Abstract
Objective
We sought to measure retention in care and identify predictors of non-retention among patients receiving ART with streamlined delivery during the first year of the ongoing SEARCH “test-and-treat” trial (NCT 01864603) in rural Uganda and Kenya.
Design
Prospective cohort of patients in the intervention arm of the SEARCH Study.
Methods
We measured retention in care at 12 months among HIV-infected adults who linked to care and were offered ART regardless of CD4 cell count, following community-wide HIV-testing. Kaplan-Meier estimates and Cox proportional hazards modeling were used to calculate the probability of retention at one year and identify predictors of non-retention.
Results
Among 5,683 adults (age ≥ 15) who linked to care, 95.5% (95% CI: 92.9 – 98.1%) were retained in care at 12 months. The overall probability of retention at one year was 89.3% (95% CI: 87.6 – 90.7%) among patients newly linking to care and 96.4% (95% CI: 95.8 – 97.0%) among patients previously in care. Younger age and pre-ART CD4 below country treatment initiation guidelines were predictors of non-retention among all patients. Among those newly linking, taking more than 30 days to link to care after HIV diagnosis was additionally associated with non-retention at one year. HIV viral load suppression at 12 months was observed in 4,227/4736 (89%) of patients retained with valid viral load results.
Conclusion
High retention in care and viral suppression after 1 year were achieved in a streamlined HIV care delivery system in the context of a universal test-and-treat intervention.
Keywords: HIV, Africa, Antiretroviral therapy, Healthcare, Retention in care
Introduction
Antiretroviral therapy (ART) reduces morbidity and mortality in patients with all CD4+ Tcell count levels[1, 2] and decreases the probability of HIV transmission to uninfected partners[3]. In order to realize these individual and public health benefits, in 2015 the WHO guidelines were updated to recommend ART for all HIV infected individuals, regardless of CD4 count[4] (universal treatment). Retention in care will be critical to the success of universal test-and-treat strategies, and treatment programs will be challenged to extend ART treatment to the newly eligible patients while supporting retention in care in an expanded patient population. Retention in care will also be essential to realizing the UNAIDS target of 90-90-90, particularly the final goal that 90% of the population on ART is virally suppressed.
Significant questions remain about whether high retention in a universal test-and-treat system is possible in the setting of increasing patient volumes, an increasing proportion of asymptomatic patients, and known barriers to retention. Current estimates of retention in sub-Saharan Africa are widely variable and the estimated regional average 12-month retention of 76% (range 65% – 89%) is insufficient to achieve these targets [5]. In addition, current retention estimates are likely biased due to incomplete ascertainment of outcomes and do not reflect retention under universal treatment. Individual factors including younger age[6–13], male gender[8–18], lower education[16], occupation[11, 17], and mobility[19] have all been associated with lower levels of retention in ART care and could affect retention under the test-and-treat paradigm. Additionally, clinic factors contributing to disengagement in care, including long wait times, negative staff attitudes and frequent visits[9, 20–22] could be exacerbated as ART access is expanded.
With the advent of large scale efficacy trials of the universal test-and-treat strategy being conducted in sub-Saharan Africa[23, 24], new research is needed to bridge existing clinical and implementation science knowledge gaps around retention in care in these ambitious universal treatment strategies. The intervention arm of the ongoing Sustainable East Africa Research on Community Health (SEARCH) test-and-treat trial (NCT01864603) provides ART within a streamlined care delivery system, which was designed to offer patient-centered and efficiently delivered care to minimize many of the traditional barriers to retention. We sought to characterize predictors and barriers to retention in care in the intervention arm of the SEARCH trial during the first year of implementation of universal testing and treatment utilizing a streamlined model of ART care.
Methods
SEARCH Study Design
The SEARCH HIV test-and-treat study is a community cluster-randomized controlled trial in 32 communities in three regions: western Kenya, southwestern Uganda, and eastern Uganda. All communities received a community census and population-wide HIV testing at baseline in 2013 – 2014. These are rural communities composed of geopolitical units just above the village level (termed a ‘parish’ in Uganda and a ‘sublocation’ in Kenya) with a population of about 10,000 people each, within the catchment area of a government supported ART clinic(s). Using a hybrid mobile HIV testing approach in which 2-week multi-disease community health campaigns (CHCs) are followed by home-based testing (HBT) of CHC non-participants, 89% of the population was tested at baseline[25]. In the 16 SEARCH intervention communities, all HIV-infected individuals were referred immediately upon HIV diagnosis to HIV care and then offered immediate ART initiation or continuation of ART therapy at the clinic within a streamlined model of care.
Streamlined care
The streamlined care model was developed using the PRECEDE framework[26] and was designed to reduce patient barriers to care. It featured ART start at first linkage to clinic, visits with reduced wait time [27], quarterly (as opposed to monthly) follow-up visits for stable patients, a patient-centered approach to care in which staff were trained to provide care in a welcoming and empathetic environment, a telephone hotline for patients with medical questions and appointment scheduling concerns, appointment reminders by phone or SMS, and provision of viral load results through a structured viral load counseling protocol [28, 29]. Patients who missed a clinic visit were tracked using a “tiered” approach performed by nurses in Uganda and community health workers in Kenya. Under the “tiered” tracking approach the patient was called after the first missed visit to determine the reason for the missed visit and reschedule the appointment. If the patient also missed the rescheduled appointment, or the patient was not reached by phone, a tracker visited the patient at home or an alternative location of their choice. In some communities the patient was offered ART in an alternative location of the patient’s choosing. All patients initiating ART at CD4 T- cell counts above country-treatment guidelines who missed a clinic visit were tracked from the beginning of the study; retention tracking was expanded to include all patients starting in March 2014 in Uganda and in June 2014 in Kenya. No financial incentives for retention in care were provided.
Measures
Patient demographics were obtained during a baseline home-to-home census enumeration. Patients with a Ministry of Health HIV medical record at the time of the baseline CHC were considered previously linked to care, and those with an ART start date indicated on their medical record were classified as prescribed ART at baseline. A viral load was obtained at least 6 months after the start of streamlined care, either during follow-up year 1 hybrid mobile testing (CHC or HBT one year after baseline testing) or in clinic. Plasma HIV-1 RNA viral load was measured from finger-prick capillary[30] or venous blood collection by commercial real-time PCR assays at multiple reference laboratories.
Retention in care was defined as not more than 90 days late to a scheduled 12-month follow-up. Patients were considered out of care (non-retention) if they were found alive, in the community and not enrolled in HIV care, moved out of the community without a documented transfer, or were lost to follow-up. Patients with a documented transfer and patients who died were censored in time-to-event analysis. Patients were considered virally suppressed if their HIV viral load was < 500 copies/ml at the time of follow-up year 1 hybrid testing or, if none available, clinic viral load performed closest in time to 12 months.
