400 MHz) δ: 7.45–7.25 (m, 10H, CHarom); 5.58 (s, 1H, PhCH); 4.98–4.95 (m, 2H, H-1, PhCHH), 4.78 (d, 1H, J = 11.6 Hz, PhCHH); 4.69 (d, 1H, J = 1.6 Hz, H-1); 4.24 (dd, 1H, J = 4.0 Hz, 9.6 Hz, H-6); 4.14–4.12 (m, 2H, H-3, H-4); 4.07–4.02 (m, 2H, H-3′, H-5′); 3.88–3.82 (m, H-2, H-2′, H-5, H-6); 3.55 (d, 1H, J = 1.6 Hz, H-4′); 3.32 (s, 3H, OCH3); 0.94 (s, 9H, (CH3)3CSi); 0.90 (s, 9H, (CH3)3CSi); 0.79 (d, 3H, J = 6.4 Hz, H-6′); 0.16, 0.13, 0.13, 0.03 (4x s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.4, 137.8 (Cq,arom); 128.9, 128.3, 128.1, 127.9, 127.7, 126.2, 125.8 (CHarom); 101.9 (PhCH); 100.7 (C-1); 96.1 (C-1′); 77.3 (C-3, C-4); 75.7 (C-2, C-5); 75.3 (C-4′); 73.9 (C-3, C-4); 73.9 (PhCH2); 71.4 (C-3′, C-5′); 68.9 (C-6); 68.0 (C-3′, C-5′); 64.4 (C-2, C-5); 61.8 (C-2′); 54.8 (OCH3); 26.3, 26.1 ((CH3)3CSi); 18.6, 18.5 (CqSi); 16.8 (C-6′); −3.4, −4.0, −4.5, −4.6 (CH3Si). IR (thin film) ν: 2953, 2857, 2106, 1254, 1179, 1121, 1099, 1049, 1028. HRMS: [M + Na]+ calcd for C39H61N3O9Si2Na 794.3839, found 794.3839.
Methyl 3-O-(2-Azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α/β-l-fucopyranosyl)-2-O-benzyl-4,6-O-benzylidene-α-d-mannopyranoside (G6)
The title compounds (α/β 10:1) were obtained after column chromatography (hexane/Et2O, 1:0 → 4:1) in 64% yield (48 mg, 0.064 mmol). NMR data are reported for the α-isomer only. 1H NMR (400 MHz) δ: 7.46–7.24 (m, 15H, CHarom); 5.58 (s, 1H, PhCH); 5.00–4.92 (m, 3H, H-1′, 2× PhCHH); 4.78 (d, 1H, J = 11.6 Hz, PhCHH); 4.70 (d, 1H, J = 1.6 Hz, H-1); 4.50 (d, 1H, J = 10.8 Hz, PhCHH); 4.42 (dd, 1H, J = 4.0 Hz, 9.6 Hz, H-6); 4.16–4.06 (m, 4H, H-3, H-3′, H-4, H-5′); 3.90 (dd, 1H, J = 3.2 Hz, 10.2 Hz, H-2′); 3.88–3.79 (m, 3H, H-2, H-5, H-6); 3.36–3.32 (m, 4H, H-4′, OCH3); 0.96 (s, 9H, (CH3)3Si); 0.80 (d, 3H, J = 6.4 Hz, H-6′); 0.21 (s, 3H, CH3Si); 0.15 (s, 3H, CH3Si). 13C-APT NMR (100 MHz) δ: 138.7, 138.3, 137.7 (Cq,arom); 128.9, 128.6, 128.4, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 127.5, 127.4, 126.2, 125.8 (CHarom); 101.9 (PhCH); 100.5 (C-1); 96.5 (C-1′); 81.0 (C-4′); 77.2 (C-4); 75.8 (C-2); 75.6 (PhCH2); 74.4 (C-3 or C-3′); 73.8 (PhCH2); 71.8 (C-3 or C-3′); 68.9 (C-6); 66.9 (C-5′); 63.3 (C-5); 62.3 (C-2′); 54.8 (OCH3); 25.9 ((CH3)3CSi); 18.0 (CqSi); 16.1 (C-6′); −4.2, −5.0 (CH3Si). IR (thin film) ν: 2930, 2857, 2106, 1454, 1364, 1260, 1117, 1098, 1047, 1028. HRMS: [M + Na]+ calcd for C40H53N3NaO9Si 770.3443, found 770.3442.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl methyl(2-azido-4-O-(2-azido-4-O-benzyl-2-deoxy-3-O-(tert-butyldimethylsilyl)-α-d-fucopyranosyl)-3-O-benzyl-2-deoxy-α-d-galactopyranosiduronate) (20)
To a solution of d-6 (0.29 g, 0.54 mmol, 2.0 equiv), Ph2SO (0.11 g, 0.54 mmol, 2.0 equiv), and TTBP (0.27 g, 1.08 mmol, 4.0 equiv) in CH2Cl2 (5.4 mL, 0.1 M relative to donor) were added flame-dried, rod-shaped, 3 Å MS. After ∼30 min, the mixture was cooled to −80 °C, Tf2O (91 μL, 0.54 mmol, 2.0 equiv) was added, and the mixture was warmed to −70 °C. The mixture was recooled to −80 °C, and a solution of acceptor 19 (0.17 g, 0.27 mmol, 1.0 equiv) in CH2Cl2 (0.54 mL, 0.5 M) was added via the wall of the flask. The mixture was warmed to −40 °C and kept at this temperature for ∼2 h, after which the reaction was quenched by addition of pyridine (0.4 mL), filtered over a pad of Celite, washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (hexane/EtOAc, 19:1 → 7:3 v/v) yielded the title disaccharide in 68% yield (0.17 g, 0.167 mmol). 1H NMR (500 MHz, 323 K) δ: 7.43–7.23 (m, 20H, CHarom); 5.16 (s, 2H, PhCH2); 5.05 (d, 1H, J = 3.0 Hz, H-1); 4.99–4.97 (m, 2H, H-1′, PhCHH); 4.82 (d, 1H, J = 11.5 Hz, PhCHH); 4.66 (d, 1H, J = 12.0 Hz, PhCHH); 4.60 (s, 1H, H-4); 4.51–4.48 (m, 3H, PhCH2); 4.31 (s, 1H, H-5); 4.17 (q, 1H, J = 6.5 Hz, H-5′); 4.07 (dd, 1H, J = 2.5 Hz, 10.5 Hz, H-3′); 3.90 (bd, 1H, J = 10.5 Hz, H-3); 3.81 (s, 3H, CO2CH3); 3.74 (dd, 1H, J = 3.5 Hz, 10.5 Hz, H-2′); 3.63 (bs, 1H, OCHHpentyl); 3.61 (dd, 1H, J = 3.5 Hz, 10.5 Hz, H-2); 3.47 (bs, 1H, OCHHpentyl); 3.40 (s, 1H, H-4′); 3.22 (bs, 2H, NCH2,pentyl); 1.52 (bs, 4H, CH2,pentyl); 1.34–1.27 (m, 2H, CH2,pentyl); 0.99–0.93 (m, 12H, H-6′, C(CH3)3Si); 0.21, 0.18 (s, 3H, CH3Si). 13C-APT NMR (125 MHz, 323 K) δ: 168.6 (C-6); 138.7, 138.0, 137.3 (Cq,arom); 128.8, 128.5, 128.4, 128.4, 128.2, 127.9, 127.8, 127.8, 127.7, 127.5, 127.2 (CHarom); 99.2 (C-1′); 98.2 (C-1); 81.0 (C-4); 75.6 (PhCH2); 75.0 (C-3); 73.8 (C-4); 72.0 (PhCH2); 71.1 (C-3′); 70.1 (C-5); 68.8 (OCH2,pentyl); 67.6 (C-5′); 67.1 (PhCH2); 62.0 (C-2′); 59.4 (C-2); 52.4 (CO2CH3); 50.5 (PhCH2); 29.0 (CH2,pentyl); 25.9 (C(CH3)3Si); 23.2 (CH2,pentyl); 18.1 (CqSi); 16.5 (C-6′); −3.9, −5.0 (CH3Si). 13C-GATED NMR (125 MHz, 323 K) δ: 99.2 (d, J = 170 Hz, C-1′); 98.2 (d, J = 172 Hz, C-1). IR (thin film) ν: 2930, 2106, 1730, 1697, 1454, 1254, 1125, 1067, 1042. HRMS: [M + Na]+ calcd for C53H69N7NaO11Si 1030.4717, found 1030.4721.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-4-O-benzyl-2-deoxy-α-d-fucopyranoside (23)
To a stirred solution of donor d-6 (0.80 g, 1.5 mmol, 1.0 equiv), Ph2SO (0.39 g, 1.95 mmol, 1.3 equiv), N-methylmaleimide (0.25 g, 2.25 mmol, 1.5 equiv), and TTBP (0.93 g, 3.75 mmol, 2.5 equiv) in CH2Cl2 (30 mL, 0.05 M) were added, flame-dried, rod-shaped, 3 Å MS. After being stirred for ∼30 min, the mixture was cooled to −80 °C, Tf2O (0.33 mL, 1.95 mmol, 1.3 equiv) was added, and the mixture was warmed to −70 °C, after which TLC analysis (toluene/EtOAc, 9:1 v/v) indicated complete activation of the donor. The mixture was recooled to −80 °C, after which Bu4NI (as a 1 M solution in CH2Cl2, 7.5 mL, 7.5 mmol, 5.0 equiv) was added, upon which the reaction mixture assumed a maroon color. After 5 min at −80 °C, a solution of acceptor 21 (as a 0.5 M solution in CH2Cl2/1,4-dioxane, 1:1 v/v, 3.0 mmol, 2.0 equiv) was slowly added via the wall of the flask, and the mixture was allowed to warm to room temperature. After the mixture was stirred for ∼18 h, TLC analysis (toluene/EtOAc, 9:1 v/v) indicated complete conversion of the starting material. The reaction was quenched by addition of NEt3, diluted with CH2Cl2, filtered over a pad of Celite, washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (toluene/Et2O, 1:0 → 9:1) delivered 22 (α/β ∼ 7:1) as an inseparable mixture in 85% yield (0.90 g, 1.27 mmol). To a stirred solution of 22 (0.90 g, 1.27 mmol, 1.0 equiv) in THF (4 mL, 0.3 M) was added Bu4NF (as a 1 M solution in THF, 2.5 mL, 2.5 mmol, 2.0 equiv). After TLC analysis (PE/EtOAc, 7:3 v/v) indicated complete consumption of the starting material (∼2 h), the reaction was quenched by addition of NaHCO3 (satd aq) and extracted with EtOAc (3×). The combined organic phases were washed (H2O 1×, brine 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (PE/EtOAc, 19:1 → 4:1 v/v) delivered the title compound as an oil in 63% yield (0.47 g, 0.80 mmol). 