Abstract
The translation of photodynamic therapy (PDT) to the clinical setting has primarily been limited to easily accessible and/or superficial diseases, for which traditional light delivery can be performed noninvasively. Cerenkov radiation, as generated from medically relevant radionuclides, has been suggested as a means to deliver light to deeper tissues noninvasively to overcome this depth limitation. This article investigates the utility of Cerenkov radiation, as generated from the radionuclide yttrium-90, for activating the PDT process using clinically approved aminolevulinic acid at 1.0 mm and also the more efficient porphyrin-based photosensitizer mesotetraphenylporphine with two sulfonate groups on adjacent phenyl rings (TPPS2a) at 1.2 μM. Experiments were conducted with monolayer cultured glioma and breast tumor cell lines. Although aminolevulinic acid proved to be ineffective for generating a therapeutic effect at all but the highest activity levels, TPPS2a produced at least a 20% therapeutic effect at activities ranging from 6 to 60 μCi/well for the C6 glioma cell line. Importantly, these results demonstrate for the first time, to our knowledge, that Cerenkov radiation generated from a radionuclide can be used to activate PDT using clinically relevant photosensitizers. These results therefore provide evidence that it may be possible to generate a phototherapeutic effect in vivo using Cerenkov radiation and clinically relevant photosensitizers.
Keywords: Cerenkov radiation, photodynamic therapy
I. INTRODUCTION
Photodynamic therapy (PDT) is a form of medical treatment that uses photosensitizers to convert ultraviolet, visible, and near-infrared radiation into chemical energy—primarily in the form of reactive oxygen species—that destroys targeted cells and tissues. With a large selection of applicable photosensitizers to use, PDT has been investigated as a treatment for a wide range of diseases including tumors,1 skin diseases,2 and macular degeneration. 3 Aside from skin care treatment, for where it has seen widespread clinical adoption, PDT has not translated into standard care in the clinical setting for many of these diseases due to a variety of limitations. One of the most significant constraints of PDT is the limited penetration depth below the tissue surface at which light can noninvasively be delivered using traditional light sources, such as light-emitting diodes (LEDs) and lasers.4 This is primarily due to the fundamental absorption and scattering of light in tissue, which can be somewhat compensated by the use of longer wavelengths. However, diseased tissue in the deepest parts of the body will still present a challenge. Although PDT can be delivered to these locations with traditional light sources, it is strictly limited to a short time window during an invasive surgical procedure. To accommodate such time constraints in the surgical setting, higher fluence rates must be used to reduce total light treatment time. This, however, can lead to oxygen depletion and photobleaching and is an undesirably less efficient process than when lower fluence rates are used.5,6 Interstitial light-delivery methods using fiber optics and/or diffusers can provide light to deeper regions of tissue, but they must still be implemented as part of an invasive procedure and due to the irregular geometry of tumors, presenting difficulties in treating the entire tumor and/or its margins.7 Ultimately, for all traditional light sources the dose of light will still vary significantly with depth from the source due to scattering and absorption of light in tissue, making it difficult to determine and deliver a consistent light dose to the entire diseased tissue region.
To overcome these limitations, a variety of noninvasive deep light sources have been investigated. The two most studied thus far include nanoscintillators and Cerenkov radiation. Nanoscintillators use nanoparticles containing high-Z elements that, when excited with external beam photon radiation, generate small amounts of light to activate the PDT process. Presently, this has been used to treat subcutaneous tumors in mice,8 but safely and systemically delivering these nanoscintillators still remains a challenge. Alternatively, Cerenkov radiation delivers a small amount of ultraviolet and visible light that is generated when high-energy charged particles, such as those generated from external beam radiotherapy and clinically relevant radionuclides used in imaging and therapy, travel through a dielectric medium at greater than the phase velocity of light for that material. Cerenkov radiation has most commonly been used for small animal imaging9 and as an excitation source for fluorophores10,11 and phosphors12–14 using external beam radiotherapy. In addition, it has more recently been studied to activate the phototherapeutic process in subcutaneous tumors in mice.15
Each of these two light sources have the potential to generate light more homogeneously throughout diseased tissue by emitting light at the molecular level, which in addition to simplifying light dosimetry, can also help to negate the effects of poor photosensitizer specificity. These light sources are also well suited to PDT because they emit light at shorter wavelengths, where photosensitizers, especially those that are porphyrin based, often have stronger absorption. The primary limitation to these light sources is that they emit at very low fluence rates and can therefore require significantly more time to deliver their total dose. Whereas this ensures minimal oxygen depletion and photobleaching of the photosensitizer, new treatment strategies may need to be developed to compensate for the limited amount of light available to use when compared with traditional PDT light sources.
