Abstract
Cancer survivors diagnosed during infancy and adolescence may be at risk for chemotherapy-related cognitive impairments (CRCI), however the effects of pediatric chemotherapy treatment on adulthood cognitive function are not well understood. Impairments in memory, attention and executive function affect 15–50% of childhood leukemia survivors related to methotrexate exposure. Systemic cisplatin is used to treat a variety of childhood and adult cancers, yet the risk and extent of cognitive impairment due to platinum-based chemotherapy in pediatric patients is unknown. Systemic cisplatin penetrates the CNS, induces hippocampal synaptic damage, and leads to neuronal and neural stem/progenitor cell (NSC) loss. Survivors of non-leukemic cancers may be at risk for significant cognitive impairment related to cisplatin-driven neurotoxicity. We sought to examine the long-term effects of systemic cisplatin administration on cognitive function when administered during infancy and adolescence in a rat model.
We performed cognitive testing in adult rats exposed to systemic cisplatin during either infancy or adolescence. Rats treated as adolescents showed significantly poor retrieval of a novel object as compared to controls. Further, cisplatin-treated infants and adolescents showed poor contextual discrimination as compared to controls, and an impaired response to cued fear conditioning. Ultimately, systemic cisplatin exposure resulted in more profound impairments in cognitive function in rats treated during adolescence than in those treated during infancy. Further, exposure to cisplatin during adolescence affected both hippocampus and amygdala dependent cognitive function, suggesting a more global cognitive dysfunction at this age.
Keywords: Cisplatin (CDDP), Chemotherapy-related cognitive, impairment (CRCI), Memory, Pediatric
1. Introduction
Cognitive impairment is a well-described consequence of cancer treatment, with 17–75% of cancer survivors reporting persistent memory problems years after completion of therapy [1,2]. Cognitive abnormalities observed in cancer survivors typically include impairments in memory, attention, processing speed and executive function [3–6]. A variety of causes including direct chemotherapy neurotoxicity as well as indirect toxicity related to hormonal abnormalities, oxidative stress, treatment-associated metabolic changes, inflammatory activation, cancer-related symptoms (pain, fatigue) and medical co-morbidities (anemia, renal dysfunction, cardiotoxicity) have been implicated in the pathogenesis of cancer-related cognitive impairment [7,8]. CNS-penetrating chemotherapy, particularly high doses of systemic and intrathecal methotrexate used in pediatric acute lymphoblastic leukemia treatment regimens, can cause chronic leukoencephalopathy and have been most strongly implicated in chemotherapy-related cognitive impairment (CRCI) [9–11]. The clinical impact of other non-antimetabolite chemotherapeutic agents on cognition in children and adolescents is not known [12–14].
Cisplatin is a CNS penetrating chemotherapeutic agent used to treat a number of malignancies including common pediatric and young adult cancers such as neuroblastoma, hepatoblastoma, germ cell tumors and primary central nervous system neoplasms [15,16]. Recent National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) data confirm that the peak incidences of the above mentioned cancers occur during infancy and/or adolescence, thereby making these patient populations at particular risk of developing side effects attributable to cisplatin [17].
To examine, the effects of early-life cisplatin exposure on cognition function in young adulthood, we developed an infant and adolescent rodent model of CRCI. Rats have a brief and accelerated childhood compared to humans [18]. The most common method to assess infancy in rats is based on weaning age. Rat pups are weaned at post-natal day 21 (P21) and the average weaning age for humans is approximately 6 months. Rats reach peak adolescence at approximately 38 days (P38), and transition into adulthood at approximately P63. In contrast, humans reach adolescence at approximately 11.5 years, and enter adulthood at 18 years of age. Based on these developmental stages, we administered cisplatin (2 mg/kg/day) to Sprague Dawley rats at P25-P29 or P35-P39, and examined cognitive function in young adulthood (P65). These rat developmental stages correlate with human infancy (0–2 years) and adolescence (11–18 years), respectively [18,19].