1H NMR (500 MHz, 323 K) δ: 7.36–7.20 (m, 15H, CHarom); 5.17 (s, 2H, PhCH2); 4.82 (d, 1H, J = 3.0 Hz, H-1); 4.77 (d, 1H, J = 11.5 Hz, PhCHH); 4.70 (d, 1H, J = 11.5 Hz, PhCHH); 4.48 (s, 2H, PhCH2); 4.03 (bd, 1H, J = 8.0 Hz, H-3); 3.92 (q, 1H, J = 6.0 Hz, H-5); 3.62–3.56 (m, 2H, H-4, OCHHpentyl); 3.15–3.39 (m, H-2, OCHHpentyl); 3.24 (bs, NCH2,pentyl); 1.54 (bs, 4H, CH2,pentyl); 1.31 (bs, 2H, CH2,pentyl); 1.24 (d, 3H, J = 6.5 Hz, H-6). 13C-APT NMR (125 MHz, 323 K) δ: 138.0, 136.9 (Cq,arom); 128.6, 128.5, 128.4, 128.0, 127.9, 127.8, 127.5, 127.2, 126.9 (CHarom); 98.2 (C-1); 80.3 (C-4); 76.1 (PhCH2); 68.5 (C-3); 68.1 (OCH2,pentyl); 67.2 (PhCH2); 66.5 (C-5); 61.0 (C-2); 50.5 (NCH2,pentyl); 29.1, 23.4 (CH2,pentyl); 16.7 (C-6). 13C-GATED NMR (125 MHz, 323 K) δ: 98.2 (d, J = 170 Hz, C-1). IR (thin film) ν: 2934, 2108, 1690, 1454, 1421, 1229, 1171, 1067. HRMS: [M + H]+ calcd for C33H41N4O6 589.3021, found 589.3022.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-3-O-(2-azido-4-O-benzyl-2-deoxy-α-l-fucopyranosyl)-4-O-benzyl-α-d-fucopyranoside (25)
To a stirred solution of donor 6 (0.43 g, 0.80 mmol, 2.0 equiv), Ph2SO (0.16 g, 0.80 mmol, 2.0 equiv), and TTBP (0.40 g, 1.60 mmol, 4.0 equiv) in CH2Cl2 (8 mL, 0.1 M relative to donor) were added flame-dried, rod-shaped, 3 Å MS. After being stirred for ∼30 min, the mixture was cooled to −80 °C, and Tf2O (0.13 mL, 0.80 mmol, 2.0 equiv) was added. After the mixture was allowed to warm to −70 °C, it was recooled to −80 °C, and a solution of acceptor 23 (0.40 mmol, 1.0 equiv, in 0.8 mL CH2Cl2, dried by triple coevaporation with toluene) was added via the wall of the flask. The mixture was warmed to −60 °C, left at this temperature for 15 min, and the reaction was quenched by addition of NEt3. The bright yellow solution was filtered over Celite, washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) yielded 24 as a mixture of disaccharides (α/β ∼ 7:1) in 73% yield (0.27 g, 0.28 mmol). 1H NMR for the α-anomer (500 MHz, 323 K) δ: 7.41–7.21 (m, 20H, CHarom); 5.24 (d, 1H, J = 3.5 Hz, H-1′); 5.16 (bs, 2H, PhCH2); 5.03 (d, 1H, J = 11.5 Hz, PhCHH); 4.91 (d, 1H, J = 11.5 Hz, PhCHH); 4.88 (d, 1H, J = 3.5 Hz, H-1); 4.62 (d, 1H, J = 11.0 Hz, PhCHH); 4.55 (d, 1H, J = 11.0 Hz, PhCHH); 4.48 (bs, 2H, PhCH2); 4.10 (dd, 1H, J = 3.0 Hz, 10.5 Hz, H-3′); 4.06 (dd, 1H, J = 3.0 Hz, 11.0 Hz, H-3); 3.99, 3.92 (q, 1H, J = 6.5 Hz, H-5, H-5′); 3.84–3.81 (m, 2H, H-2, H-2′); 3.60–3.56 (m, 2H, H-4, OCHHpentyl); 3.47 (d, 1H, J = 1.5 Hz, H-4′); 3.40 (bs, 1H, OCHHpentyl); 3.23 (bs, 2H, NCH2,pentyl); 1.53 (m, 4H, CH2,pentyl); 1.30 (m, 2H, CH2,pentyl); 1.20, 1.16 (d, 3H, J = 6.5 Hz, H-6, H-6′); 0.96 (s, 9H, (CH3)3CSi); 0.20, 0.14 (s, 3H, CH3Si). 13C-APT NMR (125 MHz, 323 K) δ: 138.7, 138.5, 138.1 (Cq,arom); 128.5, 128.4, 128.4, 128.3, 127.9, 127.9, 127.8, 127.7, 127.6, 127.6, 127.3 (CHarom); 99.5 (C-1′); 98.3 (C-1); 80.8 (C-4′); 79.9 (C-4); 76.2 (C-3); 75.6, 75.2 (PhCH2); 71.5 (C-3′); 68.3 (OCH2,pentyl); 67.8 (C-5 or C-5′); 67.2 (PhCH2); 66.9 (C-5 or C-5′); 61.8, 60.4 (C-2, C-2′); 29.2 (CH2,pentyl); 25.9 ((CH3)3CSi); 23.4 (CH2,pentyl); 18.1 (CqSi); 16.8, 16.7 (C-6, C-6′); −4.1, −5.0 (CH3Si). Diagnostic 1H NMR signal for the β-anomer (500 MHz, 323 K) δ: 4.32 (d, 1H, J = 8.0 Hz, H-1′). Disaccharide 24 was dissolved in dry THF (1.4 mL, 0.2 M), and Bu4NF (as 1 M solution in THF, 0.34 mL, 0.34 mmol, 1.2 equiv) was added. After 4 h, TLC analysis (PE/EtOAc, 7:3 v/v) indicated complete consumption of the starting material and the appearance of two more polar products. The reaction was quenched by addition of satd aq NaHCO3, the mixture was extracted (CH2Cl2, 3×), and the combined organics were washed (H2O, 1×; brine, 1×), dried over MgSO4, and concentrated in vacuo. Purification by column chromatography (hexane/EtOAc, 19:1 → 4:1 v/v) furnished the title disaccharide in 71% yield (0.17 g, 0.20 mmol). 1H NMR (500 MHz, 323 K) δ: 7.36–7.19 (m, 20H, CHarom); 5.20 (d, 1H, J = 4.0 Hz, H-1′); 5.17 (bs, 2H, PhCH2); 4.89 (d, 1H, J = 3.0 Hz, H-1); 4.79 (d, 1H, J = 11.5 Hz, PhCHH); 4.75 (d, 1H, J = 11.5 Hz, PhCHH); 4.72–4.67 (m, 2H, 2× PhCHH); 4.49 (bs, 2H, PhCH2); 4.04 (dd, 1H, J = 3.0 Hz, 10.8 Hz, H-3); 3.93–3.90 (m, 3H, H-3′, H-5, H-5′); 3.84 (dd, 1H, J = 3.5 Hz, 10.5 Hz, H-2); 3.59 (bs, 1H, OCHHpentyl); 3.55 (d, 1H, J = 2.5 Hz, H-4); 3.52 (d, 1H, J = 2.5 Hz, H-4′); 3.49 5(dd, 1H, J = 3.5 Hz, 10.8 Hz, H-2′); 3.41 (bs, 1H, OCHHpentyl); 3.24 (bs, 2H, NCH2pentyl); 1.55 (bs, 4H, 2× CH2,pentyl); 1.31 (bs, 2H, CH2,pentyl); 1.23–1.20 (m, 6H, H-6, H-6′). 13C-APT NMR (125 MHz, 323 K) δ: 138.4, 138.0, 137.9, 136.9 (Cq,arom); 128.6, 128.5, 128.4, 128.4, 128.1, 128.1, 127.9, 127.8, 127.7, 127.6, 127.3 (CHarom); 99.8 (C-1′); 98.2 (C-1); 80.4 (C-4); 79.8 (C-4′); 76.2 (C-3); 76.0, 75.5 (PhCH2); 68.7 (C-3′, C-5 or C-5′); 68.2 (OCH2,pentyl); 67.4 (C-3′, C-5 or C-5′); 67.2 (PhCH2); 67.0 (C-3′, C-5 or C-5′); 61.0 (C-2′); 60.4 (C-2); 29.1 (2× CH2,pentyl); 23.4 (CH2,pentyl); 16.8, 16.8 (C-6, C-6′). 13C-GATED NMR (125 Hz) δ: 99.8 (d, J = 170 Hz, C-1′); 98.2 (d, J = 168 Hz, C-1). IR (thin film) ν: 2936, 2106, 1694, 1454, 1422, 1092, 1036, 1028. HRMS: [M + NH4]+ calcd for C46H59N8O9 867.4400, found 867.4403.