Gender, age, education, occupation, mobility, access to a mobile phone, and HIV testing location (CHC versus home-based testing) were obtained during the baseline year. Education was categorized as no school, any primary or completed primary school, and any secondary or further education. The 13 individuals who answered “don’t know/refused” on the baseline census were considered to have attended no school in the analysis. The 20 occupational categories at baseline were further classified as formal (student, teacher, government worker, military worker, health worker, factory worker), informal-high risk (fisherman, bar owner, bar worker, truck/taxi/motorcycle/bike/boat driver, or tourism), informal-low risk (farmer, shopkeeper, market vendor, hotel worker, household worker, construction worker), no job (unemployed, disabled), or other. Individuals were considered a stable resident if they reported having resided within the community for at least 6 months out of the 12 months prior to census enumeration.
Statistical Analysis
The objective of this analysis was to describe 12-month retention in care and predictors of non-retention among adults who linked to care in a SEARCH intervention community during the first year of the intervention. The analysis was thus restricted to adults (≥ 15 years) who had at least one clinic visit after baseline hybrid testing. Individuals whose first clinic visit occurred <12 months before database closure date were also excluded. For the time-to-event analysis, patients entered the risk group (T0) at their first clinic visit after baseline hybrid testing. Time to attrition was calculated as the time between T0 and a patient’s last scheduled clinic visit. Kaplan Meier survival estimates were used to calculate probability of retention at one year. Hazard ratios for non-retention were also computed using Cox proportional hazards modeling. Follow up continued until attrition, censoring due to death or transfer, or 365 days after linkage. A secondary analysis in which death was treated as a competing risk was also performed. The proportional hazards assumption was assessed graphically and with Schoenfeld residuals. To evaluate predictors of requiring enhanced retention support in order to stay in care, we used logistic regression to evaluate adjusted predictors of the need for retention tracking after the date all patients became eligible for retention tracking (i.e. after March 1, 2014 in Uganda and after June 1, 2014 in Kenya). Multivariate models included region, sex, and age based on a priori determination. Covariates that were significant in univariate analysis were added in stepwise progression and included in the model if they contributed significantly to the fit of the model using a likelihood ratio test with p<0.1. In the proportional hazards model, age was stratified into three categories: 15–24, 25–29, and >30 years (because retention was homogenous within these categories and did not violate the proportional hazards assumption). Community was included as a fixed effect in all models and cluster-robust standard errors with household as the unit of independence were used to control for clustering by community and household. All analyses were stratified by care status at the time of baseline hybrid testing (previously in care vs. newly linking). Stata v14 (College Station, Texas) was used for analysis.
Ethics
The Makerere University School of Medicine Research and Ethics Committee (Uganda), the Ugandan National Council on Science and Technology (Uganda), the Kenya Medical Research Institute Ethical Review Committee (Kenya), and the University of California San Francisco Committee on Human Research (USA) approved the study protocol including the consent procedures. All participants provided verbal informed consent in their preferred language with fingerprint biometric confirmation of agreement.
Results
Study population
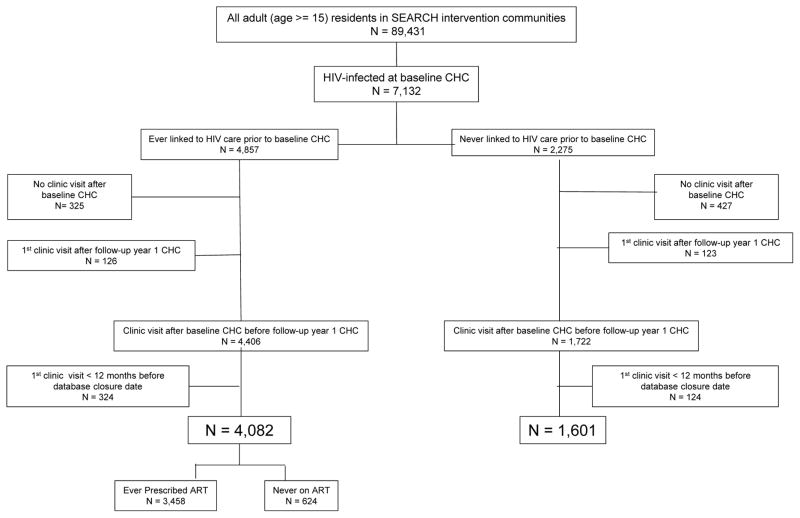
Between April 2, 2013 and June 8, 2014, 89,431 adults (≥ 15 years) were enumerated in the 16 intervention communities[25]. Among the 7,132 (8%) who were found to be HIV-infected at the time of baseline hybrid mobile testing, 6,128 (86%) had at least one clinic visit after the baseline CHC and before follow-up year one CHC. An additional 448 (6%) were excluded because their first visit occurred <12 months before database closure date resulting in 5,683 (80%) included in analysis. At baseline, 4,082 (72%) had a history of HIV care and 3,458 (61%) had ever been prescribed ART, while 1602 (28%) were linking to care for the first time [Figure 1].
Figure 1.
Study population
Demographics
Of the 5,683 patients who linked to HIV care, 3,703 (65.2%) were from Kenya, 1,306 (23.0%) were from western Uganda, and 674 (11.9%) were from eastern Uganda; 3,820 (67.2%) were female; 603 (10.6%) were between 15–24 years of age and 1,494 (26.3%) were under age 30; and almost all were stable residents (5,544, 97.5%). One thousand two hundred fifty seven (22.1%) had a pre-ART CD4 above country treatment guidelines at the time of ART start. Four thousand three hundred eight two (77.1%) had tested at the CHC vs. 23.9% at HBT. Regional differences were seen; Kenyan participants were more likely to be younger, have lower levels of education, work in the fishing industry, and to have tested during home-based testing (vs. at CHCs). Kenyans were also more likely to have a history of previous HIV care and ART [Table 1].
Table 1.