The neurotoxic effects of a variety of chemotherapeutic agents (cisplatin, methotrexate, cyclophosphamide, carmustine, 5-florouracil, cytarabine) have been examined in the pre-clinical setting and are associated with neurotoxicity and cognitive impairment [3,4,20–23]. Specifically, we have previously reported that low-dose in-vitro cisplatin exposure induces apoptosis in cultured rat hippocampal neurons and neural stem/progenitor cells (NSC) [22]. Further, we showed that acute in-vivo cisplatin (6 mg/kg, 10 mg/kg) exposure causes a time-dependent loss of hippocampal dendritic branching and dendritic spine density in adult rats [22]. When treated with a chronic cisplatin regimen (5 mg/kg/week for 4 consecutive weeks), adult male rats exhibited significant impairments in three cognitive tasks [24]. Additional behavioral studies in the adult rodent population have yielded data showing the development of hippocampal-dependent cognitive impairment. One reported pre-clinical model has explored the development of cognitive deficits in pre-weanling rodents exposed to chemotherapy (methotrexate and cytarabine mimicking childhood acute lymphoblastic leukemia treatment); however, a paucity of models exploring the late cognitive effects of non-antimetabolite chemotherapy, specifically with a pediatric focus during infancy and adolescence, exists [25]. Given the common use of cisplatin to treat a variety of pediatric cancers and the known effect of cisplatin on neural structures, we sought to develop a pre-clinical pediatric model examining the long-term cognitive function of infant and adolescent rodents treated with cisplatin.
2. Methods
2.1. Animals
Animal studies were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine. All experiments were approved by the IACUC and conformed to National Institutes of Health standards.
2.2. Chemotherapy application in-vivo
Thirty-nine male Sprague-Dawley rats (Charles River, San Diego, CA, USA) were obtained weaned from their mother at post-natal day 21 (P21). Treated rats received intraperitoneal cisplatin dissolved in 0.9% saline (Fresenius Kabi USA, LLC) dosed at 2 mg/kg/day for 5 consecutive days beginning at post-natal day 25 (P25, infancy) or post-natal day 35 (P35, adolescence). Age-matched controls received 0.9% saline of a similar volume. Mannitol (APP Pharmaceuticals, 125 mg/kg, intraperitoneal) was administered 1 h prior to CDDP, to minimize renal toxicity and increase diuresis. Additional intraperitoneal injections of 0.9% saline were given as needed for supportive care.
2.3. Behavioral testing
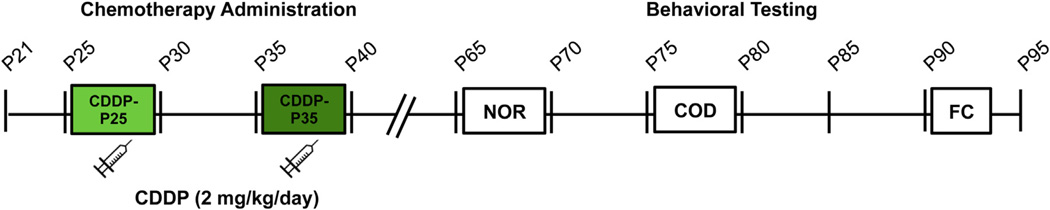
Cognitive testing was completed during adulthood (post-natal day >65) including a novel object recognition task (NOR), context object discrimination (COD) task, and fear conditioning (FC) (Fig. 1) [26–30]. Each cognitive task was performed in different rooms and arenas, and the objects used for NOR were distinct from those used for COD. A pilot experiment included 3 rats in each group (n = 9) treated as above and tested via the NOR task at post-natal day 65–70. A second experiment included 5 rats per treatment group (n = 15). Three rats in the adolescent group (CDDP-P35) died of complications of chemotherapy exposure (renal toxicity). The remainder of the rats completed the NOR task at post-natal days 65–68 (P65–P68), COD task at post-natal days 75–78 (P75–P78) and FC task at post-natal day 93–94 (P93–P94). A third experimental group was added to this study, including 5 control, 5 infant and 8 adolescent rats treated and tested using the same conditions as the second cohort. Three control rats were excluded from the FC analysis due to lack of response to conditioning; they failed to freeze in response to the foot-shocks administered during training, and as a result did not exhibit to freeing behavior during the post-training session, context test, nor cue-test.
Fig. 1. Experimental Design.
Rats received CDDP (2 mg/kg/day) for five consecutive days at P25–P29 (infancy) or P35–P39 (adolescence). Age-matched controls received 0.9% saline of similar volume. Cognitive testing was completed during adulthood, including novel object recognition (NOR) at P65–P70, context object discrimination (COD) at P75–P78, and fear conditioning (FC) at P93–P94.