Methyl (2-Azido-3,4-di-O-benzyl-2-deoxy-1-O-(N-phenyl-2,2,2-trifluoroacetimidoyl)-α/β-d-mannopyranosiduronate) (28)
To a stirred, ice-cooled solution of phenyl 2-azido-3-O-benzyl-4,6-O-benzylidene-2-deoxy-1-thio-α-d-mannopyranoside60 (4.9 g, 10.3 mmol, 1.0 equiv) in CH2Cl2 (30 mL, 0.3 M) was added, under argon, BH3.THF (as 1 M solution in THF, 30 mL, 3.0 equiv), followed by Bu2BOTf (as 1 M solution in CH2Cl2, 10 mL, 1.0 equiv) and the reaction mixture was kept at 0 °C. After 1 h, TLC analysis (PE/EtOAc, 4:1 v/v) indicated complete conversion of the starting material, and the reaction was quenched by sequential addition of NEt3 (4 mL) and MeOH (added dropwise until gas evolution ceased). The mixture was concentrated in vacuo and the residue was coevaporated with MeOH (3×). Purification by column chromatography (PE/EtOAc, 19:1 → 9:1 v/v) delivered phenyl 2-azido-3,4-di-O-benzyl-2-deoxy-1-thio-α-d-mannopyranoside as a colorless oil (4.6 g, 9.7 mmol, 94% yield). 1H NMR (400 MHz) δ: 7.42–7.22 (m, 15H, CHarom); 5.40 (s, 1H, H-1); 4.90 (d, 1H, J = 10.8 Hz, PhCHH); 4.75 (s, 2H, PhCH2); 4.66 (d, 1H, J = 11.2 Hz, PhCHH); 4.15–4.04 (m, 3H, H-2, H-3, H-5); 3.92 (t, 1H, J = 9.6 Hz, H-4); 3.77 (bs, 2H, H-6); 1.88 (bs, 1H, 6-OH). 13C-APT NMR (100 MHz) δ: 137.9, 137.3, 132.9 (Cq,arom); 132.1, 129.2, 128.6, 128.4, 128.1, 128.0, 127.9 (CHarom); 86.2 (C-1); 79.8 (C-2, C-3 or C-5); 75.4 (PhCH2); 74.2 (C-4); 73.2 (C-2, C-3 or C-5); 72.7 (PhCH2); 62.7 (C-2, C-3 or C-5); 61.7 (C-6). IR (thin film) ν: 2872, 2102, 1454, 1265, 1096, 1084, 1026. HRMS: [M + Na]+ calcd for C26H27N3NaO4S 500.1615, found 500.1611. The primary alcohol (1.17 g, 2.6 mmol, 1.0 equiv) was dissolved in CH2Cl2 (8 mL), and H2O (4 mL) and tert-butyl alcohol (1 mL, final concentration 0.2 M) were added. Under vigorous stirring were added AcOH (15 μL, 0.26 mmol, 0.1 equiv), TEMPO (81 mg, 0.52 mmol, 0.2 equiv), and PhI(OAc)2 (2.09 g, 6.5 mmol, 2.5 equiv), and the resulting red mixture was stirred until TLC analysis (PE/EtOAc/AcOH, 75:20:5 v/v/v) indicated complete conversion of the starting material into a lower running spot (∼90 min). The reaction was quenched by addition of satd aq Na2S2O3, and the resulting light yellow mixture was extracted (CH2Cl2, 2×). The combined organic fractions were washed (H2O 1×, brine 1×), dried over MgSO4, filtered, and concentrated in vacuo. After coevaporation with toluene (1×), the residue was dissolved in DMF (9 mL, 0.3 M), and MeI (0.32 mL, 5.2 mL, 2.0 equiv) and K2CO3 (0.72 g, 5.2 mmol, 2.0 equiv) were added. The reaction was stirred overnight, after which TLC analysis (PE/EtOAc/AcOH, 70:30:5 v/v/v) indicated complete conversion of the starting material. The reaction mixture was partitioned between Et2O and H2O, and after separation, the aqueous phase was extracted with Et2O (2×). The combined ethereal phases were washed (brine 1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (PE/EtOAc, 1:0 → 9:1 v/v) to deliver methyl (phenyl 2-azido-3,4-di-O-benzyl-2-deoxy-1-thio-α-d-mannopyranosiduronate) as an oil in 79% yield (1.04 g, 2.06 mmol). 1H NMR (400 MHz) δ: 7.62–7.60 (m, 2H, CHarom); 7.38–7.25 (m, 13H, CHarom); 5.61 (d, 1H, J = 7.6 Hz, H-1); 4.69–4.59 (m, 5H, H-5, PhCH2); 4.21 (dd, 1H, J = 4.4 Hz, 5.6 Hz, H-4); 3.93 (dd, 1H, J = 3.2 Hz, 5.6 Hz, H-3); 3.72 (dd, J = 2.4 Hz, 7.6 Hz, H-2); 3.54 (s, 3H, OCH3). 13C-APT NMR (100 MHz) δ: 169.3 (C-6); 137.3, 136.8 (Cq,arom); 132.4 (CHarom); 132.0 (Cq,arom); 128.9, 128.5, 128.4, 128.1, 128.0, 127.8, 127.7); 74.6 (C-4); 73.0 (PhCH2); 72.9 (C-5); 58.7 (C-2); 52.2 (OCH3). The C-1 and C-3 were not observable at room temperature due to signal broadening. IR (thin film) ν: 2870, 2102, 1749, 1454, 1439, 1265, 1119, 1094, 1078, 1024. HRMS: [M + Na]+ calcd for C27H27N3NaO5S 528.1564, found 528.1559. To a solution of the methyl uronate (0.56 g, 1.12 mmol, 1.0 equiv) in acetone (8.4 mL) was added H2O (2.8 mL, final concentration 0.1 M). At 0 °C, NBS (0.60 g, 3.35 mmol, 3.0 equiv) was added, and the reaction mixture assumed an orange-brown color. After ∼90 min, a second portion of NBS (0.60 g, 3.35 mmol, 3.0 equiv) was added, and the mixture was stirred for an additional 15 min, after which TLC (PE/EtOAc, 7:3 v/v) indicated complete conversion of the starting material. The reaction was quenched by addition of satd aq Na2SO3, and the mixture was extracted (EtOAc, 3×). The combined organic phases were washed (H2O 1×, brine 1×), dried over MgSO4, filtered, and concentrated in vacuo. Methyl 2-azido-3,4-di-O-benzyl-2-deoxy-α/β-d-mannopyranosiduronate was obtained after column chromatography (PE/EtOAc, 9:1 → 7:3 v/v), with the β-product predominating (α/β ∼ 1:9) in 82% yield (0.38 g, 0.92 mmol). NMR data are reported for the β-isomer only. 1H NMR (400 MHz, 323 K) δ: 7.29–7.22 (m, 10H, CHarom); 5.42 (d, 1H, J = 3.6 Hz, H-1); 4.65 (s, 2H, PhCH2); 4.62–4.54 (m, 2H, PhCH2); 4.49 (d, 1H, J = 5.6 Hz, H-5); 4.33 (bs, 1H, 1-OH); 4.12 (t, 1H, J = 5.6 Hz, H-4); 3.96 (bd, 1H, J = 3.2 Hz, H-3); 3.76 (bs, 1H, H-2); 3.55 (s, 3H, OCH3).13C-APT NMR (100 MHz, 323 K) δ: 169.7 (C-6); 137.5, 137.3 (Cq,arom); 128.5, 128.4, 128.3, 128.1, 127.9, 127.8, 127.7 (CHarom); 92.1 (C-1); 77.4 (C-3); 75.2 (C-4); 73.5, 72.8 (PhCH2); 72.3 (C-5); 61.0 (C-2); 52.1 (OCH3). 13C-GATED NMR (100 MHz, 323 K) δ: 92.1 (d, J = 170 Hz, H-1). IR (thin film) 3439, 2104, 1747, 1454, 1437, 1281, 1240, 1123, 1093, 1072, 1024. HRMS: [M + Na]+ calcd for C21H23N3NaO6 436.1479, found 436.1476. To a solution of the reducing sugar (0.30 g, 0.73 mmol, 1.0 equiv) in acetone (2.5 mL, 0.3 M) were added N-phenyl 2,2,2-trifluoroimidoyl chloride (0.17 mL, 1.1 mmol, 1.5 equiv) and Cs2CO3 (0.29 g, 0.88 mmol, 1.2 equiv), and the mixture was stirred until TLC analysis (PE/EtOAc, 4:1 v/v) indicated complete conversion of the starting material (∼2 h). The reaction mixture was diluted with acetone, filtered, and concentrated in vacuo. Purification of the residue by column chromatography (PE/EtOAc/NEt3, 100:0:1 → 90:10:1) delivered the title compound as a mixture of anomers and/or conformers, in 88% yield (0.38 g, 0.64 mmol). 1H NMR (major isomer, 400 MHz, 323 K) δ: 7.34–7.25 (m, 13H, CHarom); 7.11 (t, 1H, J = 7.6 Hz, CHarom); 6.80 (d, 2H, J = 8.0 Hz, CHarom); 6.30 (bs, 1H, H-1); 4.77–4.64 (m, 4H, PhCH2); 4.38 (d, 1H, J = 7.2 Hz, H-5); 4.18 (t, 1H, J = 7.6 Hz, H-4); 4.02 (dd, 1H, J = 2.8 Hz, 7.6 Hz, H-3); 3.87 (bs, 1H, H-2); 3.66 (s, 3H, OCH3). 13C-APT NMR (100 MHz, 323 K) δ: 168.5 (C-6); 143.1, 137.5, 137.1 (Cq,arom); 128.8, 128.6, 128.5, 128.3, 128.2, 128.1, 128.0, 127.8, 124.6, 124.4, 119.4 (CHarom); 94.4 (C-1); 77.8 (C-3); 74.9 (C-4); 74.6 (PhCH2); 73.8 (C-5); 73.5 (PhCH2); 59.8 (C-2); 52.4 (OCH3). Diagnostic 1H NMR signals for the minor isomer (400 MHz, 323 K) δ: 6.00 (bs, 0.2H, H-1); 4.30 (t, 0.2H, J = 6.8 Hz, H-4); 3.91 (bs, 0.2H, H-2); 3.62 (s, 0.6H, OCH3). IR (thin film) ν: 2110, 1751, 1717, 1317, 1207, 1161, 1115, 1026. HRMS: [M + Na]+ calcd for C29H27F3N4NaO6 607.1775, found 607.1777.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-3-O-benzoyl-4-O-benzyl-2-deoxy-β-d-fucopyranoside (29)