The fluence rate of Cerenkov radiation generated from radionuclides is primarily dependent on the amount of radionuclide present, the index of refraction of the targeted tissue, and the intrinsic energy distribution of the particles emitted by the radionuclide.16 Due to its energetic β particles and longer half-life of 64.1 h, 90Y represents perhaps the upper limit of the amount of Cerenkov radiation that can be delivered for phototherapeutic purposes using a radionuclide as an illumination source.17,18 Providing further significance, 90Y is currently implemented in radioimmunotherapy19 and selective internal radiation therapy20 as a clinically approved treatment. Despite these advantages, 90Y has not been empirically investigated as an illumination source for photochemical processes.
This work aims to determine whether Cerenkov radiation generated from the radionuclide 90Y can be used to activate the PDT process and generate a phototherapeutic effect using a clinically approved photosensitizer as well as a commercially available more effective porphyrin-based photosensitizer. For a clinically approved photosensitizer, the prodrug aminolevulinic acid (ALA) was used, because it is one of the most studied photosensitizers in the clinic. We selected mesotetraphenylporphine with two sulfonate groups on adjacent phenyl rings (TPPS2a) for the more effective porphyrin-based photosensitizer.21,22 Although photosensitizers are available that are even more effective than TPPS2a, they are often in the form of nanoparticles, wherein the size, coatings, and shape can modify their photochemical properties, biocompatibility, pharmacokinetics, and toxicology, thus making them less reproducible and more challenging for the clinical setting.23 Furthermore, the relative efficiency of ALA and TPPS2a has previously been well characterized with illumination sources representative of Cerenkov radiation, for which TPPS2a was found to be approximately 16 times more effective than ALA.22 This article explores the utility of 90Y as an illumination source for PDT with a clinically approved photosensitizer as well as a more efficient porphyrin based-photosensitizer.
II. MATERIALS AND METHODS
A. Cell Culture
The C6 glioma cell line (purchased through the American Type Culture Collection [ATCC], Manassas, VA) and MDA-MB-231-luc-D3H1 breast tumor cell line (purchased from Perkin Elmer Inc., Waltham, MA) were cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s minimum essential medium (Gibco, Grand Island, NY) without phenol red indicator and with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL). These cancer cell lines were chosen as representative deep tumors that would be well suited for PDT treatment using noninvasive deep light sources.
B. PDT Treatments
Cells were seeded in 100 μL/well of media on white, clear bottom, 96-well plates at 2 × 103 cells/well. For ALA (Sigma-Aldrich Co., St. Louis, MO) studies, cells were incubated in 1.0 mm ALA with reduced FBS concentration (2%) media for 6 h. A lower FBS concentration was chosen, because serum is known to reduce the amount of PpIX accumulation in cells and hence reduces the PDT effect.24 For TPPS2a studies (Frontier Scientific Inc., Logan, UT) cells were incubated for 18 h at a range of concentrations (0.040 to 4.0 μM), also at a reduced FBS concentration. After incubation with the photosensitizer, buffered 90YCl3 (Perkin Elmer Inc.) was added at 6.0, 18, or 60 μCi/well. The cells were then incubated with the radionuclide for 72 h. This amount of time allowed the 90Y to deliver 54% of the total Cerenkov radiation that it can produce, if allowed to completely decay, and also is reasonably representative of how long the 90Y would be retained in vivo. To ensure that cells had adequate ALA, additional ALA was added again at 24 and 48 h after injection of the radionuclide. Cell media was then removed and replaced with complete media for the regrowth period. For the early wash experiments, cell media was replaced with fresh media to remove excess TPPS2a immediately before the addition of 90YCl3. After 90YCl3 addition, the Cerenkov radiation of each plate was measured on a VICTOR3 Multilabel Counter plate reader (Perkin Elmer Inc.); this served to validate the amount of activity in the wells, ensure no well-to-well cross-talk, and determine the residual amount of radioactivity after the media change. All experimental procedures after the addition of the photosensitizers were completed in a dark room with dim, deep red, LED sources in order to see.