2.3.1. Novel object recognition (NOR)
First, rats were tested for recognition of a novel object [29,30]. Rats were individually placed in a quiet room in an open Plexiglas square arena (60 cm × 60 cm × 60 cm) lined with black cardboard. After habituation to the arena, each rat was given 5 min per day on 2 consecutive days to explore 2 identical objects in the arena. The test trial was performed 24 h later such that each rat was presented with one familiar object paired with a novel object for 5 min.
2.3.2. Context object discrimination (COD)
Individual rats were exposed to 2 different environmental arenas (Arena A and Arena B) located in adjacent rooms. Each rat was given 5 min in each arena per day on 2 consecutive days to freely explore. Between training sessions each rat was returned to the home cage for a 20 min interval [28]. Each environment had 2 identical objects unique to the environment. The order of context presentation was counterbalanced between subjects and across days. On day three each rat was tested for 5 min in a modified environment (Arena A’) where one of the objects from Arena B replaced one of the objects from Arena A. The total time each rat explored each object was recorded.
2.3.3. Fear conditioning (FC)
The fear conditioning task was administered at post-natal day 93–94 (P93–P94). Two similar but distinct chambers, each housed within a sound-attenuating chamber, were used for fear conditioning [31]. The floor of the conditioning chamber consisted of 18 steel rods wired to a shock generator (Coulborn Instruments) for foot-shock delivery. The fear-conditioning paradigm used was based on previous studies on chemotherapy-treated rodents [26,27]. Specifically, the fear-conditioning task consisted of three distinct phases: a training phase, a context test phase and a cue test phase. During fear conditioning rats were individually placed in a chamber for 5 min (baseline), exposed to 5 tone pairings (90 db, 2000 Hz-shock, [1 mA, 1 s]) for 5 min and then left in the conditioning chamber for an additional 5 min (post-training). The following day the rats were returned to the conditioning chamber for 5 min to assess conditioned freezing to the context (context test). No tone or shock was administered during the context test. When memory for the context-shock conditioned association is intact rats spend a significant portion of the context test freezing. The cue test was then administered 1 h after the context test. For cue testing, rats were placed in a novel context for 1 min (pre-cue test) followed by a 3- min tone presentation (cue test) and an additional 1-min (post-cue test). Freezing behavior was defined as complete immobility with the exception of breathing movements and scored as a percent of the overall time for each phase.
3. Statistical analysis
Graphs and statistical analyses were prepared using GraphPad Prism 5.0 Software (GraphPad Software, La Jolla, CA, USA). Results are expressed as mean ± SEM. Comparisons between treatment groups were made by unpaired Student’s t-test or two-way ANOVA. Fear conditioning analysis was performed using two-way RM ANOVA. Statistical significance levels were set at 0.05. Post hoc analysis was made by Bonferroni correction.
4. Results
4.1. Cisplatin treatment during adolescence causes impaired novel object recognition
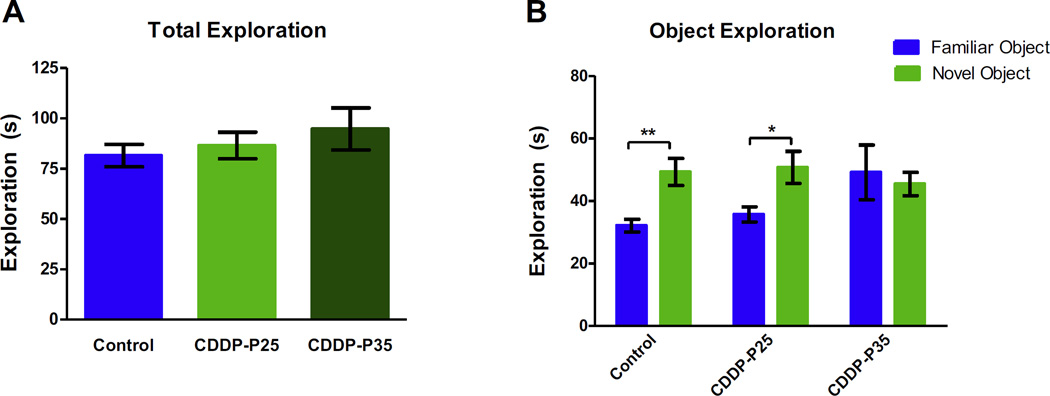
The NOR task utilizes the natural tendency of animals to explore novel objects relative to familiar objects [32]. During NOR testing, all groups showed similar total object exploration times indicating similar engagement and activity level (Fig. 2A). Controls and rats exposed to cisplatin during infancy spent significantly more time exploring the novel object compared to the familiar object (p = 0.0015 and p = 0.0141); however, rats exposed to cisplatin during adolescence displayed no preference in exploring either object (p = 0.4341). A two-way ANOVA revealed a significant difference in object exploration (F(1,72) = 5.574, p = 0.0209), but no significant difference between treatment groups (F(2,72) = 0.9079, p = 0.4079), nor a difference in interaction between treatment groups and object exploration (F(2,72) = 2.718, p = 0.0728) (Fig. 2B). Cisplatin treatment during adolescence, but not infancy, impaired the ability to recognize a novel object from a previously experienced familiar object. Together these data suggest that cisplatin treatment may have an age-dependent effect on cognitive function.