A three-necked, 250 mL flask, equipped with a thermometer, rubber septa, an argon balloon, and a gas outlet, was charged with a solution of donor d-4 (2.86 g, 5.5 mmol, 1.0 equiv), Ph2SO (1.44 g, 7.1 mmol, 1.3 equiv), and TTBP (3.39 g, 13.4 mmol, 2.5 equiv) in CH2Cl2/Et2O (110 mL, 1:1 v/v, 0.05 M). Flame-dried 3 Å MS were added, and the mixture was stirred at room temperature for ∼30 min. After the mixture was cooled to −80 °C, Tf2O (1.2 mL, 7.1 mmol, 1.3 equiv) was added and the mixture was allowed to warm to −60 °C. After the mixture was recooled to −80 °C, a solution of 21 (3.58 g, 10.9 mmol, 2.0 equiv) in CH2Cl2/Et2O (20 mL, 1:1 v/v) was added via cannula transfer (reaction mixture temperature did not exceed −70 °C during addition). The mixture was allowed to warm to −40 °C, after which the reaction was quenched by addition of NEt3 (4 mL), filtered over a pad of Celite, diluted with CH2Cl2, washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. Column chromatography (PE/EtOAc, 49:1 →9:1 v/v) first furnished a mixture of α/β products (0.77 g, 1.1 mmol, 20% yield, α/β 1:3); when all higher-running α-product had eluted off, further elution (PE/EtOAc, 4:1 v/v) delivered pure β-product as a colorless oil (2.43 g, 3.5 mmol, 60%). NMR data are reported for the pure β-isomer. 1H NMR (400 MHz, 323 K) δ: 8.06 (d, 2H, J = 7.6 Hz, CHarom); 7.59 (t, 1H, J = 7.6 Hz, CHarom); 7.46 (t, 2H, J = 7.6 Hz, CHarom); 7.30–7.17 (m, 14H, CHarom); 5.17 (bs, 2H, PhCH2); 4.93 (dd, 1H, J = 2.8 Hz, 10.8 Hz, H-3); 4.68 (d, 1H, J = 11.6 Hz, PhCHH); 4.55 (d, 1H, J = 11.6 Hz, PhCHH); 4.49 (s, 2H, PhCH2); 4.29 (d, 1H, J = 8.0 Hz, H-1); 3.96–3.91 (m, 2H, H-2, OCHHpentyl); 3.78 (d, 1H, J = 2.0 Hz, H-4); 3.63 (q, 1H, J = 6.4 Hz, H-5); 3.48 (bs, 1H, OCHHpentyl); 3.23 (CH2Npentyl); 1.61 (bs, 4H, 2× CH2,pentyl); 1.35 (bs, 2H, 2H, CH2,pentyl); 1.25 (d, 3H, J = 6.4 Hz, H-6). 13C-APT NMR (100 MHz, 323 K) δ: 165.9 (COBz); 138.1, 137.7 (Cq,arom); 133.5, 129.9 (CHarom); 129.4 (Cq,arom); 128.6, 128.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.3 (CHarom); 102.3 (C-1); 76.3 (C-4); 75.5 (PhCH2); 75.0 (C-3); 70.6 (C-5); 69.8 (OCH2,pentyl); 67.2 (PhCH2); 61.6 (C-2); 50.7 (PhCH2); 29.2, 23.3 (CH2,pentyl); 16.7 (C-6). IR (thin film) ν: 2937, 2110, 1719, 1695, 1452, 1421, 1265, 1096, 1069, 1026. HRMS: [M + Na]+ calcd for C40H44N4NaO7 715.3102, found 715.3097.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-4-O-benzyl-2-deoxy-β-d-fucopyranoside (30)
To a stirred solution of 29 (2.43 g, 3.5 mmol, 1.0 equiv) in dry MeOH (18 mL, 0.2 M) was added a chip of Na metal. The reaction mixture was stirred at room temperature until TLC analysis (PE/EtOAc, 7:3 v/v) indicated complete conversion of the starting material. The reaction mixture was neutralized by addition of Amberlite IR-120 (H+ form), filtered, and concentrated in vacuo. The title compound was obtained after column chromatography (PE/EtOAc) as a colorless oil in 95% yield (1.94 g, 3.3 mmol). 1H NMR (400 MHz, 323 K) δ: 7.36–7.22 (m, 15H, CHarom); 5.17 (s, 2H, PhCH2); 4.80 (d, 1H, J = 11.6 Hz, PhCHH); 4.72 (d, 1H, J = 11.6 Hz, PhCHH); 4.48 (s, 2H, PhCH2); 4.16 (d, 1H, J = 7.2 Hz, H-1); 3.86 (bs, 1H, OCHHpentyl); 3.53–3.43 (m, 5H, OCHHpentyl, H-2, H-3, H-4, H-5); 3.23 (bs, 2H, NCH2,pentyl); 2.17 (d, 1H, J = 9.2 Hz, 3-OH); 1.57–1.53 (m, 4H, CH2,pentyl); 1.44–1.43 (m, 2H, CH2,pentyl); 1.26 (d, 3H, J = 7.2 Hz, H-6). 13C-APT NMR (100 MHz, 323 K) δ: 138.1 (Cq,arom); 128.5, 128.5, 128.4, 128.2, 128.0, 127.9, 127.3 (CHarom); 102.3 (C-1); 78.6 (C-3, C-4, or C-5); 76.0 (PhCH2); 73.2, 70.9 (C-3, C-4 or C-5); 69.7 (OCH2,pentyl); 67.2 (PhCH2); 64.8 (C-2); 29.3, 23.3 (CH2,pentyl); 16.9 (C-6). IR (thin film) ν: 2934, 2108, 1690, 1454, 1421, 1229, 1171, 1067. HRMS: [M + Na]+ calcd for C33H40N4NaO6 611.2840, found 611.2833.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-3-O-(2-azido-2-deoxy-3,4-di-O-(tert-butyldimethylsilyl)-α-l-fucopyranosyl)-4-O-benzyl-β-d-fucopyranoside (31)
To a stirred solution of 5 (1.22 g, 2.2 mmol, 2.0 equiv), Ph2SO (0.44 g, 2.2 mmol, 2.0 equiv), and TTBP (1.09 g, 4.4 mmol, 4.0 equiv) in CH2Cl2 (22 mL, 0.1 M relative to donor) were added flame-dried, rod-shaped, 3 Å molecular sieves. After 30 min at room temperature, the reaction mixture was cooled to −80 °C, after which Tf2O (0.37 mL, 2.2 mmol, 2.0 equiv) was added. The reaction mixture was allowed to warm to −70 °C, after which it was recooled to −80 °C. A solution of acceptor 30 (dried by triple coevaporation with toluene, 0.65 g, 1.1 mmol, 1.0 equiv) in CH2Cl2 (2.2 mL) was added via the wall of the flask, and the mixture was allowed to warm to −60 °C, after which it was stirred at this temperature for 15 min. The reaction was stopped by addition of NEt3 (1 mL), and the mixture filtered over a pad of Celite, washed with brine (1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification of the residue by column chromatography (toluene/EtOAc, 1:0 → 9:1 v/v) and size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) furnished the title compound as an oil (0.83 g, 0.84 mmol, 76%). 