Abstract
Background
Maintaining folate stability during sample handling is important, yet challenging.
Objective
We investigated the effects of suboptimal preanalytical conditions on serum folate stability.
Design
Using an HPLC-tandem MS method we measured folates [5-methyltetrahydrofolate (5-methylTHF), folic acid, and MeFox (5-methylTHF oxidation product), minor forms at or below limit of detection] in human serum exposed to suboptimal conditions.
Results
Whole blood samples (n = 21) stored at 32°C for ≤3 d (experiment 1 – delayed processing) showed significant decreases in serum total folate [(tFOL; sum of folate forms) 11–32%, 5.5–15.9 nmol/L] and 5-methylTHF (36–62%, 14.5–25.1 nmol/L) and a significant increase in MeFox (346–415%, 7.17–8.63 nmol/L). Serum samples (n = 21) stored at 11°C for 7–14 d (experiment 2 – delayed freezing) also showed significant decreases in tFOL (4.6–10.4%, 2.3–5.1 nmol/L) and 5-methylTHF (8.4–29%, 3.4–11.6 nmol/L) and significant increases in MeFox (88–320%, 1.82–6.62 nmol/L). The molar loss in 5-methylTHF exceeded the gain in MeFox in these 2 experiments. When we exposed 3 serum pools (tFOL 16.7–58.3 nmol/L) for 24 h to 37°C elevated temperature (experiment 3), the significant decrease in 5-methylTHF (33% on average) was compensated by an equimolar gain in MeFox. Repeated freeze/thawing (≤3 cycles) of serum [closed (experiment 4) and open (experiment 5) vials] showed generally stable folates with small (<1 nmol/L) changes. Long-term (≤12 mo) exposure of 3 serum pools (tFOL 17.5–63.7 nmol/L) to suboptimal (−20°C) freezing temperature (experiment 6) showed significant decreases in tFOL (5% on average) already after 3 mo. The molar loss in 5-methylTHF exceeded the gain in MeFox. Folic acid generally showed good stability.
Conclusions
To avoid folate losses, unprocessed whole blood should be protected from elevated temperature and serum should not be refrigerated for >2 d or long-term stored at −20°C.
Keywords: Folate, MeFox, stability, degradation, preanalytical, delayed processing, frozen storage, freeze/thawing, and HPLC-MS/MS
INTRODUCTION
Suboptimal environmental conditions during preanalytical steps such as sample collection, processing, shipping, and storage can negatively affect analytical results. Knowledge about analyte stability during these steps is therefore critical. Serum folate is an important biomarker of folate status and it has been measured as part of the U.S. NHANES for several decades (1). While the susceptibility of reduced folates to oxidative degradation is well-known (2,3), only limited information is available on the behavior of serum folate forms during the full spectrum of preanalytical conditions. Hannisdal et al. have so far provided extensive information on the oxidative loss of 5-methyltetrahydrofolate (5-methylTHF)4, the major circulating portion of total folate (tFOL), to the pyrazino-s-triazine derivative of 4α-hydroxy-5-methylTHF [also known as MeFox (4)], in a short-term (1 wk) kinetic study of serum and plasma exposed to room temperature (5) and in a long-term (up to 29 y) cross-sectional study of serum frozen at suboptimal (−25°C) temperature (6). Our group has provided information on a broad range of micronutrients, including serum tFOL measured by microbiologic assay, for 2 unfavorable preanalytical conditions that may be encountered in resource-limited situations: delayed processing of whole blood exposed to elevated temperature sometimes encountered in the field (32°C) for up to 3 d and delayed freezing of serum exposed to slightly cooled temperature achievable with cold packs (11°C) for up to 14 d (7).
The main objective of the present study was to investigate the effects of a series of suboptimal preanalytical conditions on folate stability, covering the full spectrum from whole blood processing to repeated freeze/thawing of samples as part of the laboratory analysis. Serum folate forms were measured with a sensitive and specific HPLC-tandem MS (HPLC-MS/MS) method (4,8,9) in a series of experiments: experiment 1 – delayed (≤3 d) processing of freshly collected whole blood incubated at 32°C; experiment 2 – delayed (≤14 d) freezing of freshly obtained serum stored at 11°C; experiment 3 – short-term (≤24 h) exposure of serum to elevated temperature (room temperature and 37°C); experiments 4 and 5 – repeated (≤3 cycles) freeze/thawing of serum in either closed or open vials; experiments 6 and 7 – long-term (≤12 mo) exposure of serum to suboptimal storage temperatures (−20°C and 5°C). Results from this study will help to refine preanalytical procedures and therefore improve the measurement accuracy of serum folate forms.
MATERIALS AND METHODS
REAGENTS, MATERIALS, AND SERUM SAMPLES
Folate monoglutamate standards [(6S)-5-methylTHF, (6S)-MeFox, folic acid, (6S)-tetrahydrofolate (THF), (6S)-5-formyltetrahydrofolate (5-formylTHF), and (6R)-5,10-methenyltetrahydrofolate (5,10-methenylTHF)] together with their 13C5-labeled analogues were purchased from Merck Cie (Switzerland). All other reagents and solvents were of ACS grade (4,8,9). We used in-house prepared quality control (QC) pools using serum purchased from a U.S. blood bank. Other serum samples were from a CDC study using volunteer blood collection (7), and from a large CDC study (convenience samples). Participants for both studies provided informed consent and the study protocols were approved by the CDC Institutional Review Board and the CDC Research Ethics Review Board.