Fig. 2. Novel Object Recognition.
During NOR testing all groups showed similar total exploration times (A). As expected, control (n = 13) and CDDP-P25 (n = 13) rats explored a novel object (NO) significantly more than a familiar object **p < 0.005 and *p < 0.02; however, exploration between objects for the CDDP-P35 (n = 13) rats was not different (B). Error bars are SEM.
4.2. Cisplatin treatment during infancy and adolescence causes diminished contextual discrimination
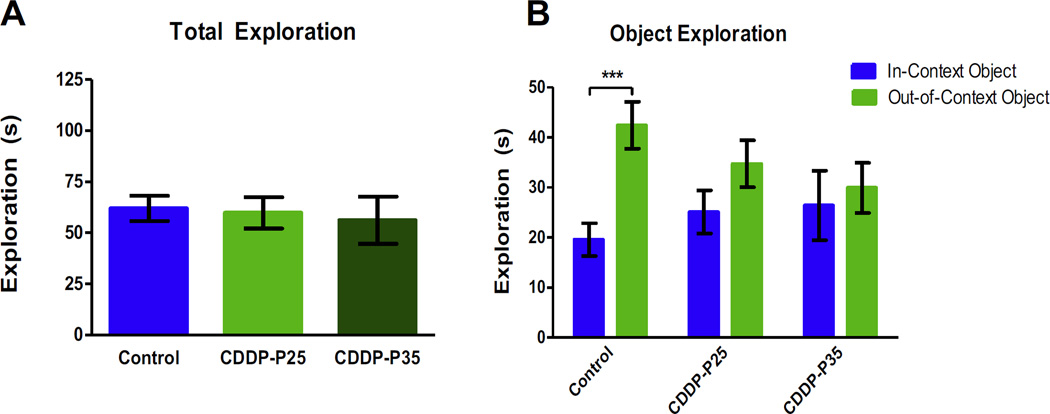
The COD task tests for context discrimination, which requires hippocampal-dependent pattern separation skill [30]. During the COD testing all groups showed similar total object exploration times (Fig. 3A). The control group explored the out-of-context object more than the in-context object (p = 0.0009); however, rats exposed to cisplatin during infancy and adolescence showed no bias between objects (infants: p = 0.7142, adolescents: p = 0.6867). A two-way ANOVA revealed a significant difference between treatment groups (F(1,54) = 8.822, p = 0.0044), but no significant difference between object exploration (F(2,54) = 0.1681, p = 0.8457), nor a difference in interaction between treatment group and object exploration (F(2,54) = 2.004, p = 0.1447) (Fig. 3B). Cisplatin treatment diminished the ability to distinguish a previously experienced object placed in a different context. This effect was most prominent in the subjects treated during adolescence.
Fig. 3. Context Object Discrimination.
During COD testing all groups showed similar total exploration times (A). As expected, control (n = 10) rats explored an out-of-context object significantly more than a familiar object; (***p = 0.001); however, exploration between objects for the CDDP-P25 (n = 10) and CDDP-P35 (n = 10) rats was not different (B). Error bars are SEM.