Baseline characteristics among adult (age ≥ 15 years) residents of SEARCH intervention communities who linked to care after baseline CHC and before follow-up year 1 CHC (N = 5683)
| Uganda-West (N = 1306) | Uganda-East (N = 674) | Kenya (N = 3703) | Total (N = 5683) | |
|---|---|---|---|---|
| Sex | ||||
| Male [n (%)] | 219 (32.5%) | 219 (32.5%) | 1163 (31.4%) | 1863 (32.8%) |
| Female [n (%)] | 455 (67.5%) | 455 (67.5%) | 2540 (68.6%) | 3820 (67.2%) |
| Age [years, median (IQR)] | 36 (29–44) | 39 (31–46) | 37 (29–46) | |
| 15–19 years | 42 (3.2%) | 20 (3.0%) | 75 (2.0%) | 137 (2.4%) |
| 20–24 years | 130 (10.0%) | 43 (6.4%) | 293 (7.9%) | 466 (8.2%) |
| 25–29 years | 189 (14.5%) | 83 (12.3%) | 619 (16.7%) | 891 (15.7%) |
| 30–34 years | 232 (17.8%) | 91 (13.5%) | 604 (16.3%) | 927 (16.3%) |
| 35–39 years | 207 (15.9%) | 118 (17.5%) | 617 (16.7%) | 942 (16.6%) |
| 40–44 years | 187 (14.3%) | 125 (18.6%) | 447 (12.1%) | 759 (13.4%) |
| > 45 years | 319 (24.4%) | 194 (28.8%) | 1048 (28.3%) | 1561 (27.5%) |
| Education | ||||
| No School | 230 (17.6%) | 123 (18.3%) | 184 (5%) | 537 (9.5%) |
| Primary or less | 794 (60.8%) | 405 (60.1%) | 3214 (86.8%) | 4413 (77.7%) |
| Any Secondary or further | 282 (21.6%) | 144 (21.4%) | 293 (7.9%) | 313 (5.5%) |
| Don’t know/refused to answer | 0 | 1 (0.2%) | 12 (0.3%) | 13 (0.2%) |
| Occupation | ||||
| Farming | 858 (65.7%) | 485 (72.0%) | 2118 (57.2%) | 3461 (65.7%) |
| Fishing | 1 (0.1%) | 3 (0.5%) | 417 (11.3%) | 421 (7.4%) |
| Shopkeeper/Vendor | 103 (7.9%) | 30 (4.5%) | 425 (11.5%) | 558 (9.8%) |
| Household worker | 50 (3.8%) | 30 (4.5%) | 175 (4.7%) | 255 (4.5%) |
| Transport worker | 26 (2.0%) | 13 (1.9%) | 33 (0.9%) | 72 (1.3%) |
| Student | 18 (1.4%) | 12 (1.8%) | 42 (1.1%) | 72 (1.3%) |
| Other | 194 (14.9%) | 78 (11.6%) | 299 (8.1%) | 571 (10.1%) |
| No Job | 56 (4.3%) | 23 (3.4%) | 194 (5.2%) | 273 (4.8%) |
| Mobility status | ||||
| Stable n(%) | 1258 (96.3%) | 658 (97.6%) | 3628 (98%) | 5544 (97.5%) |
| Mobile n(%) | 48 (3.7%) | 16 (2.4%) | 75 (2.0%) | 139 (2.5%) |
| Access to mobile phone [n (%)] | 758 (58.0%) | 306 (45.4%) | 2657 (71.8%) | 3721 (65.5%) |
| CD4 at baseline CHC | ||||
| <50 cells/mm3 | 10 (0.8%) | 8 (1.2%) | 24 (0.7%) | 42 (0.7%) |
| 50–200 cells/mm3 | 93 (7.1%) | 59 (8.8%) | 188 (5.1%) | 340 (6.0%) |
| 200–350 cells/mm3 | 231 (17.7%) | 127 (18.8%) | 543 (14.4%) | 892 (15.7%) |
| 350–500 cells/mm3 | 326 (25.0%) | 166 (24.6%) | 783 (21.1%) | 1275 (22.4%) |
| >500 cells/mm3 | 588 (45.0%) | 281 (41.7%) | 1827 (49.3%) | 2696 (47.4%) |
| Missing baseline CD4 | 58 (4.4%) | 33 (4.9%) | 347 (9.4%) | 438 (7.7%) |
| Pre-ART CD4 above country treatment guidelines§ | 408 (31.2%) | 196 (29.1%) | 653 (17.6%) | 1257 (22.1%) |
| HIV RNA < 500 copies/ml | 431 (33.0%) | 190 (28.2%) | 1656 (44.7%) | 2277 (40.1%) |
| Missing baseline viral load | 381 (29.2%) | 193 (28.6%) | 873 (23.6%) | 1245 (21.9%) |
| Previous linkage to care [n (%)] | 743 (56.9%) | 396 (58.8%) | 2943 (79.5%) | 4082 (71.8%) |
| On ART before baseline CHC [n (%)] | 585 (44.8%) | 318 (47.2%) | 2359 (63.7%) | 3262 (57.4%) |
| HIV Testing Location | ||||
| CHC* | 1075 (82.3%) | 606 (89.9%) | 2701 (72.9%) | 4382 (77.1%) |
| HBT** | 228 (17.5%) | 60 (8.9%) | 998 (26.7%) | 1286 (22.6%) |
| Missing | 3 (0.2%) | 8 (1.2%) | 4 (0.1%) | 15 (0.3%) |
CHC = Community Health Campaign
HBT = Home Based Testing
Country guidelines for treatment were to initiate ART at CD4 < 350 cells/ml until December 2013 in Uganda and until June 2014 in Kenya, after which country treatment guidelines were changed to reflect the 2013 WHO guidelines of ART initiation at CD4 < 500 cells/ml
Retention in care outcomes
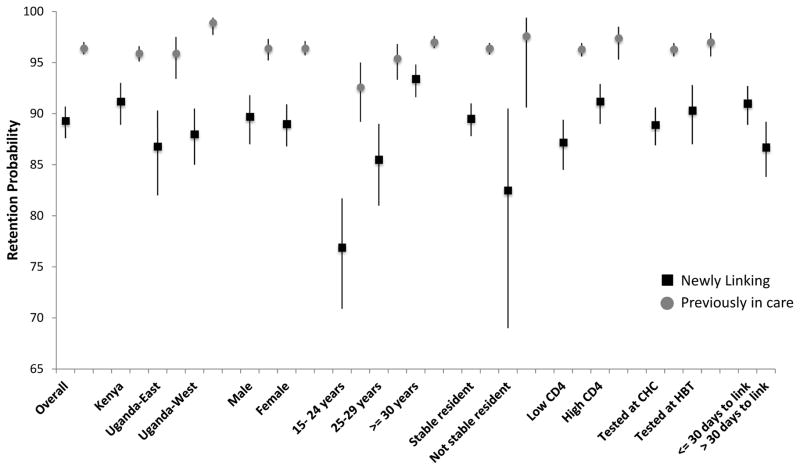
Of the 5,683 adults who linked to care during the eligible period, 5,058 (88.6%) were retained at their original clinic and 260 (4.6%) had a documented transfer to an alternative site. Sixty (1.1%) patients died, 108 (1.9%) were known to be alive and in the community, and 64 (1.1%) moved out of the community without a documented transfer [Appendix 1]. Of the 60 deaths, 15 were due to illness, 1 was due to an accident, and 44 were unknown. The overall probability of retention at one year, adjusted for out-transfers and deaths, was 95.5% (95% CI: 92.9 – 98.1%). The probability of retention at one year was 89.3% (95% confidence interval (CI) 87.6 – 90.7%) among patients newly linking to care and 96.4% (95% CI 95.8 – 97.0%) among patients previously in care. Probability of retention at one year was higher among those previously in care compared to those newly linking across all subgroups. The lowest observed retention (76.3%; 95% CI 70.9 – 81.7%) was among adults age 15–24 linking to care for the first time [Figure 2].
Figure 2.