Sample processing for preanalytical experiments
All preanalytical experiments are described in Table 1 and additional information to experiments 1 and 2 has been presented previously (7). Folate calibrators for 5-methylTHF, folic acid, THF, 5-formylTHF, 5,10-methenylTHF, and MeFox and their respective 13C5-labeled internal standards were prepared as described earlier (8–9). To measure serum folate forms, we processed samples using an automated reversed phase solid phase extraction method and analyzed sample extracts (all conditions from the same study participant in the same run) by HPLC-MS/MS (4). For experiments 6 and 7 (conducted earlier) we used our previous HPLC-MS/MS method (8,9), which did not separate the 2 isobaric compounds MeFox and 5-formylTHF. Because we spiked the serum QC pools used in those 2 experiments with 5-formylTHF at the time of pool preparation and we confirmed the target concentrations more recently with our updated HPLC-MS/MS method (which separates MeFox and 5-formylTHF via different mass transitions), we were able to subtract the correctly measured 5-formylTHF amount from the sum of MeFox and 5-formylTHF measured in experiments 6 and 7 to obtain an estimate for MeFox. All sample processing steps were conducted under gold fluorescent lights. To evaluate the quality of the analytical run, each run included 3 serum QC samples measured in duplicate, bracketing the unknown samples. We calculated serum tFOL as the sum of the individual folate forms including MeFox [using an imputed value of limit of detection (LOD) divided by the square root of 2 for results <LOD]. We are not reporting results for the 2 minor folate forms 5-formylTHF and 5,10-methenylTHF because their concentrations were <LOD. We are only reporting THF results in the text when concentrations were >LOD.
Table 1.
Summary of experiments to study folate stability during suboptimal preanalytical conditions
| Experiment | Design | Samples | Laboratory analysis1 | Statistical analysis |
|---|---|---|---|---|
| 1. Delayed processing of whole blood | Blood tubes stored at 32°C for 1, 2, and 3 d prior to processing and freezing at −70°C; reference: serum from promptly processed whole blood frozen at −70°C | Whole blood from 21 volunteer blood donors from CDC | 1 replicate/condition batch analyzed by HPLC-MS/MS | 1-way (time) ANOVA |
| 2. Delayed freezing of serum | Serum stored at 11°C for 2, 7, 10, and 14 d prior to freezing at −70°C; reference: serum from promptly processed whole blood frozen at −70°C | Serum from 21 volunteer blood donors from CDC | 1 replicate/condition batch analyzed by HPLC-MS/MS | 1-way (time) ANOVA |
| 3. Short-term exposure of serum to elevated temperatures | Serum stored at 22°C and 37°C for 2, 4, 6, and 24 h prior to freezing at −70°C; reference: serum stored at −70°C | Low, medium, and high serum QC pools | 4 replicates/condition batch analyzed by HPLC-MS/MS | 2-way (time, pool level) ANOVA |
| 4. Repeated freeze/thawing of serum (closed vials) | Serum exposed to up to 3 freeze/thawing cycles (2 h/cycle at room temperature in closed vials); reference: serum stored at −70°C | Low, medium, and high serum QC pools | 4 replicates/condition batch analyzed by HPLC-MS/MS | 2-way (time, pool level) ANOVA |
| 5. Repeated freeze/thawing of serum (open vials) | Serum from same vial exposed to up to 4 analyses (2 h/analysis at room temperature in open vials); reference: 1st analysis | 30 pooled samples from serum from a large CDC study | 1 replicate/condition batch analyzed by HPLC-MS/MS | 1-way (time) repeated measures ANOVA |
| 6. Long-term frozen storage of serum | Serum stored at −20°C for 3, 6, 9, and 12 mo prior to freezing at −70°C; reference: serum stored at −70°C | Low, medium, and high serum QC pools | 6 replicates/condition batch analyzed by HPLC-MS/MS | 2-way (time, pool level) ANOVA |
| 7. Long-term refrigerated storage of serum | Serum stored at 5°C for 6 and 12 mo prior to freezing at −70°C; reference: serum stored at −70°C | Low, medium, and high serum QC pools | 6 replicates/condition batch analyzed by HPLC-MS/MS | 2-way (time, pool level) ANOVA |
HPLC-MS/MS method used for experiments 6 and 7 did not separate the two isobaric compounds MeFox (pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate) and 5-formyltetrahydrofolate
Statistical analysis
Statistical analyses were performed using SAS (version 9.2, SAS Institute Inc., Cary, NC) software. We used a log10 transformation to account for the right-skewed distributions and for the increasing variance with increasing folate concentration. We calculated least squares geometric mean concentrations using back-transformation (95% CI) for folate forms using 1-way (time) or 2-way (time and pool) ANOVA, depending on the experiment (Table 1). As a consequence of the log transformation, differences relative to the reference condition were expressed as percent change of geometric means (95% CI) rather than an absolute difference in log transformed values and evaluated for significance in pairwise comparisons using a 2-sided level of α = 0.05. If the interaction between time and pool was significant (experiments 3 and 6 for MeFox), we reported the percent change results separately for each pool; otherwise we reported the mean percent change across the 3 pools. To facilitate the interpretation of molar changes in 5-methylTHF vs. MeFox, we also calculated the absolute change in geometric mean concentrations.