4.3. Cisplatin treatment during infancy and adolescence causes decreased response to a conditioned cued stimulus
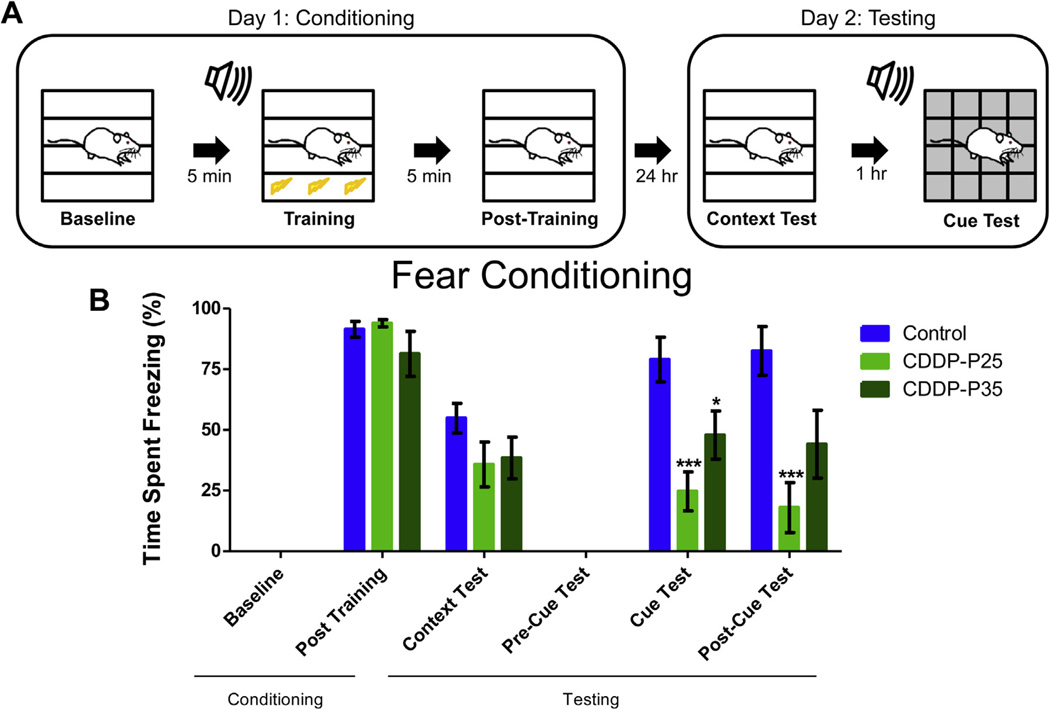
Fear conditioning assesses the ability of rodents to learn and remember an association between environmental cues and aversive experiences via evaluation of freezing behavior in response to a conditioned context or cue [33]. After being tested on novel object recognition and contextobject discrimination, the animals were tested on the fear conditioning task (Fig. 4A). While contextual fear conditioning requires an intact hippocampus, cued fear conditioning relies on the amygdala [34,35]. A two-way RM ANOVA revealed a main effect of testing phase (F(5,120) = 74.61, p < 0.0001), treatment group (F(2,120) = 6.120, p = 0.0071), and treatment group by phase interaction (F(10,120) = 4.678, p < 0.0001) for the percent time spent freezing during the fear conditioning task. During the context-test phase, cisplatin-treated infant and adolescence rats spent decreased percentages of time freezing compared with the control animals, although not significant (infants: p = 0.1405; adolescents: p = 0.1737). Surprisingly, cisplatin treatment affected cued-memory such that both cisplatin-treated infants and adolescents spent significantly decreased percentages of time freezing in response to 5 tone-shock pairings, as compared to the control subjects (infants: p = 0.0003; adolescents: p = 0.0412). The groups did not differ significantly in the freezing behavior across baseline, post-training, context-test, and pre-cue, suggesting that the deficits may be selective to the amygdala-dependent cue memory phase of the task - the tone to which the foot-shock was paired.
Fig. 4. Fear Conditioning.
Schematic of Fear Conditioning paradigm (A). During context and cued fear conditioning both CDDP-P25 (n = 10) and CDDP-P35 (n = 10) rats showed a trend toward decreasing freezing response during the context test (p = 0.14 and p = 0.17) as compared to controls (n = 7). During the Cue and post-Cue Test, CDDP-P25 rats showed significant decrease in freezing (***p < 0.001). During the Cue Test, CDDP-P35 rats showed significant decreased freezing response (*p < 0.05) which persisted to a lesser degree in the Post–Cue Test (p = 0.06) (B). Error bars are SEM.