Probability and 95% confidence intervals of being retained in care at one year among patients previously in care and newly linking to care in SEARCH intervention communities, overall and by subgroup
Low CD4 = pre-ART CD4 less than country treatment guidelines
High CD4 = pre-ART CD4 above country treatment guidelines
CHC = Community Health Campaign
HBT = Home-based testing
Virologic Outcomes
Overall, at follow-up viral load testing 4,455/5,683 (78.5%) had a suppressed viral load, 621/5,683 (10.9%) had a viral load > 500 copies/ml, and 610/5,683 (10.7%) did not have viral load results. Patients with viral load results available were more likely to be retained in care (p<0.001), live in East Uganda (p = 0.002), be age 30 years or older (p < 0.001), and have a pre-ART CD4 count above country treatment guidelines (p<0.001). Among the 5,058 retained in care, follow-up viral load data were available for 4,736 (93.6%); of these, 4,227 (89%) were suppressed.
Predictors of retention
Among both those newly linking to care and those previously in care, there was no significant association between non-retention and sex, education level, occupation, mobility, or whether HIV testing was completed at the CHC or in HBT. Non-retention was significantly more likely among younger patients, with those age 15–24 years being the least likely to be retained in care at 12 months among those newly linking to care (aHR 3.78, 95% CI 2.48 – 5.76) and those previously in care (aHR 2.70, 95% CI 1.70 – 4.29). Additionally, among patients newly linking to care, non-retention was more likely among residents of eastern Uganda (aHR 2.52, 95% CI 1.04 – 6.12) and those who took more than 30 days to link to care (aHR 1.41, 95% CI 1.03 – 1.95). In addition to young age, not having access to a mobile phone (aHR 1.94, 95% CI 1.36 – 2.77) was the only other predictor associated with increased risk of non-retention in multivariate analysis among patients who had a history of HIV care [Table 2]. Results were similar when death was treated as a competing risk rather than censoring event [Appendix 2].
Table 2.
Predictors of non-retention at 12 months in adult residents of SEARCH intervention communities who linked to HIV care after baseline CHC and before follow-up year 1 CHC (N =5683)
| Newly Linking to care (N = 1601) | Previously in Care (N = 4082) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictor | Hazard Ratio | 95% CI | Adj Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Adj Hazard Ratio | 95% CI |
| Region | ||||||||
| Kenya | ref | ref | ref | ref | ||||
| Uganda-East | 1.90 | 0.82–4.43 | 2.52 | 1.04–6.12 | 0.64 | 0.15–2.79 | 0.53 | 0.12–2.32 |
| Uganda-West | 0.95 | 0.36–2.51 | 0.88 | 0.32–2.42 | 0.28 | 0.13–0.60 | 0.25 | 0.12–0.52 |
| Sex | ||||||||
| Male | ref | ref | ref | ref | ||||
| Female | 1.08 | 0.80–1.47 | 0.76 | 0.54–1.06 | 1.01 | 0.71–1.43 | 0.84 | 0.59–1.21 |
| Age | ||||||||
| 15–24 | 3.94 | 2.72–5.70 | 3.78 | 2.48–5.76 | 2.48 | 1.57–3.90 | 2.70 | 1.70–4.29 |
| 25–29 | 2.39 | 1.61–3.55 | 2.51 | 1.67–3.77 | 1.48 | 0.95–2.32 | 1.59 | 1.01–2.51 |
| >= 30 | ref | ref | ref | ref | ||||
| Education | ||||||||
| No School | 0.53 | 0.30–0.94 | 0.78 | 0.44 – 1.40 | 0.83 | 0.43–1.60 | ||
| Primary | ref | ref | ref | |||||
| Any secondary or further | 0.78 | 0.50–1.23 | 0.76 | 0.48 – 1.21 | 0.88 | 0.48–1.61 | ||
| Occupation | ||||||||
| Formal | ref | ref | ref | |||||
| Informal – high risk | 1.71 | 0.37–7.86 | 1.37 | 0.28 – 6.73 | 1.01 | 0.29–3.49 | ||
| Informal – low risk | 2.16 | 0.67–7.01 | 1.79 | 0.51 – 6.28 | 1.10 | 0.44–2.80 | ||
| No job | 4.61 | 1.30–16.4 | 2.79 | 0.72 – 10.7 | 2.41 | 0.83–6.94 | ||
| Other | 2.32 | 0.59–9.2 | 1.79 | 0.42 – 7.58 | 0.68 | 0.18–2.59 | ||
| Access to a mobile phone | ||||||||
| Yes | ref | ref | ref | |||||
| No | 1.27 | 0.92–1.76 | 1.94 | 1.35–2.77 | 1.94 | 1.36 – 2.77 | ||
| Mobility | ||||||||
| Stable resident | ref | ref | ||||||
| Not stable resident | 1.77 | 0.87–3.60 | 0.82 | 0.20–3.39 | ||||
| Pre-ART CD4 | ||||||||
| Below country treatment guidelines | ref | ref | ref | ref | ||||
| Above country treatment guidelines | 0.68 | 0.50–0.93 | 0.62 | 0.45–0.84 | 0.75 | 0.40–1.38 | 0.63 | 0.34–1.17 |
| Site of testing | ||||||||
| CHC | ref | ref | ||||||
| HBT | 0.93 | 0.61–1.40 | 0.90 | 0.56–1.42 | ||||
| Time to link | ||||||||
| <= 30 days | ref | ref | ||||||
| > 30 days | 1.52 | 1.11–2.10 | 1.41 | 1.03–1.95 | ||||
Abbreviations: CI = Confidence Interval; CHC = Community Health Campaign; HBT = Home-based Testing
Country guidelines for treatment were to initiate ART at CD4 < 350 cells/ml until December 2013 in Uganda and until June 2014 in Kenya, after which country treatment guidelines were changed to reflect the 2013 WHO guidelines of ART initiation at CD4 < 500 cells/ml
Predictors of retention tracking
Patients newly linking to care were more likely to require retention tracking to stay in care compared to those previously in care (31.1% vs. 9.3%, p<0.001). Patients who lived in Uganda, younger patients, men, and those with a pre-ART CD4 count above country treatment guidelines were more likely to require retention tracking to stay in care. Testing site (CHC vs. HBT) and time to link were not associated with retention tracking [Table 3].
Table 3.