1H NMR (400 MHz, 323 K) δ: 7.34–7.21 (m, 15H, CHarom); 5.36 (d, 1H, J = 3.6 Hz, H-1′); 5.16 (s, 2H, PhCH2); 4.97 (d, 1H, J = 11.6 Hz, PhCHH); 4.58 (d, 1H, J = 11.2 Hz, PhCHH); 4.48 (s, 2H, PhCH2); 4.17 (d, 1H, J = 8.0 Hz, H-1); 4.99 (bd, 1H, J = 10.0 Hz, H-3′); 3.94 (q, 1H, J = 6.8 Hz, H-5′); 3.85–3.80 (m, 2H, OCHHpentyl, H-2); 3.77–3.71 (m, 2H, H-2′, H-4′); 3.52–3.42 (m, 4H, OCHHpentyl, H-3, H-4, H-5), 3.23 (bs, 2H, NCH2,pentyl); 1.53 (bs, 4H, 2× CH2,pentyl); 1.34 (bs, 2H, CH2,pentyl); 1.23 (d, 3H, J = 6.4 Hz, H-6 or H-6′); 1.19 (d, 3H, J = 6.4 Hz, H-6 or H-6′); 0.93, 0.93 (s, 9H, CH3,tBuSi); 0.16, 0.14, 0.11, 0.09 (s, 3H, CH3,MeSi). 13C-APT NMR (100 MHz, 323 K) δ: 151.5 (COCbz); 138.3, 138.1 (Cq,arom); 128.5, 128.4, 128.3, 127.9, 127.9, 127.8, 127.7, 127.2 (CHarom); 102.8 (C-1); 99.3 (C-1′); 78.8, 78.7 (C-4, C-5); 75.0 (PhCH2); 74.9 (C-4); 71.4 (C-3′); 70.8 (C-3); 69.7 (OCH2,pentyl); 69.0 (C-5′); 67.2 (PhCH2); 63.8 (C-2); 61.2 (C-2′); 50.5 (PhCH2); 29.2 (CH2,pentyl); 26.2, 26.1 (CH3,tBuSi); 23.3 (PhCH2); 18.6, 18.5 (Cq,tBuSi); 17.3, 16.9 (C-6, C-6′); −3.4, −3.6, −4.5, −4.6 (CH3,MeSi). IR (thin film) ν: 2930, 2857, 2114, 1697, 1252, 1177, 1105, 1067, 1042, 1026. HRMS: [M + Na]+ calcd for C51H77N7NaO9Si2 1010.5214, found 1010.5216.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-3-O-(2-azido-3-O-benzoyl-2-deoxy-α-l-fucopyranosyl)-4-O-benzyl-β-d-fucopyranoside (33)
To a stirred solution of disaccharide 31 (0.83 g, 0.84 mmol, 1.0 equiv) in THF (4 mL, 0.2 M) was added Bu4NF (as 1 M solution in THF, 2.0 mL, 2.4 equiv), and the resulting yellow reaction mixture was stirred until TLC analysis (toluene/EtOAc, 4:1 v/v) indicated complete conversion of the starting material (∼2 h). The mixture was diluted with EtOAc, and the reaction quenched by addition of satd aq NaHCO3. After separation of the layers, the aqueous layer was extracted with EtOAc (1×), and the combined organics were washed (H2O, 1×; brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was coevaporated once with dry MeCN before dissolution in MeCN (4 mL, 0.2 M). Added were, in succession, 2-aminoethyl diphenylborinate 30 (18 mg, 0.08 mmol, 0.1 equiv), DIPEA (0.3 mL, 1.68 mmol, 2.0 equiv), and BzCl (0.2 mL, 1.68 mmol, 2.0 equiv). The reaction vessel was stirred, under exclusion of light, until TLC analysis (toluene/EtOAc, 4:1 v/v) indicated complete conversion of the starting material (∼16 h). The reaction mixture was diluted with EtOAc, washed (H2O, 2×; brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (toluene/EtOAc, 1:0 → 17:3 v/v) furnished the title compound as a colorless oil in 67% yield over two steps (0.48 g, 0.56 mmol). 1H NMR (500 MHz, 323 K) δ: 8.10–8.09 (m, 2H, CHarom); 7.59 (t, 1H, J = 7.5 Hz, CHarom); 7.48–7.21 (m, 17H, CHarom); 5.46 (dd, 1H, J = 3.0 Hz, 11.5 Hz, H-3′); 5.42 (d, 1H, J = 3.5 Hz, H-1′); 5.16 (bs, 2H, PhCH2); 4.93 (d, 1H, J = 12.0 Hz, PhCHH); 4.77 (d, 1H, J = 11.5 Hz, PhCHH); 4.48 (bs, 2H, PhCH2); 4.20 (d, 1H, J = 7.5 Hz, H-1); 3.99 (bs, 1H, H-4′); 3.95 (q, 1H, J = 6.5 Hz, H-5′); 3.90–3.86 (m, 2H, H-2, OCHHpentyl); 3.82 (dd, 1H, J = 3.5 Hz, 11.3 Hz, H-2′); 3.55–3.45 (m, 4H, H-3, H-4, H-5, OCHHpentyl); 3.22 (bs, 2H, NCH2,pentyl); 1.59–1.21 (m, 6H, 3× CH2,pentyl); 1.22 (d, 3H, J = 6.5 Hz, H-6); 1.14 (d, 3H, J = 6.5 Hz, H-6′). 13C-APT NMR (125 MHz, 323 K) δ: 165.5 (COBz); 138.2, 138.1 (Cq,arom); 133.5, 129.9 (CHarom); 129.5 (Cq,arom); 128.6, 128.5, 128.4, 128.1, 127.9, 127.8, 127.3 (CHarom); 102.8 (C-1); 99.8 (C-1′); 78.9, 78.3 (C-3, C-4 or C-5); 75.2 (PhCH2); 71.4 (C-3′); 71.0 (C-3, C-4 or C-5); 70.2 (C-4′); 69.8 (OCH2,pentyl); 67.2 (PhCH2); 66.5 (C-5′); 63.8 (C-2); 57.4 (C-2′); 29.3, 23.3 (CH2,pentyl); 17.1. 16.1 (C-6, C-6′). IR (thin film) ν: 2117, 1717, 1273, 1072. HRMS: [M + Na]+ calcd for C46H53N7NaO10 886.3746, found 886.3743.
5-(Benzyl(benzyloxycarbonyl)amino)pentyl 2-Azido-3-O-(2-azido-3-O-benzoyl-2-deoxy-4-O-(methyl 2-azido-3,4-di-O-benzyl-2-deoxy-β-d-mannopyranosiduronate)-α-l-fucopyranosyl)-4-O-benzyl-2-deoxy-β-d-fucopyranoside (27)
To a stirred solution of donor 28 (0.75 g, 1.28 mmol, 4.0 equiv) and acceptor 33 (0.28 g, 0.32 mmol, 1.0 equiv) in CH2Cl2 (3.2 mL, 0.1 M) were added flame-dried, rod-shaped, 3 Å MS. After ∼30 min, the mixture was cooled to −80 °C, and TBSOTf (60 μL, 0.26 mmol, 0.8 equiv) was added. The reaction mixture was allowed to warm to −55 °C and was stirred at this temperature using an immersion cooler. After the mxiture was stirred for ∼5.5 h, TLC analysis (toluene/acetone, 4:1 v/v) indicated complete disappearance of the acceptor, and the reaction was quenched by addition of NEt3 (0.2 mL). The mixture was diluted with CH2Cl2, filtered over a small bed of Celite, washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by size-exclusion chromatography (CH2Cl2/MeOH, 1:1v/v) followed by column chromatography (toluene/EtOAc, 1:0 → 9:1) furnished the title trisaccharide as a colorless oil in 65% yield (260 mg, 0.21 mmol). 1H NMR (500 MHz, 323 K) δ: 8.11–8.09 (m, 2H, CHarom); 7.57 (t, 1H, J = 7.5 Hz, CHarom); 7.47–7.14 (m, 27H, CHarom); 5.41–5.39 (m, 2H, H-1′, H-3′); 5.16 (bs, 2H, PhCH2); 4.95 (d, 1H, J = 11.5 Hz, PhCHH); 4.76 (d, 1H, J = 11.0 Hz, PhCHH); 4.72–4.68 (m, 3H, PhCH2); 4.55 (d, 1H, J = 11.0 Hz, PhCHH); 4.49–4.48 (m, 3H, H-1″, PhCH2); 4.21 (d, 1H, J = 3.0 Hz, H-4′); 4.18 (d, 1H, J = 8.0 Hz, H-1); 4.02–3.96 (m, 3H, H-2, H-2″, H-5′); 3.94 (t, 1H, J = 9.0 Hz, H-4″); 3.86 (bs, 1H, OCHHpentyl); 3.83 (dd, 1H, J = 8.0 Hz, 10.5 Hz, H-2); 3.55 (d, 1H, J = 10.0 Hz, H-5″); 3.52–3.46 (m, 5H, H-3, H-3″, H-4, H-5, OCHHpentyl); 3.22 (bs, 2H, NCH2,pentyl); 3.13 (s, 3H, OCH3); 1.58–1.38 (m, 6H, CH2,pentyl); 1.30 (d, 3H, J = 6.0 Hz, H-6); 1.16 (d, 3H, J = 7.0 Hz, H-6′). 13C-APT NMR (125 MHz, 323 K) δ: 166.9, 166.1 (C-6″, COBz); 138.2, 138.1, 137.4 (Cq,arom); 133.0, 130.1 (CHarom); 129.9 (Cq,arom); 128.6, 128.5, 128.5, 128.4, 1283, 128.2, 128.1, 127.9, 127.9, 127.7, 127.7, 127.5, 127.3 (CHarom); 102.8 (C-1); 101.2 (C-1″); 99.5 (C-1′); 79.9 (C-3″); 79.0 (C-3); 78.3 (C-4); 76.5 (C-4′); 75.3, 75.2 (PhCH2); 75.2, 75.2 (C-4″; C-5″); 72.3 (PhCH2); 70.9 (C-5); 69.8 (OCH2,pentyl); 69.5 (C-3″); 67.2 (PhCH2); 66.4 (C-5′); 63.8 (C-2); 61.3 (C-2″); 57.4 (C-2′); 51.9 (OCH3,methyl); 50.9 (NCH2,pentyl); 29.3, 23.3 (CH2,pentyl); 17.1 (C-6); 16.6 (C-6′). 13C-GATED NMR (125 MHz, 323 K) δ: 102.8 (d, J = 159 Hz, C-1); 101.2 (d, J = 160 Hz, C-1″); 99.5 (d, J = 173 Hz, C-1′). IR (thin film) ν: 2934, 2110, 1749, 1717, 1699, 1273, 1103, 1069, 1038. HRMS: [M + Na]+ calcd for C67H74N10NaO15 1281.5227, found 1281.5228.