C. Cell Viability
After a 72-h regrowth period, cell viability was assessed with the WST-1 proliferation assay (Roche Diagnostics, Indianapolis, IN) following the manufacturer’s protocol. To determine cell viabilities, treatment groups were normalized to controls. Experiments were repeated three times, and the data presented herein are the mean ± standard error of the mean (sem) of all experiments. The Student’s t-test was used to determine statistical significance.
III. RESULTS and DISCUSSION
A. Photosensitizer Comparison
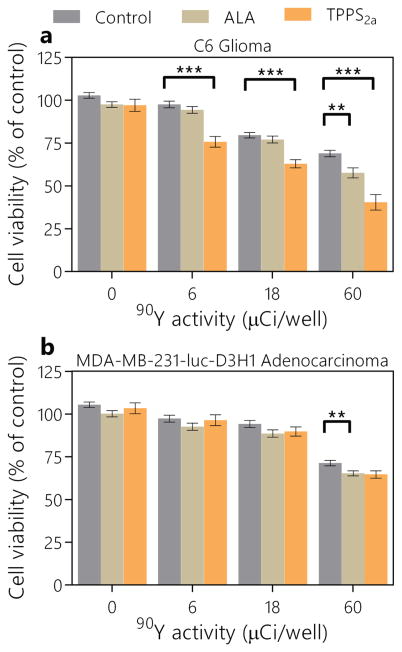
Initial experiments were conducted with the two different cell lines and two photosensitizers, each at a single concentration. The concentrations used were determined based from previous experiments using LED light sources representative of Cerenkov radiation.22 The results from these experiments are shown in Figure 1. The radiotherapeutic response from 90Y alone was roughly similar for each cell line, although the C6 glioma cell line appeared to have a more gradual trend as dose increased, whereas the breast tumor cell line showed no effect until 60 μCi/well. When ALA was used, no significant phototherapeutic effect—defined here as the difference between viabilities at a single 90Y activity level with and without photosensitizer—was observed for either cell line for <60 μCi/well of 90Y. At this activity level, the additional phototherapeutic effect was only 6% and 11% for the breast and glioma cell lines, respectively. Whereas ALA had a similar effect for each of the cell lines, TPPS2a was largely ineffective with the breast tumor cells but generated phototherapeutic effects at all activity levels down to 6 μCi/well. Across the different activity levels, TPPS2a produced an average of 22% additional phototherapeutic effect beyond the radiotherapeutic effect, making it ~11 times more effective than ALA. Interestingly, the additional phototherapeutic effect was not proportional to the activity per well and therefore the amount of Cerenkov radiation being generated; rather, it was somewhat consistent across activities. This nonlinear trend, however, is consistent with previous results using Cerenkov radiation as an excitation source for in vitro PDT.15 The variability among cell lines agrees with previous results, which also found that TPPS2a’s uptake and effectiveness are cell-line dependent.22,25 Although phototherapeutic effects using ALA were on the fringe of detection, TPPS2a’s phototherapeutic effect was roughly proportional to the radiotherapeutic effect and merited further investigation.
FIG. 1.
Comparison between the efficacy of ALA and TPPS2a as photosensitizers activated with Cerenkov radiation generated from 90Y. Cell viabilities are plotted for C6 glioma cells (a) and MDA-MB-231-luc-D3H1 breast cancer cells (b). Values are mean ± sem. **p < 0.01; ***p < 0.001.