Predictors of requiring tracking for retention support among patients retained in care at one year (N = 5318)
| Newly Linking to care (N = 1412) | Previously in Care (N = 3906) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictor | OR | 95% CI | Adj OR | 95% CI | OR | 95% CI | Adj OR | 95% CI |
| Region | ||||||||
| Kenya | ref | ref | ref | ref | ||||
| Uganda-East | 4.76 | 2.89 – 7.85 | 12.0 | 4.80 – 30.1 | 8.15 | 3.39 – 19.6 | 6.85 | 2.82 – 16.7 |
| Uganda-West | 1.51 | 0.84 – 2.71 | 1.25 | 0.50 – 3.15 | 2.0 | 1.51 – 2.66 | 0.75 | 0.30 – 1.85 |
| Sex | ||||||||
| Male | ref | ref | ref | ref | ||||
| Female | 0.85 | 0.66 – 1.08 | 0.68 | 0.52 – 0.89 | 0.98 | 0.76 – 1.27 | 0.70 | 0.52 – 0.95 |
| Age | ||||||||
| 15–24 years | 1.97 | 1.27 – 3.03 | 2.55 | 1.59 – 4.10 | 2.76 | 1.77 – 4.31 | 2.39 | 1.46 – 3.91 |
| 25–29 years | 1.84 | 1.24 – 2.75 | 2.00 | 1.30 – 3.07 | 2.05 | 1.38 – 3.07 | 1.90 | 1.21 – 2.99 |
| 30–34 years | 1.28 | 0.85 – 1.92 | 1.40 | 0.90 – 2.16 | 1.88 | 1.28 – 2.77 | 1.85 | 1.22 – 2.81 |
| 35–39 years | 1.35 | 0.90 – 2.05 | 1.44 | 0.93 – 2.24 | 1.28 | 0.87 – 1.89 | 1.25 | 0.81 – 1.92 |
| 40–44 years | 0.96 | 0.61 – 1.52 | 1.09 | 0.67 – 1.78 | 1.08 | 0.71 – 1.64 | 1.06 | 0.67 – 1.66 |
| > 45 years | ref | ref | ref | ref | ||||
| Education | ||||||||
| No School | 0.87 | 0.60 – 1.28 | 1.18 | 0.76 – 1.82 | 1.02 | 0.69 – 1.49 | 1.24 | 0.80 – 1.92 |
| Primary | ref | ref | ref | ref | ||||
| Any secondary or further | 0.92 | 0.65 – 1.32 | 0.97 | 0.65 – 1.42 | 0.84 | 0.58 – 1.22 | 0.99 | 0.66 – 1.47 |
| Occupation | ||||||||
| Formal | ref | ref | ||||||
| Informal – high risk | 1.22 | 0.54 – 2.75 | 1.64 | 0.75 – 3.58 | ||||
| Informal – low risk | 0.96 | 0.50 – 1.84 | 1.43 | 0.73 – 2.82 | ||||
| No job | 1.27 | 0.58 – 2.78 | 1.70 | 0.75 – 3.87 | ||||
| Other | 0.58 | 0.22 – 1.55 | 1.64 | 0.63 – 4.27 | ||||
| Own a mobile phone | ||||||||
| Yes | ref | ref | ref | ref | ||||
| No | 1.26 | 0.97 – 1.63 | 1.17 | 0.89 – 1.55 | 1.25 | 0.97 – 1.62 | 1.30 | 0.98 – 1.73 |
| Mobility | ||||||||
| Stable resident | ref | ref | ref | ref | ||||
| Not stable resident | 0.34 | 0.12 – 0.93 | 0.33 | 0.13 – 0.84 | 0.76 | 0.29 – 1.97 | 0.83 | 0.26 – 2.63 |
| Pre-ART CD4 | ||||||||
| Below country treatment guidelines | ref | ref | ref | ref | ||||
| Above country treatment guidelines | 3.19 | 2.43 – 4.19 | 3.16 | 2.39 – 4.18 | 10.1 | 7.49 – 13.6 | 9.52 | 7.00 – 12.9 |
| Site of testing | ||||||||
| CHC | ref | ref | ref | ref | ||||
| HBT | 0.85 | 0.63 – 1.15 | 0.87 | 0.62 – 1.20 | 1.13 | 0.82 – 1.55 | 1.08 | 0.77 – 1.53 |
| Time to link | ||||||||
| <= 30 days | ref | ref | ||||||
| > 30 days | 1.28 | 0.99 – 1.66 | 1.25 | 0.95 – 1.64 | ||||
Abbreviations: CI = Confidence Interval; CHC = Community Health Campaign; HBT = Home-based Testing
Country guidelines for treatment were to initiate ART at CD4 < 350 cells/ml until December 2013 in Uganda and until June 2014 in Kenya, after which country treatment guidelines were changed to reflect the 2013 WHO guidelines of ART initiation at CD4 < 500 cells/ml
Discussion
In one of the first evaluations of retention in HIV care in the setting of a universal test-and-treat program, we found 95% of patients who linked to care were still in care one year after introducing universal ART delivered via streamlined care in two country public health systems. This included demographic groups who have historically demonstrated lower retention, such as men, those with lower educational levels, and persons who work in high-risk occupations (e.g. fisherfolk). In contrast to concerns about retention among those with high CD4 counts (patients who will increasingly be initiating ART under new universal ART guidelines), these patients demonstrated high retention with 91% of patients newly linking patients and 97% of those previously in care retained in care at one year.
The streamlined model of care employed in the SEARCH-supported clinics might have contributed to the high levels of observed retention, as it addresses many of the traditional barriers to retention, such as negative staff attitudes, frequent clinic visits, and long waiting times. Staff were trained to deliver care in a warm and welcoming environment and to support adherence and retention in an empathetic manner with patients[29]. Decreased wait times and decreased frequency of visits from monthly to every three months increases the clinic capacity to see more patients, which will be critical for treatment programs to scale-up under universal test-and-treat[28]. Reducing wait times may mitigate stigma associated with queuing outside HIV clinics for some patients. Also, the provision of viral load results and structured viral load counseling provided a tool for adherence assessment and retention support.
Active outreach, especially immediately after or within one week of missed appointments, is known to improve retention [31–33]. Retention tracking that was initiated immediately following a missed visit was part of the streamlined care package, and used a tiered approach to adapt to the resources and needs of the communities as well as the individual patients. Patients in Uganda, men, younger patients, and those newly linking to care were more likely to require retention tracking to stay in care, possibly reflecting a higher risk of attrition among these groups. Although mobility has been associated with lower retention[19], unstable residents who were retained in care had a lower probability of requiring retention tracking. It may be that the unstable residents who successfully link to care are highly motivated to stay in care. While patients with high CD4 were also more likely to have been tracked, early during the study follow up period only high CD4 patients were eligible for tracking and may have continued to be prioritized throughout the follow-up period even after all patients became eligible.
In contrast to other settings in sub-Saharan Africa in which men have lower retention rates than women[7–10, 12, 13, 17, 18, 34], we did not observe any significant gender disparity. The streamlined care system employed by the clinics, which decreased the frequency and improved the efficiency of visits, could have particularly benefited men by reducing any disruption to employment or other work. In these communities in rural East Africa a large proportion of the men work as farmers or fishermen, which requires them to be away from their communities for extended periods, and has been a demonstrated to deter men from to facility-based testing[35]. Men did require additional effort through retention tracking to stay in care, and may have benefited from this individualized outreach.