RESULTS
Experiment 1 – Delayed processing of whole blood
Freshly collected whole blood samples (n = 21) stored at 32°C for 1–3 d prior to processing showed substantial and significant decreases in serum tFOL (5.5–15.9 nmol/L, up to ~30%) and 5-methylTHF (14.5–25.1 nmol/L, up to ~60%) and increases in MeFox (7.17–8.63 nmol/L, up to ~400%) (Table 2 and Supplemental Figure 1, panels A and B). The gain in MeFox represented only a fraction of the loss in 5-methylTHF at either time point. Serum folic acid concentrations were stable under these conditions. Serum THF concentrations (0.647 nmol/L at baseline) decreased by ~0.1–0.4 nmol/L (data not shown).
Table 2.
Effect of delayed processing of whole blood stored at 32°C for up to 3 d prior to processing on serum folate concentrations1
| Analyte | Serum folate concentration2 | Relative change3 | |||||
|---|---|---|---|---|---|---|---|
| Reference | 1 d | 2 d | 3 d | 1 d | 2 d | 3 d | |
| nmol/L | % | ||||||
| tFOL | 49.5 (40.4, 60.7) | 44.0 (35.9, 53.9) | 38.7 (31.6, 47.5) | 33.6 (27.4, 41.2) | −11.1** (−13.7, −8.38) | −21.7** (−24.0, −19.3) | −32.0** (−34.0, −29.9) |
| 5-MethylTHF | 40.3 (32.6, 49.9) | 25.8 (20.9, 31.9) | 19.7 (15.9, 24.4) | 15.2 (12.3, 18.8) | −36.0** (−42.7, −28.4) | −51.2** (−56.3, −45.4) | −62.3** (−66.3, −57.9) |
| Folic acid | 2.21 (1.13, 4.31) | 2.26 (1.16, 4.42) | 2.24 (1.14, 4.37) | 2.28 (1.17, 4.45) | 2.44 (−2.03, 7.12) | 1.37 (−3.05, 6.00) | 3.21 (−1.30, 7.92) |
| MeFox | 2.07 (1.65, 2.60) | 9.24 (7.37, 11.6) | 10.4 (8.26, 13.0) | 10.7 (8.51, 13.4) | 346** (294, 405) | 400** (342, 466) | 415** (356, 483) |
Serum from promptly processed whole blood frozen at −70°C was used as reference; statistical analysis by 1-way ANOVA. 5-methylTHF, 5-methyltetrahydrofolate; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; tFOL, total folate.
Values are geometric means (95% CI), n = 21
Values are percent change (95% Cl) compared to reference
P <0.0001
Experiment 2 – Delayed freezing of serum
Freshly prepared serum samples (n = 21) kept for 2 d at 11°C showed no change in tFOL or 5-methylTHF, but a small and significant increase in MeFox (0.38 nmol/L) (Table 3 and Supplemental Figure 1, panels C and D). Longer delays in freezing serum samples (7–14 d) resulted in moderate-to-substantial significant decreases in tFOL (2.3–5.1 nmol/L, up to ~10%) and 5-methylTHF (3.4–11.6 nmol/L, up to ~30%) and increases in MeFox (1.82–6.62 nmol/L, up to ~300%). The gain in MeFox only partially compensated for the loss in 5-methylTHF. The decrease in serum folic acid was significant but small (≤0.17 nmol/L, ~5%). Serum THF concentrations (0.647 nmol/L at baseline) also decreased by ~0.2–0.3 nmol/L (data not shown).
Table 3.
Effect of delayed freezing of serum stored at 11°C for up to 14 d on serum folate concentrations1
| Analyte | Serum folate concentration2 | Relative change3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | 2 d | 7 d | 10 d | 14 d | 2 d | 7 d | 10 d | 14 d | |
| nmol/L | % | ||||||||
| tFOL | 49.5 (40.8, 60.1) | 48.8 (40.2, 59.3) | 47.2 (38.9, 57.3) | 47.2 (38.9, 57.2) | 44.4 (36.6, 53.8) | −1.33 (−3.23, 0.61) | −4.61** (−6.45, −2.74) | −4.70** (−6.45, −2.82) | −10.4** (−12.1, −8.59) |
| 5-MethylTHF | 40.3 (34.5, 47.1) | 39.6 (34.0, 46.3) | 36.9 (31.6, 43.1) | 34.6 (29.6, 40.4) | 28.7 (24.6, 33.5) | −1.70 (−5.00, 1.70) | −8.43** (−11.5, −5.26) | −14.2** (−17.1, −11.2) | −28.9** (−31.3, −26.5) |
| Folic acid | 2.21 (1.11, 4.39) | 2.12 (1.06, 4.21) | 2.04 (1.02, 4.05) | 2.09 (1.05, 4.16) | 2.11 (1.06, 4.20) | −4.08* (−7.13, −0.94) | −7.70** (−10.6, −4.68) | −5.27* (−8.27, −2.17) | −4.25* (−7.28, −1.11) |
| MeFox | 2.07 (1.68, 2.55) | 2.45 (2.00, 3.02) | 3.89 (3.16, 4.78) | 5.57 (4.53, 6.85) | 8.69 (7.07, 10.7) | 18.5* (4.70, 34.2) | 87.8** (65.8, 113) | 169** (138, 205) | 320** (271, 375) |
Serum from promptly processed whole blood frozen at −70°C was used as reference; statistical analysis by 1-way ANOVA. 5-methylTHF, 5-methyltetrahydrofolate; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; tFOL, total folate.