5-Aminopentyl 2-Acetamido-3-O-(2-acetamido-3-O-acetyl-4-O-(2-acetamido-2-deoxy-β-d-mannopyranosiduronyl)-2-deoxy-α-l-fucopyranosyl)-2-deoxy-β-d-fucopyranoside (26)
A solution of 27 (50 mg, 0.040 mmol, 1.0 equiv) in THF was treated with a freshly prepared solution of KOOH (H2O2, 30% w/w in H2O, was added to 0.5 M aq KOH solution), and the slightly turbid mixture was allowed to stir for 2 days until TLC analysis (toluene/EtOAc/AcOH, 60:40:5 v/v/v) indicated complete conversion of the starting material. The mixture was acidified to pH 3 with 1 M aq HCl and extracted (CH2Cl2, 5×), and the combined organic fractions were washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (toluene/EtOAc/AcOH, 90:10:5 → 70:30:5 v/v/v) gave free uronic acid 34 as a milky solid (30 mg, 0.026 mmol, 66%). 1H NMR (500 MHz, 323 K, CD3CN + acetic acid-d4) δ: 7.40–7.18 (m, 25H, CHarom); 5.22 (d, 1H, J = 3.5 Hz, H-1′); 5.12 (s, 2H, PhCH2); 4.85 (d, 1H, J = 11.0 Hz, PhCHH); 4.77–4.75 (m, 3H, H-1″, PhCH2); 4.66–4.61 (m, 3H, PhCH2); 4.46 (s, 2H, PhCH2); 4.34 (d, 1H, J = 2.5 Hz, H-2″); 4.24 (d, 1H, J = 7.0 Hz, H-1); 4.03 (q, 1H, J = 6.5 Hz, H-5′); 3.96 (d, 1H, J = 9.0 Hz, H-5″); 3.94–3.89 (m, 2H, H-3′, H-4′); 3.87 (t, 1H, J = 9.5 Hz, H-4″); 3.78 (dd, 1H, J = 3.5 Hz, 9.0 Hz, H-3″); 3.75–3.74 (m, 1H, OCHHpentyl); 3.63–3.54 (m, 4H, H-2, H-3, H-4, H-5); 3.48–3.36 (m, 1H, OCHHpentyl); 3.39 (dd, 1H, J = 3.5 Hz, 10.5 Hz, H-2′); 3.27 (t, 2H, J = 7.5 Hz, NCH2,pentyl); 1.53–1.50 (m, 4H, CH2,pentyl); 1.34–1.20 (m, 8H, H-6, H-6′, CH2,pentyl). HRMS: [M + H]+ calcd for C59H69N10O14 1141.4989, found 1141.4994. The uronic acid 32 (17 mg, 0.015 mmol, 1.0 equiv) was dissolved in pyridine (0.6 mL), and at 0 °C, Ac2O (0.15 mL) was slowly added. The mixture was stirred until TLC analysis (toluene/EtOAc/AcOH, 60:40:5 v/v/v) indicated complete conversion of the starting material. The reaction was quenched by slow addition of water, and after ∼15 min, the mixture was extracted (CH2Cl2, 5×). The organic phases were washed (brine, 1×), dried over MgSO4, filtered, and concentrated in vacuo. The residue was coevaporated with toluene (2×) to remove excess acetic acid and pyridine. 1H NMR (500 MHz, CD3CN + acetic acid-d4) δ: 7.39–7.22 (m, 25H, CHarom); 5.32 (d, 1H, J = 3.5 Hz, H-1′); 5.14 (dd, 1H, J = 3.0 Hz, 11.5 Hz, H-3′); 5.10 (bs, 2H, PhCH2); 4.87 (d, 1H, J = 10.0 Hz, PhCHH); 4.79–4.74 (m, 2H, PhCH2); 4.67 (d, 1H, J = 1.0 Hz, H-1″); 4.65–4.60 (m, 2H, PhCH2); 4.54 (d, 1H, J = 11.0 Hz, PhCHH); 4.44 (s, 2H, PhCH2); 4.31 (d, 1H, J = 2.5 Hz, H-2″); 4.24 (bs, 1H, H-1); 4.11 (d, 1H, J = 3.0 Hz, H-4′); 4.08 (q, 1H, J = 6.5 Hz, H-5′); 3.83–3.70 (m, 5H, H-2′, H-3″, H-4″, H-5″, OCHHpentyl); 3.58–3.53 (m, 4H, H-2, H-3, H-4, H-5); 3.44 (bs, 2H, OCHHpentyl); 3.20 (bs, 2H, NCH2,pentyl); 2.01 (s, 3H, CH3,Ac); 1.51–1.49 (m, 4H, CH2,pentyl); 1.32–1.19 (m, 8H, H-6, H-6′, CH2,pentyl). The crude O-acetate (12 mg, 0.01 mmol, 1.0 equiv) was dissolved in pyridine (0.5 mL, degassed by sonication before use), and added was freshly distilled AcSH (0.5 mL). The reaction was stirred for 3 days after which LC–MS analysis (MeCN/H2O/TFA, 50:50:0.1 → 90:10:0.1 v/v/v, tR: 6.80 min) indicated complete conversion of the starting material. The mixture was diluted with pyridine (∼1 mL) and concentrated in vacuo. The crude mixture was subjected to size-exclusion chromatography (CH2Cl2/MeOH, 1:1 v/v) to isolate the intermediate 35 in 57% yield (7 mg, 0.0057 mmol). 1H NMR (500 MHz, 323 K, CD3CN + acetic acid-d4) δ: 7.42–7.21 (m, 25H, CHarom); 5.11 (s, 2H, PhCH2); 4.97–4.90 (m, 4H, H-1″, H-2″, H-3′, PhCHH); 4.79–4.77 (m, 2H, PhCH2); 4.69–4.67 (m, 2H, H-1′, PhCHH); 4.58 (d, 1H, J = 10.5 Hz, PhCHH); 4.48–4.46 (m, 3H, PhCH2); 4.41 (dd, 1H, J = 3.5 Hz, 11.5 Hz, H-2′); 4.25 (d, 1H, J = 9.0 Hz, H-1); 4.10–4.05 (m, 3H, H-2, H-4′, H-5′); 3.81–3.60 (m, 6H, H-3, H-3″, H-4″, H-5, H-5″, OCHHpentyl); 3.56 (d, 1H, J = 2.5 Hz, H-4); 3.37–3.35 (m, 1H, OCHHpentyl); 3.21 (t, 1H, J = 7.0 Hz, NCH2,pentyl); 2.03, 1.95, 1.91, 1.81 (CH3,Ac); 1.50–1.45 (m, 4H, CH2,pentyl); 1.27–1.18 (m, 8H, H-6, H-6′, CH2,pentyl). 13C-APT NMR (125 MHz, 323 K, CD3CN + acetic acid-d4) δ: 171.9 (C-6″); 129.6, 129.5, 129.4, 129.4, 129.3, 129.0, 129.0, 128.8, 128.8, 128.6, 128.3 (CHarom); 102.3 (C-1); 101.5 (C-1′); 100.4 (C-1″); 80.9 (C-3″, C-4″ or C-5″); 80.4 (C-4); 78.8 (C-3); 77.3, 77.0 (C-4 and C-3″, C-4″ or C-5″); 76.2, 75.8 (PhCH2); 75.6 (C-3″, C-4″ or C-5″); 72.0 (PhCH2); 71.5 (C-5); 71.0 (C-3′); 69.9 (OCH2,pentyl); 68.1 (C-5′); 67.9 (PhCH2); 53.0 (C-2); 50.3 (C-2″); 47.8 (C-2′); 30.1, 24.1 (CH2,pentyl); 23.5, 23.3, 23.3, 21.3 (CH3,Ac); 17.5, 16.8 (C-6, C-6′). HRMS: [M + H]+ calcd for C67H83N4O18 1231.5697, found 1231.5704. The trisaccharide 33 (4 mg, 0.003 mmol) was dissolved in THF, tert-butyl alcohol, and H2O (1:1:3 v/v/v, 0.002 M). Added was a drop of AcOH and the mixture was degassed (freeze–thaw, 3 cycles) and backfilled with argon. Pd(OH)2 (20 weight% on carbon, 50% H2O) was added and the mixture was purged with argon (balloon), followed by H2 (balloon). After purging, the mixture was stirred under H2 atmosphere for 2 days. The mixture was filtered over a fritted syringe filled with Celite, the residue was washed with THF/H2O (1:1 v/v) and concentrated in vacuo. After 1H NMR analysis revealed no aromatic signals, the crude trisaccharide was purified by passing over a C18 solid-phase extraction column (MeCN/H2O, 0:1 → 1:9) and subsequently lyophilized to obtain the product 26 (2.5 mg, 0.003 mmol) as a white solid. 1H NMR (500 MHz, D2O) δ: 5.03 (dd, 1H, J = 3.0 Hz, 12.0 Hz, H-3′); 5.01 (d, 1H, J = 4.0 Hz, H-1′); 4.74 (bs, 1H, H-1″); 4.59 (d, 1H, J = 4.5 Hz, H-2″); 4.41 (d, 1H, J = 8.5 Hz, H-1); 4.38 (dd, 1H, J = 4.0 Hz, 11.5 Hz, H-2′); 4.22 (d, 1H, J = 2.5 Hz, H-4′); 4.19 (q, 1H, J = 6.5 Hz, H-5′); 3.99 (t, 1H, J = 8.5 Hz, H-2); 3.91–3.86 (m, 1H, OCHHpentyl); 3.82–3.77 (m, 4H, H-3, H-3″, H-4, H-5); 3.65 (t, 1H, J = 9.5 Hz, H-4″); 3.60–3.56 (m, 2H, H-5″, OCHHpentyl); 2.14, 2.09, 2.01, 1.99 (s, 3H, CH3,Ac); 1.66 (t, 2H, J = 7.5 Hz, CH2,pentyl); 1.62–1.56 (m, 2H, CH2,pentyl); 1.41–1.38 (m, 2H, CH2,pentyl); 1.28 (d, 3H, J = 6.5 Hz, H-6); 1.25 (d, 3H, J = 6.5 Hz, H-6′). 13C-APT NMR (125 MHz, D2O) δ: 175.7, 175.6, 174.3, 174.1, 173.9 (C-6″, COAc); 101.6 (C-1); 99.9 (C-1″); 99.1 (C-1′); 78.7 (C-5″); 71.1 (C-3, C-3″, C-4 or C-5); 71.7, 70.8, 70.5 (C-3, C-3″, C-4, C-5); 70.1 (OCH2,pentyl); 69.9 (C-3′); 69.6 (C-4″); 66.9 (C-5); 53.1 (C-2″); 51.4 (C-2); 47.2 (C-2′); 39.4 (NCH2,pentyl); 28.2, 26.5 (CH2,pentyl); 22.2, 22.2, 22.1 (CH3,Ac); 22.0 (CH2,pentyl); 20.4 (CH3,Ac); 15.4, 15.3 (C-6, C-6′). HRMS: [M + H]+ calcd for C31H53N4O16 737.3451, found 737.3452.