These data also highlight the challenges adolescents and young adults face in remaining in care. Younger adults not only required more effort through retention tracking to stay in care, but those under age 30 also had significantly lower retention than older age groups. This age group faces high levels of stigma in their home environment and at school, which negatively affects retention[36]. In addition, younger adults are more likely to be mobile, traveling for education or employment. In our population, 5% of the youth (age 15–24) are mobile compared to 2.5% of the overall population. These mobile youth demonstrated the lowest overall retention, with only 81% still in care at 12 months. Retention support that addresses this vulnerable population will be required as our streamlined care model evolves. Alternative treatment sites, such as school-based treatment, longer intervals between refills to accommodate those who attend school outside of the community, and alternative methods of antiretroviral therapy, including long-acting methods, may be needed to support this population.
This analysis is subject to several limitations. These high retention rates should be interpreted in the context of the 86% who linked to care within one year. Those with delayed linkage may experience higher rates of non-retention, and those with the greatest challenges to linkage are those who are also likely to experience challenges staying in care. While the majority of patient outcomes at one year were ascertained, there were likely silent transfers that were not accounted for among those who moved out of the study community without a documented transfer or among those with missing outcomes[9, 37]. In addition, the care outcomes of the patients with an official transfer are unknown. However, data on patients transferring care in the region indicates almost all patients with an official transfer are linked to care at their destination facility within 3 months[38]. Only retention in the first year is captured so longer follow-up will be necessary to evaluate durability of retention in care. Finally, the viral suppression in this population could be underestimated, as almost 10% patients were missing viral load data at 12 months. We anticipate the completeness of viral load results will increase as viral load monitoring is expanded in East Africa.
To our knowledge, these are the first data on retention in care in a universal test-and-treat setting and demonstrate that high levels of retention in care are achievable when some of the known barriers to retention have been targeted. As universal test-and-treat is expanded, these barriers will need to be continually addressed to ensure durable retention, especially as patients’ ages, and life factors change. Specific targeted interventions to retain young adults will also be crucial to the success of test-and-treat programs, as will strengthening timely linkage to care and retention for new diagnoses, and supporting those accessing care for the first time.
Acknowledgments
Sources of support:
Research reported in this manuscript was supported by the National Institutes of Mental Health of the U.S. Public Health Service grant T32 MH19105 and the Division of AIDS, National Institute of Allergy and Infectious Diseases of the National Institutes of Health award number U01AI099959 and in part by the President’s Emergency Plan for AIDS Relief and Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, PEPFAR, or Gilead.
The SEARCH project gratefully acknowledges the Ministries of Health of Uganda and Kenya, our research team, collaborators and advisory boards, and especially all communities and participants involved. The SEARCH project also gratefully acknowledges Douglas Black and his contributions to the study and preparation of this manuscript.
L.B.B. was responsible for designing the analytic approach, data analysis and interpretation of results, and drafting the manuscript. D.V.H, M.R.K., E.D.C., T.C., and M.L.P. designed the study, oversaw implementation and contributed to interpretation of results and drafting the manuscript. V.J, T.R., G.C., and C.R.C. contributed to study design, data interpretation, and drafting of the manuscript. J.A., F.M., A.O. contributed to data collection and interpretation. E.A.B. contributed to study design and interpretation. All authors contributed to and approved the final version of the manuscript.
Appendix 1. Outcomes at 12 months among adults in SEARCH intervention communities who accessed care after baseline CHC and before follow-up year one CHC (N = 5683)
| Accessed care after baseline CHC [N] | Retained in care at original site [n (%)] | Documented transfer [n (%)] | Died [n(%)] | Alive & in the community [n(%)] | Moved out of community [n(%)] | Missing [n(%)] | |
|---|---|---|---|---|---|---|---|
| Total | 5683 | 5058 (89.0%) | 260 (4.6%) | 60 (1.1%) | 108 (1.9%) | 64 (1.1%) | 134 (2.4%) |
| New to care | |||||||
| Total | 1601 | 1329 (83.0%) | 83 (5.2%) | 25 (1.6%) | 86 (5.4%) | 45 (2.8%) | 33 (2.0%) |
| Uganda-East | 278 | 227 (81.7%) | 6 (2.2%) | 10 (3.6%) | 22 (7.9%) | 11 (4.0%) | 2 (0.7%) |
| Uganda-West | 563 | 465 (82.6%) | 27 (4.8%) | 6 (1.1%) | 35 (6.2%) | 22 (3.9%) | 8 (1.4%) |
| Kenya | 760 | 637 (83.8%) | 50 (6.6%) | 9 (1.2%) | 29 (3.8%) | 12 (1.6%) | 31 (4.0%) |
| Men | 642 | 547 (85.2%) | 22 (3.4%) | 9 (1.4%) | 44 (6.9%) | 11 (1.7%) | 9 (1.5%) |
| Women | 959 | 782 (81.5%) | 61 (6.4%) | 16 (1.7%) | 42 (4.4%) | 34 (3.6%) | 24 (2.5%) |
| Age 15–24 | 257 | 173 (67.3%) | 23 (9.0%) | 6 (2.3%) | 24 (9.3%) | 24 (9.3%) | 7 (2.7%) |