Values are geometric means (95% CI), n = 21
Values are percent change (95% Cl) compared to reference
0.0001 ≥ P < 0.05;
P <0.0001
Experiment 3 – Short-term exposure of serum to elevated temperatures
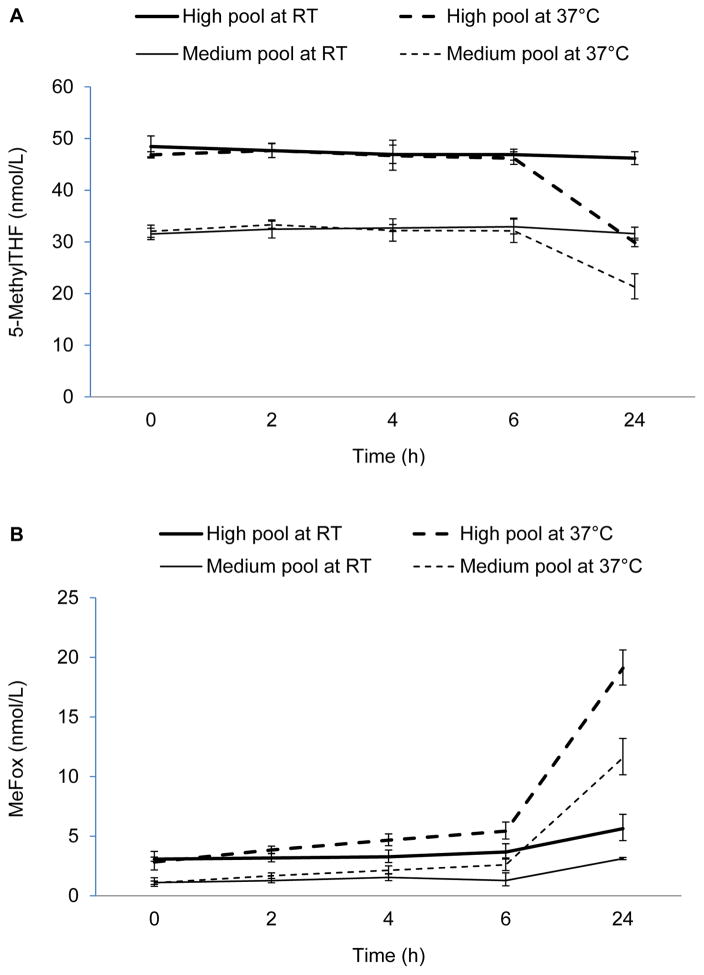
We found good stability of 5-methylTHF in 3 serum QC pools stored up to 24 h at room temperature and up to 6 h at 37°C, but we noted a significant average decrease of ~30% at 24 h (4.2, 10.8, and 17.0 nmol/L decrease for the low, medium, and high pool, respectively) (Supplemental Table 1). MeFox concentrations were fairly stable up to 6 h at room temperature, but increased significantly at 24 h by 0.83–2.56 nmol/L depending on the pool (P-interaction = 0.0072). At 37°C, MeFox concentrations increased continually with time (e.g., 4.57, 10.5, and 16.3 nmol/L increase at 24 h for the low, medium, and high pool, respectively; P-interaction = 0.0013). At both temperatures, the molar gain in MeFox was of similar magnitude as the loss in 5-methylTHF at 24 h (Fig. 1). Serum folic acid and tFOL concentrations were stable under these conditions (small but significant fluctuations in tFOL of ≤6%).
Figure 1.
Effect of short-term (≤24 h) exposure of serum QC pools to elevated temperature (room temperature and 37°C) on concentrations of 5-methylTHF (panel A) and MeFox (panel B). Values are geometric means from 4 replicates/condition. Error bars represent the 95% CI. 5-methylTHF, 5-methyltetrahydrofolate; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; QC, quality control; RT, room temperature.
Experiments 4 and 5 – Repeated freeze/thawing of serum
We first subjected 3 serum QC pools in closed vials (sample not exposed to air) to up to 3 freeze/thawing cycles. Compared to serum QC pools that were thawed only once, we found no loss of tFOL and 5-methylTHF, and only a small but significant increase in folic acid (≤1 nmol/L, 5%) after 3 freeze/thawing cycles and significant increases in MeFox (0.12–0.96 nmol/L, up to ~25%) at 1, 2, and 3 freeze/thawing cycles (Table 4). Next, we subjected 30 pooled serum samples (each pool was generated from several individual samples to provide sufficient volume) to 4 subsequent analyses (Table 5). This resulted in each pooled sample being opened 4 times and exposed to air for 2 h each. Under these conditions we found a small but significant decrease in tFOL (1.4 nmol/L, ~3%) after 3 freeze/thawing cycles and small but significant changes in 5-methylTHF and MeFox at 1, 2, and 3 freeze/thawing cycles. However, the gain in MeFox (≤0.3 nmol/L) only partially compensated for the loss in 5-methylTHF (0.9–1.5 nmol/L). We noted small (<0.2 nmol/L) fluctuations in folic acid and THF (significant at 2 and 3 freeze/thawing cycles).