Acknowledgments
We thank Fons Lefeber and Dr. Karthick Babu Sai Sankar Gupta for assistance in recording the low-temperature NMR spectra. The Netherlands Organization for Scientific Research (NWO) is acknowledged for financial support (VIDI Grant to J.D.C.C.).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.6b02593.
NMR spectra of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Emmadi M.; Kulkarni S. S. Nat. Prod. Rep. 2014, 31, 870–879. 10.1039/c4np00003j. [DOI] [PubMed] [Google Scholar]
- Berti F.; Ravenscroft N. In Carbohydrate-Based Vaccines: Methods and Protocols; Lepenies B., Ed.; Springer: New York, 2015; pp 189–209. [Google Scholar]
- Jones C. Carbohydr. Res. 2005, 340, 1097–1106. 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Liau D. F.; Hash J. H. J. Bacteriol. 1977, 131, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. V. K. N.; Melly M. A.; Harris T. M.; Hellerqvist C. G.; Hash J. H. Carbohydr. Res. 1983, 117, 113–123. 10.1016/0008-6215(83)88080-X. [DOI] [PubMed] [Google Scholar]
- Jann B.; Shashkov A. S.; Kochanowski H.; Jann K. Carbohydr. Res. 1993, 248, 241–250. 10.1016/0008-6215(93)84131-O. [DOI] [PubMed] [Google Scholar]
- Svensson M. V.; Weintraub A.; Widmalm G. Carbohydr. Res. 2011, 346, 2300–2303. 10.1016/j.carres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Anish C.; Schumann B.; Pereira C. L.; Seeberger P. H. Chem. Biol. 2014, 21, 38–50. 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Seeberger P. H.; Werz D. B. Nature 2007, 446, 1046–1051. 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- Fernández-Tejada A.; Cañada F. J.; Jiménez-Barbero J. Chem. - Eur. J. 2015, 21, 10616–10628. 10.1002/chem.201500831. [DOI] [PubMed] [Google Scholar]
- Crich D. Acc. Chem. Res. 2010, 43, 1144–1153. 10.1021/ar100035r. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Schmidt R. R. Angew. Chem., Int. Ed. 2009, 48, 1900–1934. 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- Nigudkar S. S.; Demchenko A. V. Chem. Sci. 2015, 6, 2687–2704. 10.1039/C5SC00280J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydock L. K.; Demchenko A. V. Org. Biomol. Chem. 2010, 8, 497–510. 10.1039/B916088D. [DOI] [PubMed] [Google Scholar]
- Yasomanee J. P.; Visansirikul S.; Pornsuriyasak P.; Thompson M.; Kolodziej S. A.; Demchenko A. V. J. Org. Chem. 2016, 81, 5981–5987. 10.1021/acs.joc.6b00910. [DOI] [PubMed] [Google Scholar]
- Danieli E.; Proietti D.; Brogioni G.; Romano M. R.; Cappelletti E.; Tontini M.; Berti F.; Lay L.; Costantino P.; Adamo R. Bioorg. Med. Chem. 2012, 20, 6403–6415. 10.1016/j.bmc.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Gagarinov I. A.; Fang T.; Liu L.; Srivastava A. D.; Boons G.-J. Org. Lett. 2015, 17, 928–931. 10.1021/acs.orglett.5b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visansirikul S.; Yasomanee J. P.; Pornsuriyasak P.; Kamat M. N.; Podvalnyy N. M.; Gobble C. P.; Thompson M.; Kolodziej S. A.; Demchenko A. V. Org. Lett. 2015, 17, 2382–2384. 10.1021/acs.orglett.5b00899. [DOI] [PubMed] [Google Scholar]
- Banoub J.; Boullanger P.; Lafont D. Chem. Rev. 1992, 92, 1167–1195. 10.1021/cr00014a002. [DOI] [Google Scholar]
- Mehta S.; Pinto B. M. Tetrahedron Lett. 1991, 32, 4435–4438. 10.1016/0040-4039(91)80005-Q. [DOI] [Google Scholar]
- Szeja W.; Grynkiewicz G.; Field R. A. In Handbook of Chemical Glycosylation; Demchenko A. V., Ed.; Wiley-VCH, 2008; pp 329–379. [Google Scholar]
- Mironov Y. V.; Sherman A. A.; Nifantiev N. E. Tetrahedron Lett. 2004, 45, 9107–9110. 10.1016/j.tetlet.2004.10.022. [DOI] [Google Scholar]
- Komarova B. S.; Ustyuzhanina N. E.; Tsvetkov Y. E.; Nifantiev N. E. In Modern Synthetic Methods in Carbohydrate Chemistry; Werz D. B., Vidal S., Eds.; Wiley-VCH, 2013; pp 125–159. [Google Scholar]
- Arafuka S.; Koshiba N.; Takahashi D.; Toshima K. Chem. Commun. 2014, 50, 9831–9834. 10.1039/C4CC03544E. [DOI] [PubMed] [Google Scholar]
- Guillemineau M.; Auzanneau F.-I. J. Org. Chem. 2012, 77, 8864–8878. 10.1021/jo301644w. [DOI] [PubMed] [Google Scholar]
- Mong K.-K. T.; Wong C.-H. Angew. Chem., Int. Ed. 2002, 41, 4087–4090. . [DOI] [PubMed] [Google Scholar]
- Krylov V. B.; Kaskova Z. M.; Vinnitskiy D. Z.; Ustyuzhanina N. E.; Grachev A. A.; Chizhov A. O.; Nifantiev N. E. Carbohydr. Res. 2011, 346, 540–550. 10.1016/j.carres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Gerbst A. G.; Ustuzhanina N. E.; Grachev A. A.; Zlotina N. S.; Khatuntseva E. A.; Tsvetkov D. E.; Shashkov A. S.; Usov A. I.; Nifantiev N. E. J. Carbohydr. Chem. 2002, 21, 313–324. 10.1081/CAR-120013500. [DOI] [Google Scholar]
- Gerbst A. G.; Ustuzhanina N. E.; Grachev A. A.; Khatuntseva E. A.; Tsvetkov D. E.; Whitfield D. M.; Berces A.; Nifantiev N. E. J. Carbohydr. Chem. 2001, 20, 821–831. 10.1081/CAR-100108659. [DOI] [Google Scholar]
- Gerbst A. G.; Ustuzhanina N. E.; Grachev A. A.; Tsvetkov D. E.; Khatuntseva E. A.; Nifant'ev N. E. Mendeleev Commun. 1999, 9, 114–116. 10.1070/MC1999v009n03ABEH001073. [DOI] [Google Scholar]
- Daly R.; McCabe T.; Scanlan E. M. J. Org. Chem. 2013, 78, 1080–1090. 10.1021/jo302487c. [DOI] [PubMed] [Google Scholar]