B. Optimum TPPS2a Concentration
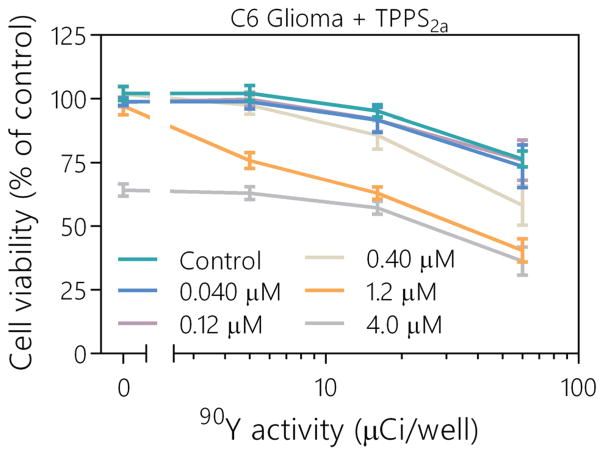
To further characterize and optimize the therapeutic effect of TPPS2a, as activated with Cerenkov radiation from 90Y, the photosensitizer’s concentration was tested at 0.040, 0.12, 0.40, 1.2, and 4.0 μM (with a standard incubation period of 72 h). This range was used to explore the lower limit of the effect, where the minimum therapeutic effect was observed, as well as the upper limit, where the photosensitizer began to produce a dark toxicity effect. Due to the limited effectiveness of TPPS2a with the MDA-MB-231-luc-D3H1 breast cancer cell line, experiments were only performed with the C6 glioma cell line. Further studies were also not conducted with ALA, because 1.0 mm has been shown to be an approximately ideal concentration to use, with doses above this providing minimal additional benefit or even reduced effect.26,27 The results from this study are presented in Figure 2. Dark toxicity from TPPS2a was observed at 4.0 μM; however, the additional toxicity from the high photosensitizer concentration did not appear to be additive at higher activities. This inconsistency is most likely due to the additional TPPS2a in the media absorbing the Cerenkov radiation before it was able to enter inside a cell, thus reducing the phototherapeutic effect. The minimum concentration at which a phototherapeutic effect was observed was 0.40 μM. The largest therapeutic effect for in vitro Cerenkov radiation activated PDT was observed at 1.2 μM.
FIG. 2.
Viabilities of C6 cells are shown for varying concentrations of TPPS2a, ranging from 0 μM as control to the upper limit of 4.0 μM, where dark toxicity was observed. 90Y was added at 0, 6.0, 18, and 60 μCi/well. Values are mean ± sem.
C. Effect of Extracellular TPPS2a
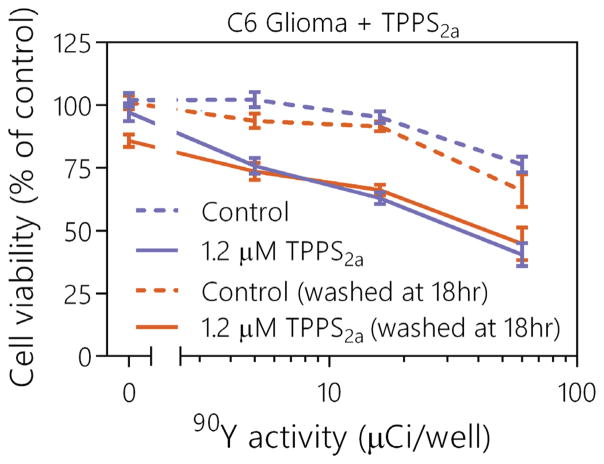
To determine if any remaining extracellular TPPS2a was absorbing a significant amount of light outside of the cells, thus absorbing Cerenkov radiation before it entered the cells, experiments were conducted in which the media was washed after the initial 18-h incubation period and just before the addition of 90Y. These experiments also confirmed that the phototherapeutic effect was generated inside of the cell, as opposed to an effect produced extracellularly from the unlocalized TPPS2a. Results from this study are shown in Figure 3. No significant differences were observed for either control or TPPS2a treatment groups when the media was washed after the 18-h incubation period. This suggests that the additional photosensitizer was not absorbing a significant amount of Cerenkov radiation outside the cells and inhibiting the phototherapeutic process. If any phototherapeutic activation of TPPS2a was occurring outside the cell, it is likely that it had minimal effects on the overall viability of the cells. By delivering low fluence rates during several days, the effect of an initial decrease in photosensitizer localization could have been dramatically minimized. Due to localization into the glioma cells and the larger phototherapeutic effect that this generated, TPPS2a would be a candidate for further investigation of Cerenkov radiation activated PDT.