5. Discussion
As most of the current pre-clinical studies on CRCI have been performed on adult rodents, we recognized a need to develop a model exploring the effects of chemotherapy on the developing brain. We have previously shown that when administered chronically to adult male Sprague Dawley rats, cisplatin (20 mg/kg) impaired performance in novel object recognition, context object discrimination, and contextual but not cued fear conditioning [24]. In the adult population, ovarian cancer patients treated with cisplatin consistently develop CRCI during and after platinum-based chemotherapy [36]. A study that examined advanced ovarian cancer patients in detailed neuro-cognitive tests detected impairments in two or more cognitive domains in 40% of cisplatin chemotherapy recipients [37]. However, studies examining the effects of platinum-chemotherapy in pediatric rodent models and in the adolescent and young adult (AYA) cancer survivor population are lacking.
Childhood and adolescence are distinct yet similarly robust periods of brain development and maturation of cognitive skills, which are vulnerable to harmful environmental influences such as illicit drug exposure [38]. Similarly, the developing rodent brain has been shown to be more vulnerable to toxins or stress as compared to the matured rodent brain [39]. These differences in vulnerability obviate the need for the development of pediatric pre-clinical models of CRCI to parallel those for adults. Here we developed a model of CRCI for examining cisplatin-induced cognitive impairments in an infant (P25) and adolescent (P35) rat model. Our current data shows that cisplatin, when administered during infancy and adolescence, causes long-term cognitive impairment; however, the degree and type of impairment appears to differ with age at time of cisplatin exposure. Understanding the age-dependent and agent-specific differences in CRCI will be important in developing effective supportive and preventative strategies to preserve cognitive function in growing cancer survivor populations.
Given that cisplatin exposure reduces dendritic branching and spine density and induces apoptosis of neurons and NSC within the hippocampal formation [24,40], it follows that cognitive tasks that require the hippocampus would be highly susceptible to cisplatin, and the effects of cisplatin on cognitive function should be explored. The hippocampus is required for spatial and contextual memory [33]. Specifically in rodents, the hippocampus participates in the recollection of where and in what context an object is encountered [33]. Further, the hippocampus is crucial for forming an association between an aversive experience (foot-shock) and the context in which it occured [34]. COD and context fear conditioning tasks evaluate for intact hippocampus function. In addition to hippocampal specific behavior testing, we utilized other tasks involving cortical brain regions and the amygdala to evaluate cognition function more globally (Table 1). The amygdala participates in memory processing and emotional reaction and plays a critical role in the formation of an association between an unconditioned stimulus and a cued stimulus [34,35]. Contextual fear conditioning requires an intact hippocampus; however, the cued fear conditioning component of the task requires intact amygdala function. The NOR task differs from all previous tests in that it assesses general memory function, which requires an intact temporal lobe including hippocampus as well as adjacent cortical structures [32].
Table 1.
Neural systems involved in behavioral tasks.
| Novel Object Recognition |
Context Object Discrimination |
Fear Conditioning |
|---|---|---|
|
|
|
6. Conclusions
Our data indicates that rats exposed to systemic cisplatin during infancy and adolescence experience impairment of cognitive function. The degree and type of cognitive dysfunction may depend on age at time of cisplatin exposure. Rodents treated during adolescence may develop greater impairment in cognitive tasks than those treated during infancy, as evidenced by impaired performance on the NOR and COD; however, neither group showed significant impairment on the contextual FC (Table 2). A more strongly powered follow-up study may help confirm these conclusions and define the subtle cognitive changes caused by cisplatin exposure at various ages. While cisplatin-associated cognitive impairment may involve the hippocampus, rats treated with cisplatin during adolescence appear to experience more global cognitive deficits. Amygdala functions are diminished after cisplatin exposure in infancy and adolescence. Previous data has shown that activation of neural circuitry pathways involving the nucleus of the amygdala contribute to cisplatin-induced malaise and energy balance dysregulation [41]. Changes in pathway activation or direct neural toxicity in the amygdala may explain the impairment in amygdala-dependent cognitive tasks in the pediatric rodents. Further, adolescent rats showed significant lack of discrimination of a novel object in the NOR testing indicating more global disruption of neural circuitry involving cortical brain regions. This was not seen in the population exposed during infancy that exhibited comparable discrimination for a novel object to controls.