- Zong C.; Li Z.; Sun T.; Wang P.; Ding N.; Li Y. Carbohydr. Res. 2010, 345, 1522–1532. 10.1016/j.carres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hua Y.; Du Y.; Yu G.; Chu S. Carbohydr. Res. 2004, 339, 2083–2090. 10.1016/j.carres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Kasai A.; Arafuka S.; Koshiba N.; Takahashi D.; Toshima K. Org. Biomol. Chem. 2015, 13, 10556–10568. 10.1039/C5OB01634G. [DOI] [PubMed] [Google Scholar]
- Garcia B. A.; Poole J. L.; Gin D. Y. J. Am. Chem. Soc. 1997, 119, 7597–7598. 10.1021/ja971067y. [DOI] [Google Scholar]
- Garcia B. A.; Gin D. Y. J. Am. Chem. Soc. 2000, 122, 4269–4279. 10.1021/ja993595a. [DOI] [Google Scholar]
- Codée J. D. C.; Litjens R. E. J. N.; Den Heeten R.; Overkleeft H. S.; Van Boom J. H.; Van der Marel G. A. Org. Lett. 2003, 5, 1519–1522. 10.1021/ol034312t. [DOI] [PubMed] [Google Scholar]
- Frihed T. G.; Bols M.; Pedersen C. M. Chem. Rev. 2015, 115, 4963–5013. 10.1021/cr500434x. [DOI] [PubMed] [Google Scholar]
- Volbeda A. G.; Kistemaker H. A. V; Overkleeft H. S.; Van der Marel G. A.; Filippov D. V.; Codée J. D. C. J. Org. Chem. 2015, 80, 8796–8806. 10.1021/acs.joc.5b01030. [DOI] [PubMed] [Google Scholar]
- Fascione M. A.; Adshead S. J.; Mandal P. K.; Kilner C. A.; Leach A. G.; Turnbull W. B. Chem. - Eur. J. 2012, 18, 2987–2997. 10.1002/chem.201102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fascione M. A.; Brabham R.; Turnbull W. B. Chem. - Eur. J. 2016, 22, 3916–3928. 10.1002/chem.201503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crich D.; Smith M.; Yao Q.; Picione J. Synthesis 2001, 2001, 323–326. 10.1055/s-2001-10798. [DOI] [Google Scholar]
- Beaver M. G.; Woerpel K. A. J. Org. Chem. 2010, 75 (4), 1107–1118. 10.1021/jo902222a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S. C.; Cumpstey I.; Fairbanks A. J.; Butters T. D.; Mackeen M.; Wormald M. R. Tetrahedron 2002, 58, 9403–9411. 10.1016/S0040-4020(02)01221-8. [DOI] [Google Scholar]
- Szurmai Z.; Balatoni L.; Lipták A. Carbohydr. Res. 1994, 254, 301–309. 10.1016/0008-6215(94)84264-7. [DOI] [PubMed] [Google Scholar]
- Bohé L.; Crich D. Carbohydr. Res. 2015, 403, 48–59. 10.1016/j.carres.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohé L.; Crich D. C. R. Chim. 2011, 14, 3–16. 10.1016/j.crci.2010.03.016. [DOI] [Google Scholar]
- Smith D. M.; Woerpel K. A. Org. Biomol. Chem. 2006, 4, 1195–1201. 10.1039/b600056h. [DOI] [PubMed] [Google Scholar]
- Romero J. A. C.; Tabacco S. A.; Woerpel K. A. J. Am. Chem. Soc. 2000, 122, 168–169. 10.1021/ja993366o. [DOI] [Google Scholar]
- Ayala L.; Lucero C. G.; Romero J. A. C.; Tabacco S. A.; Woerpel K. A. J. Am. Chem. Soc. 2003, 125, 15521–15528. 10.1021/ja037935a. [DOI] [PubMed] [Google Scholar]
- Woods R. J.; Andrews C. W.; Bowen J. P. J. Am. Chem. Soc. 1992, 114, 859–864. 10.1021/ja00029a008. [DOI] [Google Scholar]
- Van Rijssel E. R.; Van Delft P.; Lodder G.; Overkleeft H. S.; Van Der Marel G. a.; Filippov D. V.; Codée J. D. C. Angew. Chem., Int. Ed. 2014, 53, 10381–10385. 10.1002/anie.201405477. [DOI] [PubMed] [Google Scholar]
- Walvoort M. T. C.; Dinkelaar J.; Van den Bos L. J.; Lodder G.; Overkleeft H. S.; Codée J. D. C.; Van der Marel G. A. Carbohydr. Res. 2010, 345, 1252–1263. 10.1016/j.carres.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Codée J. D. C.; van den Bos L. J.; de Jong A.-R.; Dinkelaar J.; Lodder G.; Overkleeft H. S.; van der Marel G. A. J. Org. Chem. 2009, 74, 38–47. 10.1021/jo8020192. [DOI] [PubMed] [Google Scholar]
- The 1,2-cis selectivity can also be accounted for with a kinetic scenario that is based on the in situ anomerization of an α-triflate into the corresponding β-triflate and subsequent displacement of this more reactive covalent species. However, we found that addition of extra triflate ions in the condensation of 1 with cyclohexanol enhances the rate of anomerization of the reactive intermediates, leading to the formation of more β-product (α/β 2:5, compared to a ratio of 1:1 using unmodified conditions (Table 1)).
- Mulani S. K.; Hung W.-C.; Ingle A. B.; Shiau K.-S.; Mong K.-K. T. Org. Biomol. Chem. 2014, 12, 1184–1197. 10.1039/c3ob42129e. [DOI] [PubMed] [Google Scholar]
- Noti C.; de Paz J. L.; Polito L.; Seeberger P. H. Chem. - Eur. J. 2006, 12, 8664–8686. 10.1002/chem.200601103. [DOI] [PubMed] [Google Scholar]
- Chu A.-H. A.; Nguyen S. H.; Sisel J. A.; Minciunescu A.; Bennett C. S. Org. Lett. 2013, 15, 2566–2569. 10.1021/ol401095k. [DOI] [PubMed] [Google Scholar]
- Lemieux R. U.; Hendriks K. B.; Stick R. V.; James K. J. Am. Chem. Soc. 1975, 97, 4056–4062. 10.1021/ja00847a032. [DOI] [Google Scholar]
- Litjens R. E. J. N.; Leeuwenburgh M. A.; Van der Marel G. A.; Van Boom J. H. Tetrahedron Lett. 2001, 42, 8693–8696. 10.1016/S0040-4039(01)01880-9. [DOI] [Google Scholar]
- Walvoort M. T. C.; Lodder G.; Mazurek J.; Overkleeft H. S.; Codée J. D. C.; Van der Marel G. A. J. Am. Chem. Soc. 2009, 131, 12080–12081. 10.1021/ja905008p. [DOI] [PubMed] [Google Scholar]
- Walvoort M. T. C.; Lodder G.; Overkleeft H. S.; Codée J. D. C.; Van der Marel G. A. J. Org. Chem. 2010, 75, 7990–8002. 10.1021/jo101779v. [DOI] [PubMed] [Google Scholar]
- Demchenko A. V.; Stauch T.; Boons G.-J. Synlett 1997, 1997, 818–820. 10.1055/s-1997-5762. [DOI] [Google Scholar]
- Hashimoto S.; Hayashi M.; Noyori R. Tetrahedron Lett. 1984, 25, 1379–1382. 10.1016/S0040-4039(01)80163-5. [DOI] [Google Scholar]
- Lee D.; Taylor M. S. J. Am. Chem. Soc. 2011, 133, 3724–3727. 10.1021/ja110332r. [DOI] [PubMed] [Google Scholar]
- Walvoort M. T. C.; de Witte W.; van Dijk J.; Dinkelaar J.; Lodder G.; Overkleeft H. S.; Codée J. D. C.; van der Marel G. A. Org. Lett. 2011, 13, 4360–4363. 10.1021/ol2016862. [DOI] [PubMed] [Google Scholar]
- The hydrolyzed donor could be partially recovered after the glycosylation.
- Walvoort M. T. C.; Moggré G.-J.; Lodder G.; Overkleeft H. S.; Codée J. D. C.; van der Marel G. A. J. Org. Chem. 2011, 76, 7301–7315. 10.1021/jo201179p. [DOI] [PubMed] [Google Scholar]
- Shangguan N.; Katukojvala S.; Greenberg R.; Williams L. J. J. Am. Chem. Soc. 2003, 125, 7754–7755. 10.1021/ja0294919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.