Table 4.
Effect of repeated freeze/thawing of closed vials on serum folate concentrations1
| Analyte QC pool | Serum folate concentration2 | Relative change3 | |||||
|---|---|---|---|---|---|---|---|
| Reference | 1 F/T | 2 F/T | 3 F/T | 1 F/T | 2 F/T | 3 F/T | |
| nmol/L | % | ||||||
| tFOL | 1.17 (−0.59, 2.97) | −0.95 (−2.67, 0.82) | 0.83 (−0.93, 2.62) | ||||
| Low | 16.4 | 16.7 | 16.3 | 16.5 | |||
| Medium | 37.3 | 37.3 | 36.5 | 37.0 | |||
| High | 57.9 | 58.8 | 57.9 | 59.3 | |||
| 5-MethylTHF | 0.86 (−0.85, 2.60) | −1.62 (−3.29, 0.08) | −0.92 (−2.60, 0.79) | ||||
| Low | 14.6 | 14.8 | 14.5 | 14.6 | |||
| Medium | 32.6 | 32.3 | 31.7 | 31.8 | |||
| High | 46.9 | 47.9 | 46.6 | 47.0 | |||
| Folic acid | 1.20 (−3.09, 5.68) | −1.31 (−5.50, 3.06) | 5.24* (0.78, 9.90) | ||||
| Low | 0.81 | 0.84 | 0.81 | 0.87 | |||
| Medium | 3.37 | 3.52 | 3.30 | 3.48 | |||
| High | 7.96 | 7.61 | 7.78 | 8.36 | |||
| MeFox | 10.2* (1.58, 19.5) | 14.6* (5.62, 24.3) | 26.0** (16.2, 36.7) | ||||
| Low | 0.96 | 1.05 | 0.99 | 1.08 | |||
| Medium | 1.25 | 1.41 | 1.53 | 1.67 | |||
| High | 2.95 | 3.19 | 3.51 | 3.91 | |||
2 h per freeze/thawing cycle at 22°C; serum QC pools stored at −70°C were used as reference; statistical analysis by 2-way ANOVA. 5-methylTHF, 5-methyltetrahydrofolate; F/T, freeze/thawing; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; QC, quality control; tFOL, total folate.
Values are geometric means from 4 replicates/condition
Values are percent change (95% Cl) compared to reference across the QC pools (no significant interaction between time and pool)
0.0001 ≥ P < 0.05;
P <0.0001
Table 5.
Effect of repeated freeze/thawing of open vials on serum folate concentrations1
| Analyte | Serum folate concentration2 | Relative change3 | |||||
|---|---|---|---|---|---|---|---|
| Reference | 1 F/T | 2 F/T | 3 F/T | 1 F/T | 2 F/T | 3 F/T | |
| nmol/L | % | ||||||
| tFOL | 42.9 (38.6, 47.7) | 42.2 (37.9, 46.9) | 42.6 (38.3, 47.4) | 41.5 (37.3, 46.2) | −1.77* (−2.98, −0.54) | −0.83 (−2.05, 0.41) | −3.32** (−4.52, −2.12) |
| 5-MethylTHF | 37.9 (33.6, 42.6) | 37.0 (32.8, 41.7) | 37.0 (32.8, 41.6) | 36.4 (32.3, 41.0) | −2.31* (−3.50, −1.10) | −2.41* (−3.60, −1.20) | −3.94** (−5.12, −2.76) |
| Folic acid | 0.95 (0.77, 1.16) | 0.96 (0.78, 1.17) | 0.99 (0.81, 1.22) | 0.88 (0.72, 1.08) | 1.09 (−2.45, 4.77) | 5.05* (1.37, 8.87) | −7.03* (−10.3, −3.65) |
| MeFox | 1.90 (1.49, 2.43) | 2.06 (1.61, 2.63) | 2.22 (1.74, 2.84) | 2.01 (1.57, 2.57) | 8.05** (4.69, 11.5) | 16.7** (13.1, 20.5) | 5.59* (2.30, 8.98) |
Pooled serum samples were subjected to 3 freeze/thawing cycles (2 h/cycle at 22°C) beyond the first thawing to produce a total of 4 results; first result was used as reference; statistical analysis by 1-way repeated measures ANOVA. 5-methylTHF, 5-methyltetrahydrofolate; F/T, freeze/thawing; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; tFOL, total folate.