FIG. 3.
Comparison of therapeutic effects between standard photosensitizer incubation methods used for all previous TPPS2a experiments and once cells were washed after the 18-h incubation period but before the addition of 90Y. Values are mean ± sem.
IV. CONCLUSION
This work demonstrates that Cerenkov radiation as generated from 90Y can be used to activate the PDT process in vitro using photosensitizers ALA and TPPS2a. The phototherapeutic effects were significantly larger and proportional to the radiotherapeutic effect observed using the porphyrin based-photosensitizer TPPS2a, whereas only a small effect occurred with ALA and only at the highest 90Y activities. A concentration of 1.2 μM of TPPS2a was found to be the most effective photosensitizer dose to produce the largest phototherapeutic effect without causing dark toxicity. The additional photosensitizer in the media during incubation with 90Y was not found to inhibit or enhance the therapeutic process at that concentration. For the combination of 90Y and TPPS2a, the largest phototherapeutic effect that could be generated was ~22%. In all cases, however, the phototherapeutic effects were primarily seen during activities wherein a radiotherapeutic response was also observed. Thus, any phototherapeutic effect from the Cerenkov radiation–activated PDT was detected as a synergistic or additive effect rather than a standalone effect. Cerenkov radiation activated PDT, although this not necessarily intended as a primary treatment method for diseases. Rather, its role would likely be as an adjuvant treatment to radiotherapy and/or chemotherapy to assist in treating postoperative margins, undetected metastasis, and/or deep targets that cannot be reached with surgical intervention. As would be the case for all systemically injected light sources, to study this therapy in vivo, one would need to ensure that there was no undesired colocalization of the targeted 90Y with the photosensitizer in regions other than the tumor. This work provides evidence that Cerenkov radiation from 90Y is perhaps near the limit for use as an illumination source for PDT using clinically relevant photosensitizers. Given the variety of clinically approved photosensitizers and radionuclides readily available, further investigation is merited.
Acknowledgments
This work was supported in part by National Institutes of Health grant nos. R21 EB018750 and R01 EB015471.
References
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene J, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: An update. Cancer J Clin. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babilas P, Schreml S, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol Photo. 2010;26:118–32. doi: 10.1111/j.1600-0781.2010.00507.x. [DOI] [PubMed] [Google Scholar]
- 3.Silva JN, Filipe P, Morliere P, Maziere JC, Freitas JP, Gomes MM, Santus R. Photodynamic therapy: Dermatology and ophthalmology as main fields of current applications in clinic. Biomed Mater Eng. 2008;18:319–27. [PubMed] [Google Scholar]
- 4.Stolik S, Delgado JA, Perez A, Anasagasti L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B. 2000;57:90–3. doi: 10.1016/s1011-1344(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 5.Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38:489–93. doi: 10.1002/lsm.20327. [DOI] [PubMed] [Google Scholar]
- 6.Mathews MS, Angell-Petersen E, Sanchez R, Sun CH, Vo V, Hirschberg H, Madsen SJ. The effects of ultra low fluence rate single and repetitive photodynamic therapy on glioma spheroids. Lasers Surg Med. 2009;41:578–84. doi: 10.1002/lsm.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon I, Li JZ, Shim YK. Advance in photosensitizers and light delivery for photodynamic therapy. Clin Endosc. 2013;46:7–23. doi: 10.5946/ce.2013.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Wang GD, Chuang YJ, Zhen Z, Chen X, Biddinger P, Hao Z, Liu F, Shen B, Pan Z, Xie J. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015;15:2249–56. doi: 10.1021/nl504044p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355–65. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsson J, Davis SC, Gladstone DJ, Pogue BW. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med Phys. 2011;38:4127–32. doi: 10.1118/1.3592646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demers JL, Davis SC, Zhang RX, Gladstone DJ, Pogue BW. Cerenkov excited fluorescence tomography using external beam radiation. Opt Lett. 2013;38:1364–6. doi: 10.1364/OL.38.001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang RX, Glaser A, Esipova TV, Kanick SC, Davis SC, Vinogradov S, Gladstone D, Pogue BW. Cerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies. Biomed Opt Express. 2012;3:2381–94. doi: 10.1364/BOE.3.002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang RX, Davis SC, Demers JH, Glaser AK, Gladstone DJ, Esipova TV, Vinogradov SA, Pogue BW. Oxygen tomography by Cerenkov-excited phosphorescence during external beam irradiation. J Biomed Opt. 2013;18:050503. doi: 10.1117/1.JBO.18.5.050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang RX, D’Souza AV, Gunn JR, Esipova TV, Vinogradov SA, Glaser AK, Jarvis AL, Gladstone DJ, Pogue BW. Cherenkov-excited luminescence scanned imaging. Opt Lett. 2015;40:827–30. doi: 10.1364/OL.40.000827. [DOI] [PubMed] [Google Scholar]
- 15.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–9. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank I, Tamm I. Coherent visible radiation of fast electrons passing through matter. Dokl Akad Nauk SSSR. 1937;14:109–14. [Google Scholar]
- 17.Glaser AK, Zhang R, Andreozzi JM, Gladstone DJ, Pogue BW. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys Med Biol. 2015;60:6701–18. doi: 10.1088/0031-9155/60/17/6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill RK, Mitchell GS, Cherry SR. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys Med Biol. 2015;60:4263–80. doi: 10.1088/0031-9155/60/11/4263. [DOI] [PubMed] [Google Scholar]
- 19.Witzig TE, Molina A, Gordon LI, Emmanouilides C, Schilder RJ, Flinn IW, Darif M, Macklis R, Vo K, Wiseman GA. Long-term responses in patients with recurring or refractory B-cell non-hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–10. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Kessel D, Thompson P, Saatio K, Nantwi KD. Tumor-localization and photosensitization by sulfonated derivatives of tetraphenylporphine. Photochem Photobiol. 1987;45:787–90. doi: 10.1111/j.1751-1097.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartl BA, Hirschberg H, Marcu L, Cherry SR. Characterizing low fluence thresholds for in vitro photodynamic therapy. Biomed Opt Express. 2015;6:770–9. doi: 10.1364/BOE.6.000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Jajoo A, Dube A. 5-Aminolevulinic acid-induced protoporphyrin-IX accumulation and associated phototoxicity in macrophages and oral cancer cell lines. J Photochem Photobiol B. 2007;88:156–62. doi: 10.1016/j.jphotobiol.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Vikdal M, Weyergang A, Selbo PK, Berg K. Vascular endothelial cells as targets for photochemical internalization (PCI) Photochem Photobiol. 2013;89:1185–92. doi: 10.1111/php.12126. [DOI] [PubMed] [Google Scholar]
- 26.Kiesslich T, Helander L, Illig R, Oberdanner C, Wagner A, Lettner H, Jakab M, Plaetzer K. Real-time analysis of endogenous protoporphyrin IX fluorescence from δ-aminolevulinic acid and its derivatives reveals distinct time- and dose-dependent characteristics in vitro. J Biomed Opt. 2014;19:085007. doi: 10.1117/1.JBO.19.8.085007. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez L, de Bruijn HS, Di Venosa G, Mamone L, Robinson DJ, Juarranz A, Batlle A, Casas A. Porphyrin synthesis from aminolevulinic acid esters in endothelial cells and its role in photodynamic therapy. J Photoch Photobiol B. 2009;96:249–54. doi: 10.1016/j.jphotobiol.2009.07.001. [DOI] [PubMed] [Google Scholar]