| Age 25–29 | 308 | 247 (80.2%) | 13 (4.2%) | 5 (1.6%) | 23 (7.5%) | 10 (3.3%) | 10 (3.2%) |
| Age >= 30 | 1036 | 909 (87.7%) | 47 (4.5%) | 14 (1.4%) | 39 (3.8%) | 11 (1.1%) | 16 (1.5%) |
| Low CD4 | 766 | 595 (77.7%) | 59 (7.7%) | 20 (2.6%) | 39 (5.1%) | 26 (3.4%) | 27 (3.5%) |
| High CD4 | 835 | 734 (87.9%) | 24 (2.9%) | 5 (0.6%) | 47 (5.6%) | 19 (2.3%) | 6 (0.7%) |
| Stable resident | 1548 | 1288 (83.2%) | 81 (5.2%) | 24 (1.6%) | 82 (1.6%) | 42 (2.7%) | 25 (1.6%) |
| Not stable resident | 53 | 41 (77.4%) | 2 (3.8%) | 1 (1.9%) | 4 (7.6%) | 3 (5.7%) | 1 (1.9%) |
| Tested at CHC | 1173 | 965 (82.5%) | 63 (5.4%) | 21 (1.8%) | 64 (5.5%) | 33 (2.8%) | 27 (2.3%) |
| Tested in HBT | 428 | 364 (85.1%) | 20 (4.7%) | 4 (0.9%) | 22 (5.1%) | 12 (2.8%) | 6 (1.4%) |
| <= 30 days to link | 960 | 817 (85.1%) | 43 (4.5%) | 17 (1.8%) | 38 (4.0%) | 28 (2.9%) | 17 (1.8%) |
| > 30 days to link | 641 | 512 (79.9%) | 40 (6.2%) | 8 (1.3%) | 48 (7.5%) | 17 (2.7%) | 16 (2.5%) |
| Previously in care | |||||||
| Total | 4082 | 3729 (91.4%) | 177 (4.3%) | 35 (0.9%) | 22 (0.5%) | 18 (0.4%) | 101 (2.5%) |
| Uganda-East | 396 | 356 (89.9%) | 16 (4.0%) | 9 (2.3%) | 7 (1.8%) | 5 (1.3%) | 3 (0.8%) |
| Uganda-West | 743 | 653 (87.9%) | 78 (10.5%) | 4 (0.5%) | 1 (0.1%) | 3 (0.4%) | 4 (0.5%) |
| Kenya | 2943 | 2720 (92.4%) | 83 (2.8%) | 22 (0.8%) | 14 (0.5%) | 10 (0.3%) | 94 (3.2%) |
| Men | 1221 | 1119 (91.7%) | 46 (3.8%) | 14 (1.2%) | 12 (1.0%) | 4 (0.3%) | 26 (2.2%) |
| Women | 2861 | 2610 (91.2%) | 131 (4.6%) | 21 (0.7%) | 10 (0.4%) | 14 (0.5%) | 75 (2.6%) |
| Age 15–24 | 346 | 300 (86.7%) | 18 (5.2%) | 4 (1.2%) | 4 (1.2%) | 3 (0.9%) | 17 (4.9%) |
| Age 25–29 | 583 | 525 (90.1%) | 24 (4.1%) | 8 (1.4%) | 5 (22.7%) | 6 (1.0%) | 15 (2.5%) |
| Age >= 30 | 3153 | 2904 (92.1%) | 135 (4.3%) | 23 (0.7%) | 13 (0.4%) | 9 (0.3%) | 69 (2.2%) |
| Low CD4 | 3660 | 3330 (91.0%) | 166 (4.5%) | 34 (0.9%) | 15 (0.4%) | 15 (0.4%) | 100 (2.7%) |
| High CD4 | 422 | 399 (94.6%) | 11 (2.6%) | 1 (0.2%) | 7 (1.7%) | 3 (0.7%) | 1 (0.2%) |
| Stable resident | 3996 | 3651 (91.4%) | 172 (4.3%) | 34 (0.9%) | 21 (0.5%) | 17 (0.4%) | 101 (2.5%) |
| Not stable resident | 86 | 78 (90.7%) | 5 (5.8%) | 1 (1.2%) | 1 (1.2%) | 1 (1.2%) | 0 |
| Tested at CHC | 3213 | 2945 (91.7%) | 134 (4.2%) | 29 (0.9%) | 17 (0.5%) | 13 (0.4%) | 86 (2.7%) |
| Tested in HBT | 858 | 784 (91.4%) | 43 (5.0%) | 6 (0.7%) | 5 (0.6%) | 5 (0.6%) | 15 (1.7%) |
Low CD4 = Pre-ART CD4 below Country treatment guidelines
High CD4 = Pre-ART CD4 above Country treatment guidelines
CHC = Community Health Campaign
HBT = Home-based Testing
Appendix 2. Predictors of non-retention at 12 months in adult residents of SEARCH intervention communities who linked to HIV care after baseline CHC and before follow-up year 1 CHC with death as a competing risk (N = 5683)
| Newly Linking to care (N = 1601) | Previously in Care (N = 4082) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictor | Hazard Ratio |
95% CI | Adj Hazard Ratio |
95% CI | Hazard Ratio |
95% CI | Adj Hazard Ratio |
95% CI |
| Region | ||||||||
| Kenya | ref | ref | ref | ref | ||||
| Uganda-East | 1.90 | 0.82 – 4.42 | 2.51 | 1.04 – 6.07 | 0.95 | 0.47–1.91 | 0.84 | 0.47 – 1.50 |
| Uganda-West | 0.96 | 0.36 – 2.54 | 0.89 | 0.32 – 2.43 | 0.28 | 0.13–0.61 | 0.25 | 0.12 – 0.52 |
| Sex | ||||||||
| Male | ref | ref | ref | ref | ||||
| Female | 1.07 | 0.79 – 1.43 | 0.75 | 0.53 – 1.05 | 1.01 | 0.72–1.43 | 0.83 | 0.58 – 1.20 |
| Age | ||||||||
| 15–24 | 3.88 | 2.69 – 5.60 | 3.72 | 2.44 – 5.68 | 2.48 | 1.57 – 3.91 | 2.78 | 1.76 – 4.39 |
| 25–29 | 2.37 | 1.60 – 3.52 | 2.50 | 1.67 – 3.76 | 1.49 | 0.95 – 2.32 | 1.64 | 1.04 – 2.58 |
| >= 30 | ref | ref | ref | ref | ||||
| Education | ||||||||
| No School | 0.53 | 0.30 – 0.95 | 0.79 | 0.44 – 1.42 | 0.84 | 0.43 – 1.61 | ||
| Primary | ref | ref | ref | |||||
| Any secondary or further | 0.88 | 0.50 – 1.24 | 0.77 | 0.49 – 1.22 | 0.88 | 0.48 – 1.61 | ||
| Occupation | ||||||||
| Formal | ref | ref | ||||||
| Informal – high risk | 1.72 | 0.37 – 7.91 | 1.40 | 0.28 – 6.84 | 0.73 | 0.22 – 2.38 | ||
| Informal – low risk | 2.14 | 0.66 – 6.95 | 1.78 | 0.51 – 6.23 | 0.92 | 0.33 – 2.58 | ||
| No job | 4.59 | 1.29 – 16.27 | 2.80 | 0.73 – 10.8 | 2.13 | 0.70 – 6.44 | ||
| Other | 2.32 | 0.58 – 9.17 | 1.77 | 0.42 – 7.53 | 0.61 | 0.11 – 3.35 | ||
| Access to a mobile phone | ||||||||
| Yes | ref | ref | ref | |||||
| No | 1.27 | 0.92 – 1.75 | 1.93 | 1.35 – 2.76 | 1.92 | 1.35 – 2.74 | ||
| Mobility | ||||||||
| Stable resident | ref | ref | ||||||
| Not stable resident | 1.76 | 0.86 – 3.58 | 0.82 | 0.20 – 3.40 | ||||
| Pre-ART CD4 | ||||||||
| Below country treatment guidelines | ref | ref | ref | ref | ||||
| Above country treatment guidelines | 0.69 | 0.50 – 0.94 | 0.63 | 0.46 – 0.86 | 0.75 | 0.41–1.39 | 0.63 | 0.34 – 1.15 |
| Site of testing | ||||||||
| CHC | ref | ref | ||||||
| HBT | 0.93 | 0.62 – 1.40 | 0.82 | 0.55–1.22 | ||||
| Time to link | ||||||||
| <= 30 days | ref | ref | ||||||
| > 30 days | 1.53 | 1.11 – 2.11 | 1.43 | 1.04 – 1.98 | ||||
Abbreviations: CI = Confidence Interval; CHC = Community Health Campaign; HBT = Home-based testing
Footnotes
Conflicts of interest: VJ has received grant support from Gilead Sciences. DVH has received non-financial support from Gilead Sciences. CRC has received grants from the Bill & Melinda Gates Foundation, grants from CIFF, personal fees from Legal consulting about a malpractice case, and personal fees from Symbiomix. All other authors have no other conflicts of interest to disclose.