Table 2.
Summary of cognitive testing results.
| Group | NOR | COD | FC | |
|---|---|---|---|---|
| Context-Test | Cue-Test | |||
| Control | + | + | + | + |
| CDDP-P25 | + | − | + | − |
| CDDP-P35 | − | − | + | − |
+Indicates intact discrimination of objects (NOR, COD), or intact freezing behavior in FC. − Indicates impaired discrimination of object (NOR, COD), or poor freezing behavior in FC.
The differences in cognitive performance between the subjects exposed to cisplatin during infancy and adolescence may be attributed to neurodevelopmental changes that occur at these distinct stages [42]. Infancy is a critical period of robust neurodevelopment. Key developmental processes that occur in infancy in humans as well as rodents include: brain reaches 90–95% of adult weight [43] peak in synaptic density [44] and myelination rate, and neurotransmitter and receptor changes. In rodents, the critical period of synaptogenesis occurs during the first three post-natal weeks of life, peaking by P25. In addition, NMDA subunit expression peaks by P20 in the rat hippocampus and cortex [45]. During adolescence, there is specialization and strengthening of neural networks, reduced synaptic density which reaches adult levels, and refinement of cognitive-dependent circuitry [46]. The robust neurodevelopment that occurs during infancy may facilitate the recovery of cognitive function following chemotherapy treatment, whereas during adolescence, chemotherapy treatment may result in cognitive impairments, which persist long into adulthood.
In our evaluation of young rats exposed to cisplatin we were able to detect an effect of cisplatin on long-term cognitive function after rats entered adulthood. In addition to detection of hippocampal-dependent changes, we also detected a potentially more global effect of cisplatin on cognition, which may be age-dependent and include the amygdala and cortical circuitry. Further exploration of CRCI, specifically regarding the effect of cisplatin exposure on the developing brain, is imperative not only in developing a more comprehensive understanding of CRCI, but also in devising targetable mechanisms for treatment and prevention.
HIGHLIGHTS.
A pediatric rodent model for chronic cisplatin-related cognitive impairment is proposed.
Extent of cognitive impairment in the chemotherapy exposed pediatric rodent population appears to vary based on age of exposure and neurodevelopmental stage.
Rats exposed to cisplatin during infancy show hippocampal-dependent cognitive impairment.
Rats exposed to cisplatin during adolescence show more global cognitive deficits.
Acknowledgments
Funding
This work was supported by: (1) National Institute for Neurological Diseases and Stroke Award (NINDS/NIH) number NS072234 (2) National Cancer Institute UCI Cancer Center Award number P30CA062203. Additional Funding support for Naomi Lomeli - T32 NS082174/NS/NINDS NIH HHS/United States (Training Program in Stem Cell Translational Medicine for Neurological Disorders) under the PI: Leslie Thompson.
Footnotes
Ethics
Approval was obtained from University of California, Irvine Institutional Animal Care and Use Committee (IACUC) protocol #2010-2946.