Values are geometric means (95% CI), n = 30
Values are percent change (95% Cl) compared to reference
0.0001 ≥ P < 0.05;
P <0.0001
Experiments 6 and 7 – Long-term storage of serum at suboptimal conditions
When we stored 3 serum QC pools at −20°C for 3, 6, 9, and 12 mo (Table 6), we observed a continuous and significant decrease in tFOL (4.91%, 8.64%, 10.2%, and 12.2%, respectively; corresponding to 0.7–2.2, 2.0–5.1, and 3.8–7.5 nmol/L for the low, medium, and high QC pool, respectively) and 5-methylTHF (9.58%, 16.6%, 18.7%, and 20.9%, respectively; corresponding to 1.1–3.0, 3.7–7.1, and 4.7–10 nmol/L for the low, medium, and high QC pool, respectively), irrespective of the folate baseline concentration. We observed a significant increase in MeFox up to 6 mo followed by a plateauing of concentrations in all 3 QC pools (P-interaction = 0.0299): low QC: 0.41, 0.82, 0.87, and 0.80 nmol/L or 66.9%, 134%, 142%, and 131%, respectively; medium QC: 1.59, 2.04, 1.94, and 2.01 nmol/L or 187%, 239%, 227%, and 235%, respectively; high QC: 1.19, 2.80, 2.67, and 2.54 nmol/L or 63%, 148%, 141%, and 134%, respectively. The molar gain in MeFox made up only a fraction of the loss in 5-methylTHF. Serum folic acid concentrations did not change over the 12 mo storage period. Storing serum at 5°C for 6 and 12 mo (Supplemental Table 2) led to significant and drastic changes: approximately half and 2/3 of tFOL was lost and approximately ¾ and almost all of 5-methylTHF was lost, respectively. Even though MeFox concentrations nearly quadrupled after 6 mo, the gain in MeFox only compensated for a small fraction of the loss in 5-methylTHF. Under these drastic storage conditions we also found a significant but small decrease in serum folic acid at 12 mo (6.61%).
Table 6.
Effect of long-term storage at suboptimal freezing temperature (−20°C) for up to 12 mo on serum folate concentrations1
| Analyte QC pool | Serum folate concentration2 | Relative change3 | ||||
|---|---|---|---|---|---|---|
| Reference | 3 mo | 6 mo | 9 mo | 12 mo | 3 mo | |
| nmol/L | % | |||||
| tFOL | 4.91* (−7.92, −1.81) | |||||
| Low | 17.5 | 16.8 | 15.9 | 15.5 | 15.3 | |
| Medium | 40.2 | 38.2 | 36.6 | 36.3 | 35.1 | |
| High | 63.7 | 59.9 | 58.5 | 57.5 | 56.2 | |
| 5-MethylTHF | −9.58** (−12.9, −6.13) | |||||
| Low | 14.8 | 13.7 | 12.4 | 12.0 | 11.8 | |
| Medium | 32.6 | 28.9 | 27.0 | 26.6 | 25.5 | |
| High | 48.2 | 43.5 | 40.3 | 39.2 | 38.2 | |
| Folic acid | −2.13 (−7.88, 3.99) | |||||
| Low | 0.84 | 0.80 | 0.83 | 0.80 | 0.84 | |
| Medium | 3.88 | 3.96 | 3.86 | 3.96 | 3.92 | |
| High | 9.16 | 8.80 | 8.99 | 9.12 | 9.00 | |
| MeFox | ||||||
| Low | 0.62 | 1.03 | 1.44 | 1.49 | 1.42 | 66.9** (34.4, 107) |
| Medium | 0.86 | 2.45 | 2.90 | 2.80 | 2.87 | 187** (131, 256) |
| High | 1.90 | 3.09 | 4.70 | 4.57 | 4.44 | 62.7** (31.0, 102) |
Serum QC pools stored at −70°C were used as reference; statistical analysis by 2-way ANOVA. 5-methylTHF, 5-methyltetrahydrofolate; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; QC, quality control; tFOL, total folate.
Values are geometric means from 6 replicates/condition
Values are percent change (95% Cl) compared to reference across the QC pools except for MeFox where a significant interaction between time and pool (P-interaction = 0.0299) necessitated providing separate estimates for each pool
0.0001 ≥ P < 0.05;
P <0.0001
DISCUSSION
The present study investigated changes in serum folate forms in response to a series of suboptimal preanalytical experiments on folate stability, covering the full spectrum from whole blood processing to repeated freeze/thawing of samples as part of the laboratory analysis. Because 5-methylTHF makes up the major portion of circulating folates (10) and it is susceptible to oxidative degradation to MeFox (11), a folate form that is detected in most serum samples (4) but is biologically inactive (12), it is critical to understand how these 2 folate forms behave under preanalytical conditions. Folic acid is another important component in serum samples, especially in populations that are exposed to folic acid-fortified foods (10), but this vitamer is generally believed to have good stability (2,13). Not much is known about the stability of minor folate forms (THF, 5-formylTHF, and 5,10-methenylTHF) during suboptimal preanalytical conditions, particularly because these vitamers are present in serum at low concentrations and often not detectable. However, during analysis these folates are known to be either sensitive to oxidation or subject to interconversions (2,13).
The notable loss of 5-methylTHF during delayed processing of whole blood, 14.5 nmol/L (36%) after only 1 d at 32°C, was similar to the loss of tFOL (12.9 nmol/L or 30%) we reported earlier from the microbiologic assay for the same set of samples (7). This is expected because the microbiologic assay does not respond to MeFox. The tFOL measured by our HPLC-MS/MS included MeFox and as a result decreased by only 5.5 nmol/L (11%). A previous report on a 1 d serum-clot contact time at 32°C reported only a 15% loss of tFOL measured by a radioimmunoassay (14). This brings up the question whether the latter assay may have responded to MeFox and it emphasizes that findings obtained with one method have to be carefully interpreted and cannot necessarily be applied to another. Also, more research is needed to investigate whether different clinical folate assays respond to MeFox.