References
- 1.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2015 [Google Scholar]
- 5.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69:98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auld AF, Agolory SG, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep. 2014;63:1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 7.Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenga M, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health. 2014;19:1397–1410. doi: 10.1111/tmi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5:e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2015 doi: 10.1093/cid/civ1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugisha V, Teasdale CA, Wang C, Lahuerta M, Nuwagaba-Biribonwoha H, Tayebwa E, et al. Determinants of Mortality and Loss to Follow-Up among Adults Enrolled in HIV Care Services in Rwanda. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS One. 2014;9:e110252. doi: 10.1371/journal.pone.0110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, de Wit TF, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health. 2015;15:478. doi: 10.1186/s12889-015-1814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nsanzimana S, Kanters S, Remera E, Forrest JI, Binagwaho A, Condo J, et al. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. Lancet HIV. 2015;2:e208–215. doi: 10.1016/S2352-3018(15)00024-7. [DOI] [PubMed] [Google Scholar]
- 14.Mutasa-Apollo T, Shiraishi RW, Takarinda KC, Dzangare J, Mugurungi O, Murungu J, et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe’s National Antiretroviral Therapy Programme, 2007–2010. PLoS One. 2014;9:e86305. doi: 10.1371/journal.pone.0086305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takarinda KC, Harries AD, Shiraishi RW, Mutasa-Apollo T, Abdul-Quader A, Mugurungi O. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. Int J Infect Dis. 2015;30:98–105. doi: 10.1016/j.ijid.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marson KG, Tapia K, Kohler P, McGrath CJ, John-Stewart GC, Richardson BA, et al. Male, mobile, and moneyed: loss to follow-up vs. transfer of care in an urban African antiretroviral treatment clinic. PLoS One. 2013;8:e78900. doi: 10.1371/journal.pone.0078900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoboi S, Ding E, Persuad S, Wangisi J, Birungi J, Shurgold S, et al. Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS Res Ther. 2015;12 doi: 10.1186/s12981-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucciardini R, Fragola V, Abegaz T, Lucattini S, Halifom A, Tadesse E, et al. Retention in Care of Adult HIV Patients Initiating Antiretroviral Therapy in Tigray, Ethiopia: A Prospective Observational Cohort Study. PLoS One. 2015;10:e0136117. doi: 10.1371/journal.pone.0136117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Janssen S, Wieten RW, Stolp S, Cremers AL, Rossatanga EG, Klipstein-Grobusch K, et al. Factors Associated with Retention to Care in an HIV Clinic in Gabon, Central Africa. PLoS One. 2015;10:e0140746. doi: 10.1371/journal.pone.0140746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lifson AR, Demissie W, Tadesse A, Ketema K, May R, Yakob B, et al. Barriers to retention in care as perceived by persons living with HIV in rural Ethiopia: focus group results and recommended strategies. J Int Assoc Provid AIDS Care. 2013;12:32–38. doi: 10.1177/1545109712456428. [DOI] [PubMed] [Google Scholar]
- 22.Layer EH, Kennedy CE, Beckham SW, Mbwambo JK, Likindikoki S, Davis WW, et al. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014;9:e104961. doi: 10.1371/journal.pone.0104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment - a study protocol for a cluster randomised trial. Trials. 2014;15:57. doi: 10.1186/1745-6215-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwuji CC, Orne-Gliemann J, Tanser F, Boyer S, Lessells RJ, Lert F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. doi: 10.1186/1745-6215-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3:e111–119. doi: 10.1016/S2352-3018(15)00251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene LW. Toward cost–benefit evaluations of health education: some concepts, methods, and examples. Health Education Monographs. 1974;2:34–64. [Google Scholar]
- 27.Shade SBCW, Kahn JG, Mwai D, Mwanga F, Kwarisiima D, Owaranganise A, Ayieko J, Havlir DV, Kamya MR, Charlebois ED, Petersen ML, Clark TD, Bukusi EA, Cohen CR, Jain V. SEARCH Streamlined HIV Care is Associated with Shorter Wait Times Before and During Patient Visits in Ugandan and Kenyan HIV Clinics. The 21st International AIDS Conference; Durban, South Africa. 2016. [Google Scholar]
- 28.Jain V, Byonanebye DM, Amanyire G, Kwarisiima D, Black D, Kabami J, et al. Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts above 350 cells/mul in rural Uganda. Aids. 2014;28:2241–2249. doi: 10.1097/QAD.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwarisiima DJV, Owaraganise A, Mwangwa F, Byonanebye D, Ayieko J, Petersen M, Havlir DV, Kamya MR. Virologic Efficacy of ART Begun at High CD4+ Counts via Streamlined Care in East Africa. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2016. [Google Scholar]
- 30.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, Black D, et al. Assessment of Population-Based HIV RNA Levels in a Rural East African Setting Using a Fingerprick-Based Blood Collection Method. Clin Infect Dis. 2013;56:598–605. doi: 10.1093/cid/cis881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PLoS One. 2012;7:e38443. doi: 10.1371/journal.pone.0038443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakiwogga-Muwanga A, Musaazi J, Katabira E, Alamo-Talisuna S, Colebunders R. Early Tracking after a Missed Return Visit Reduces the Proportion of Untraceable Patients at a Large HIV Clinic in Kampala, Uganda. J Int Assoc Provid AIDS Care. 2014 doi: 10.1177/2325957414530471. [DOI] [PubMed] [Google Scholar]
- 34.Mekuria LA, Prins JM, Yalew AW, Sprangers MA, Nieuwkerk PT. Retention in HIV Care and Predictors of Attrition from Care among HIV-Infected Adults Receiving Combination Anti-Retroviral Therapy in Addis Ababa. PLoS One. 2015;10:e0130649. doi: 10.1371/journal.pone.0130649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camlin CSSE, Chamie G, El Ayadi AM, Kwarisiima D, Sang N, Kabami J, Charlebois E, Petersen M, Clark TD, Bukusi EA, Cohen CR, Kamya M, Havlir D. Men “missing” from population-based HIV testing: insights from qualitative research. AIDS Care. 2016 doi: 10.1080/09540121.2016.1164806. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf HT, Halpern-Felsher BL, Bukusi EA, Agot KE, Cohen CR, Auerswald CL. “It is all about the fear of being discriminated [against]…the person suffering from HIV will not be accepted”: a qualitative study exploring the reasons for loss to follow-up among HIV-positive youth in Kisumu, Kenya. BMC Public Health. 2014;14:1154. doi: 10.1186/1471-2458-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey MD, Omollo D, Salmen CR, Mattah B, Blat C, Ouma GB, et al. Movement between facilities for HIV care among a mobile population in Kenya: transfer, loss to follow-up, and reengagement. AIDS Care. 2016:1–8. doi: 10.1080/09540121.2016.1179253. [DOI] [PMC free article] [PubMed] [Google Scholar]