References
- 1.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat. Rev. 2013;39:297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol. Nurs. Forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapyin children: the past 10 years of research on brain structure and function. Pediatr. Blood Cancer. 2009;52:159–164. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 6.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J. Int. Neuropsychol. Soc.: JINS. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol. Rev. 2008;18:121–131. doi: 10.1007/s11065-008-9058-x. [DOI] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer NS, Balsamo LM, Bracken MB, Kadan-Lottick NS. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126:346–353. doi: 10.1182/blood-2015-02-627414. [DOI] [PubMed] [Google Scholar]
- 10.Duffner PK, et al. Neurocognitive and neuroradiologic central nervous system late effects in children treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) acute lymphoblastic leukemia protocols (ACCL0131): a methotrexate consequence? A report from the Children’s Oncology Group. J. Pediatr. Hematol. Oncol. 2014;36:8–15. doi: 10.1097/MPH.0000000000000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conklin HM, et al. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J. Natl. Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadan-Lottick NS, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J. Natl. Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit. Rev. Oncol. Hematol. 2007;63:183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.John TD, Sender LS, Bota DA. Cognitive impairment in survivors of adolescent and early young adult onset non-CNS cancers: does chemotherapy play a role? J. Adolesc. Young Adult Oncol. 2016;5(3):226–231. doi: 10.1089/jayao.2015.0025. [DOI] [PubMed] [Google Scholar]
- 15.Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. 6th. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011. [Google Scholar]
- 16.Nakagawa H, et al. Difference in CDDP penetration into CSF between selective intraarterial chemotherapy in patients with malignant glioma and intravenous or intracarotid administration in patients with metastatic brain tumor. Cancer Chemother. Pharmacol. 1996;37:317–326. doi: 10.1007/s002800050391. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone A, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. Apr, (based on November 2013 SEER Data Submission, Posted to the SEER Website, Last Updated December 17, 2014) 2014. [Google Scholar]
- 18.Sengupta P. The laboratory rat: relating its age with human’s. Int. J. Prevent. Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 19.Andreollo NA, Santos EF, Araujo MR, Lopes LR. Rat’s age versus human’sage: what is the relationship? Arquivos brasileiros de cirurgia digestiva, ABCD: Braz. Arch. Digest. Surg. 2012;25:49–51. doi: 10.1590/s0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- 20.Rzeski W, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann. Neurol. 2004;56:351–360. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- 21.Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol. Biochem. Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Andres AL, Gong X, Di K, Bota DA. Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp. Neurol. 2014;255:137–144. doi: 10.1016/j.expneurol.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiriz AB, et al. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2006;12:5000. doi: 10.1158/1078-0432.CCR-06-0138. [DOI] [PubMed] [Google Scholar]
- 24.Lomeli N, Kaijun D, Czerniawski J, Guzowski JF, Bota DA. Cisplatin induces mitochondrial damage and hippocampal neurotoxicity: a potential mechanism for chemotherapy-related cognitive impairment; Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16–20; New Orleans, LA. Philadelphia (PA): AACR; 2016. (Abstract nr 4782.) [Google Scholar]
- 25.Bisen-Hersh EB, Hineline PN, Walker EA. Effects of early chemotherapeutic treatment on learning in adolescent mice: implications for cognitive impairment and remediation in childhood cancer survivors. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2013;19:3008–3018. doi: 10.1158/1078-0432.CCR-12-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acharya MM, et al. Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 2015;75:676–686. doi: 10.1158/0008-5472.CAN-14-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie LA, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin. Cancer Res. 2012;18:1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 28.Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav. Immun. 2015;44:159–166. doi: 10.1016/j.bbi.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 2007;27:2957–2984. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J. Neurosci. 2014;34:12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter MG. I’ve seen it all before: explaining age-related impairments in object recognition: theoretical comment on Burke et al. (2010) Behav. Neurosci. 2010;124:706–709. doi: 10.1037/a0021029. [DOI] [PubMed] [Google Scholar]
- 33.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 35.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 36.Correa DD, Hess LM. Cognitive function and quality of life in ovarian cancer. Gynecol. Oncol. 2012;124:404–409. doi: 10.1016/j.ygyno.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Hess LM, et al. Pilot study of the prospective identification of changes in cognitive function during chemotherapy treatment for advanced ovarian cancer. J. Support Oncol. 2010;8:252–258. doi: 10.1016/j.suponc.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Konrad K, Firk C, Uhlhaas PJ. Brain development during adolescence: neuroscientific insights into this developmental period. Dtsch. Arztebl. Int. 2013;110:425–431. doi: 10.3238/arztebl.2013.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Praag H, et al. Unilateral hippocampal lesions in newborn and adult rats: effects on spatial memory and BDNF gene expression. Behav. Brain Res. 1998;92:21–30. doi: 10.1016/s0166-4328(97)00117-4. [DOI] [PubMed] [Google Scholar]
- 40.Andres AL, Gong X, Di K, Bota DA. Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp. Neurol. 2014;225:137–144. doi: 10.1016/j.expneurol.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate receptors in the central nucleus of the amygdala mediate cisplatin-induced malaise and energy balance dysregulation through direct hindbrain projections. J. Neurosci. 2015;35:11094–11104. doi: 10.1523/JNEUROSCI.0440-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 44.Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J. Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- 46.Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]