As expected, the loss of 5-methylTHF was more moderate when serum was stored at 11°C (3.4, 5.7, and 11.6 nmol/L after 7 d, 10 d, and 14 d, respectively) and similar to the loss of tFOL (1.9, 4.0, and 10.6 nmol/L) we reported earlier from the microbiologic assay (7). However, the large increase in MeFox, 1.82–6.62 nmol/L when serum was stored 7–14 d at 11°C and 7.17–8.63 nmol/L when unprocessed whole blood was stored 1–3 d at 32°C, did not compensate for the loss of 5-methylTHF. This means that while capturing MeFox as part of tFOL is important, it does not guarantee that all folate that was originally in the sample is accounted for. Following proper preanalytical conditions that protect labile folates is still important.
While the room temperature condition in our experiment on short-term exposure of serum to elevated temperature was similar to the kinetic study conducted by Hannisdal et al. (5) and confirmed their findings [5-methylTHF was stable at room temperature for 24 h (our study) and 48 h (5)], the 37°C condition was new and added to the previous findings [33% loss of 5-methylTHF after 1 d at 37°C (our study) vs. 50% loss of 5-methylTHF after 8 d at room temperature (5)]. Our observation that the 5-methylTHF loss after 1 d at either room temperature or 37°C was recovered by MeFox was similar to the conclusion by Hannisdal et al. that the 5-methylTHF loss within 2 d at room temperature was recovered by MeFox (5). This indicates that a limited exposure of serum to moderate temperature does not compromise the quantitation of tFOL as long as MeFox is also measured as part of the tFOL.
The stability of folates during repeated freeze/thawing of serum is of great concern because analyses sometimes have to be repeated due to a run being out of control or specific results violating predetermined sample QC criteria. We confirmed previous findings that folates are relatively stable (<5% loss) when exposed to a limited number of short freeze/thawing cycles. Pfeiffer et al. (9) found no loss of folates when serum was briefly (1 h) subjected to 3 freeze/thawing cycles at room temperature, but found some folate loss (~10%) when samples were kept at room temperature for extended time (5 h). Similarly, Drammeh et al. found some folate loss (<5%) when serum was subjected to 3 freeze/thawing cycles (7). The moderate increase in MeFox (<1 nmol/L) in the absence of a loss of 5-methylTHF we observed in the “closed vial” experiment 4 may be due to the structural rearrangement of residual 4α-hydroxy-5-methylTHF (the intermediary product in the oxidation of 5-methylTHF) to MeFox.
Only sparse information is available on long-term storage stability of folates (15), particularly at suboptimal temperatures, which is the case if the laboratory does not have access to a low temperature freezer. Good stability of folates in serum or plasma containing ascorbic acid (5 g/L) stored at −20°C for years was reported (16). On the other hand, a 20% decrease of folate was noted in serum samples (without added ascorbic acid) stored at −20°C for 1 y with no further decrease thereafter for up to 4 y (17). This was similar to our finding of 21% loss of 5-methylTHF when we stored serum QC samples (without added ascorbic acid) at −20°C for 1 y. One study reported an even higher (40%) loss of folate after serum storage at −20°C for 1 mo, but levels remained stable afterwards for 1 y (18). The doubling to tripling of MeFox concentrations in our experiment 6 (serum storage at −20°C for 1 y) was not able to compensate the molar loss of 5-methylTHF and confirmed findings by Hannisdal et al. who investigated the analytical recovery of folate and its degradation products during long-term storage of serum at −25°C for up to 29 y (6). While the authors found MeFox in all specimens, this oxidation product did not compensate for the complete loss of folate; substantial folate degradation was recovered as para-aminobenzoylglutamate. Not surprisingly, we found drastic 5-methylTHF losses when serum was stored at 5°C for up to 1 y, yet only 7% loss of folic acid. Folic acid showed good stability during frozen storage at −20°C for 1 y, but also during most other preanalytical experiments in our study.
Our study has several strengths. We covered a broad range of preanalytical conditions, including some extreme conditions that are rarely reported in the literature. The analyses were conducted with a sensitive, specific, and precise HPLC-MS/MS method. To enhance the power of our study, we either analyzed >20 individual samples per condition or we analyzed several replicates (>4) of the same pool per condition. A limitation of our study is that we did not apply a consistent design to all experiments. This was because we relied on already available and unique sample sets for some experiments (7), while for others we relied on well-characterized serum QC pools. It would have been desirable to also obtain information on the behavior of minor folate forms, however this is limited by the fact that in most samples those vitamer forms are not detectable.
In conclusion, the current study provides valuable information for the design of field studies. Our findings reemphasize that sample processing and storage time and temperature are critical parameters that influence serum folate stability and the accuracy of results. Prompt processing of whole blood and storage of serum at optimal temperature (−70°C) is critical to prevent the loss of folate and the oxidation of 5-methylTHF to MeFox. While the quantitation of MeFox in optimally collected, processed, and stored samples is critical to compensate for minor losses of 5-methylTHF during preanalytical steps, MeFox may not completely recover the loss of 5-methylTHF, especially when samples have undergone more extreme suboptimal processing and storage conditions.
Supplementary Material
Acknowledgments
We thank Drs. Bakary Drammeh and Mindy Zhang, CDC for coordinating the logistics to obtain specimens for experiments 1 and 2. ZF and CMP designed the overall research project; ZF conducted most of the research; MS analyzed the majority of the data; ZF wrote the initial draft, which was modified after feedback from CMP; CMP has primary responsibility for content. All authors heave read and approve the final manuscript.
Footnotes
No specific sources of financial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
Author disclosures: None of the authors have any conflicts of interest.
Supplemental Tables 1–2 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: 5-formylTHF, 5-formyltetrahydrofolate; 5,10-methenylTHF, 5,10-methenyltetrahydrofolate; 5-methylTHF, 5-methyltetrahydrofolate; HPLC-MS/MS, HPLC-tandem MS; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate; QC, quality control; THF, tetrahydrofolate.
References
- 1.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable of NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr. 2011;94:297S–302S. doi: 10.3945/ajcn.111.017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory JF., III Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Adv Food Nutr Res. 1989;33:1–101. doi: 10.1016/s1043-4526(08)60126-6. [DOI] [PubMed] [Google Scholar]
- 3.Verlinde PH, Oey I, Deborggraeve WM, Hendrickx ME, Van Loey AM. Mechanism and related kinetics of 5-methyltetrahydrofolic acid degradation during combined high hydrostatic pressure-thermal treatments. J Agric Food Chem. 2009;57:6803–14. doi: 10.1021/jf900832g. [DOI] [PubMed] [Google Scholar]
- 4.Fazili Z, Pfeiffer CM. Accounting for an isobaric interference allows correct determination of folate vitamers in serum by isotope dilution-liquid chromatography-tandem MS. J Nutr. 2013;143:108–13. doi: 10.3945/jn.112.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr. 2009;139:1415–8. doi: 10.3945/jn.109.105635. [DOI] [PubMed] [Google Scholar]
- 6.Hannisdal R, Gislefoss RE, Grimsrud TK, Hustad S, Mørkrid L, Ueland PM. Analytical recovery of folate and its degradation products in human serum stored at −25°C for up to 29 years. J Nutr. 2010;140:522–6. doi: 10.3945/jn.109.116418. [DOI] [PubMed] [Google Scholar]
- 7.Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem. 2008;54:1883–91. doi: 10.1373/clinchem.2008.108761. [DOI] [PubMed] [Google Scholar]
- 8.Fazili Z, Pfeiffer CM. Measurement of folates in serum and conventionally prepared whole blood lysates: application of an automated 96-well plate isotope-dilution tandem mass spectrometry method. Clin Chem. 2004;50:2378–81. doi: 10.1373/clinchem.2004.036541. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem. 2004;50:423–32. doi: 10.1373/clinchem.2003.026955. [DOI] [PubMed] [Google Scholar]
- 10.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assays and Bio-Rad radioassay. Clin Chem. 2007;53:781–4. doi: 10.1373/clinchem.2006.078451. [DOI] [PubMed] [Google Scholar]
- 11.Jongejan JA, Mager HIX, Berends W. Autoxidation of 5-alkyl-tetrahydropteridinesthe oxidation product of 5-methyl-THF. In: Kisliuk RL, Brown GM, editors. Chemistry and biology of pteridines: proceedings of the Sixth International Symposium on the Chemistry and Biology of Pteridines; La Jolla, California. September 25–28,1978; New York: Elsevier/North-Holland; 1979. pp. 241–6. [Google Scholar]
- 12.Thien KR, Blair JA, Leeming RJ, Cooke WT, Melikian V. Serum folates in man. J Clin Path. 1977;30:438–48. doi: 10.1136/jcp.30.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Brouwer V, Zhang G-F, Storozhenko S, Van Der Straeten D, Lambert WE. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem Anal. 2007;18:496–508. doi: 10.1002/pca.1006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DJ, Elswick RK, Miller WG, Bailey JL. Effect of serum-clot contact time on clinical chemistry lab results. Clin Chem. 1998;44:1325–33. [PubMed] [Google Scholar]
- 15.Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology. In: Bailey LB, editor. Folate in Health and Disease. 2. CRC Press. Taylor & Francis Group; 2010. pp. 517–74. [Google Scholar]
- 16.O’Broin JD, Temperly IJ, Scott JM. Erythrocyte, plasma, and serum folate: Specimen stability before microbiological assay. Clin Chem. 1980;26:522–4. [PubMed] [Google Scholar]
- 17.Ocke MC, Schrijver J, Obermann-de Boer GL, Ploemberg BPM, Haenen GR, Dromhout D. Stability of blood (pro) vitamins during four years of storage at −20°C: Consequences for epidemiologic research. J Clin Epidemiol. 1995;48:1077–85. doi: 10.1016/0895-4356(94)00232-f. [DOI] [PubMed] [Google Scholar]
- 18.Jansen EHJM, Beekhof PK, Cremers JWJM, Schenk E. Long-term (in)stability of folate and vitamin B12 in human serum. Clin Chem Lab Med. 2012;50:1761–3. doi: 10.1515/cclm-2012-0108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.