By adding a small amount of a vesicle surfactant, “unavoidable” splashing is considerably reduced on superhydrophobic surfaces.
Keywords: Superhydrophobic, liquid deposition, vesicle surfactant
Abstract
Deposition of liquid droplets on solid surfaces is of great importance to many fundamental scientific principles and technological applications, such as spraying, coating, and printing. For example, during the process of pesticide spraying, more than 50% of agrochemicals are lost because of the undesired bouncing and splashing behaviors on hydrophobic or superhydrophobic leaves. We show that this kind of splashing on superhydrophobic surfaces can be greatly inhibited by adding a small amount of a vesicular surfactant, Aerosol OT. Rather than reducing splashing by increasing the viscosity via polymer additives, the vesicular surfactant confines the motion of liquid with the help of wettability transition and thus inhibits the splash. Significantly, the vesicular surfactant exhibits a distinguished ability to alter the surface wettability during the first inertial spreading stage of ~2 ms because of its dense aggregates at the air/water interface. A comprehensive model proposed by this idea could help in understanding the complex interfacial interactions at the solid/liquid/air interface.
INTRODUCTION
Pesticide spraying is “hard” agricultural work, where more than 50% of agrochemicals are lost because of undesired bouncing and splashing behaviors on crop leaves with “waterproof” properties (1–3). In natural plants, superhydrophobic leaves are ubiquitous, and they usually get their nonwetting properties from the presence of waxy features on their surface. Particularly because of the combination of extremely low energized chemical composition and microstructured/nanostructured surface morphology, the superhydrophobic surface was demonstrated to facilitate droplets, even those with surfactant additives (4), bouncing and splashing within shortened contact time (5–17). This increases the difficulty of droplet retention (18), thus threatening ecological security and human safety: If pesticides cannot be properly deposited on crops, then pests might not be controlled and plant injury might occur (1). Redundant pesticides might contribute to soil, air, or water pollution, and human health might be negatively affected.
Although adding surfactants to the sprayed liquid is considered to be a simple method to reduce surface tension and to improve drop retention on a smooth hydrophobic surface, surfactant liquids still slide or bounce off the hydrophobic surface at a tilted angle and reduce liquid splashing on the superhydrophobic surface (19). In addition, according to the Kelvin-Helmholtz instability, the wave number kmax equals 2ρaU2/3γ, where ρa is the air density, γ is the fluid surface tension, and U is the relative velocity between gas and liquid. Therefore, reduced surface tension was believed to play a major role in the increased instability of the spilt droplet (20, 21). Enhancing liquid deposition on the superhydrophobic surface is complex and difficult work, where selected surfactants need to diffuse from the bulk to the newly created interfaces quickly, because the contact time is typically only several milliseconds, and need to decrease retraction velocity to reduce instability due to the reduced surface tension. Several studies have demonstrated the difficulty of surfactant drop deposition on the superhydrophobic surface. On the basis of these results, it would seem that surfactant additives should not be responsible for reducing high-speed liquid splashing on the superhydrophobic surface.
However, we found that this kind of “unavoidable” bouncing or splashing behavior was greatly inhibited or even completely reduced on the superhydrophobic surface at varied angles by adding a small amount of a double-chain vesicle surfactant. Unlike the micelle surfactant previously discussed, the vesicular surfactants additive demonstrated here was able to diffuse from the bulk to the newly formed interfaces during the deformation process, enter into the microstructured/nanostructured morphology, confine the motion of the liquid with the help of the wettability transition during the first inertial spreading stage of ~2 ms, decrease the retraction velocity down to nearly zero, and reduce bouncing and splashing (Fig. 1). For the first time, we have found that the vesicle surfactant could exhibit a distinguished ability to alter the surface wettability during the impact process. By taking advantage of this wettability transition, not only have liquid splashing and retraction been completely reduced but the maximum wetting area is also maintained after the impact process. This behavior is different from that observed in previous research, in which a micelle surfactant drop could bounce off the superhydrophobic surface (18), viscosity induced the splashing reduction (1), a surfactant drop partly reduced the liquid retraction on the hydrophobic surface (22–24), and nanoparticles or surface charges suppressed droplet rebound on the superhydrophobic surface (19, 25).
Fig. 1. The deposition of high-velocity impacting drops on the superhydrophobic leaf surface.
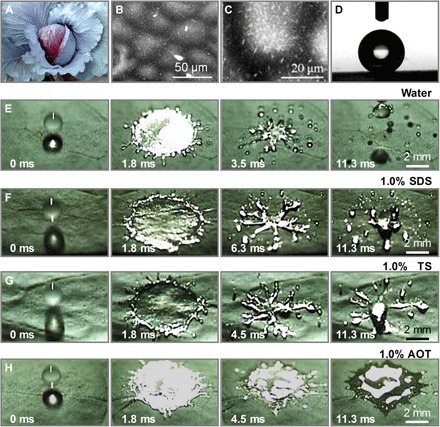
(A) Optical image of the B. oleracea L. leaf. (B and C) Environmental scanning electron microscope (SEM) images of the leaf surface with a microstructured/nanostructured morphology. (D) Water contact angle reveals the superhydrophobic property of the leaf surface. (E) Impact process of a water droplet on the superhydrophobic leaf surface. The splashing of water mainly occurs in the receding stage. (F and G) Inhomogeneous receding behavior: SDS (1%) and TS (1%) additives partially inhibit the receding splash. (H) Reduced receding behavior by the AOT surfactant: The receding splash was substantially depressed by 1% AOT additive.
RESULTS
Figure 1 shows the impacts of droplets of pure water and aqueous solutions containing the same mass fraction of the micelle surfactant sodium dodecyl sulfate (SDS), trisiloxane molecules (TSs), and the vesicle surfactant sodium bis(2-ethylhexyl) sulfosuccinate [Aerosol OT (AOT)] on a Brassica oleracea L. leaf (Fig. 1A) at a velocity of 2.53 ± 0.11 m s−1 using high-speed cameras (movie S1). In the experiment, the impact behavior was defined by measuring more than 30 different positions of the same sample and surfactant solutions. The B. oleracea L. leaf surface characterized by microstructured/nanostructured morphologies (Fig. 1, B and C) shows a water contact angle of 156.2 ± 4.3° (Fig. 1D), ensuring superhydrophobicity. The impacting water droplet, after reaching its maximum spreading at ~1.8 ms, breaks up into numerous droplets (Fig. 1E). By adding a certain amount of surfactants, the receding splash be inhibited by varying degrees (movie S1). Figure 1 (F and G) shows that aqueous droplets containing 1% SDS or 1% TSs partially reduced the receding splash but left several streams during retraction and finally broke up into several fragments. In contrast, by adding 1% AOT in the aqueous phase, the impacting drop first spread to a larger wetting area within 2 ms. Then, the liquid broke up and the receding laminar stream was substantially depressed (Fig. 1H) even after the maximum spreading stage (movie S1). Finally, a large wetting area was achieved and maintained. Partial and scarce receding behavior at low-speed impact was found in the micelle and vesicle regions (fig. S1), respectively. Furthermore, on artificial superhydrophobic surfaces with varied structures and tilted angles, aqueous droplets containing 1% AOT would spread properly (movies S2 to S4). Notably, this kind of liquid deposition behavior is in contrast to previous works, where surfactant drops have been shown to bounce off the superhydrophobic surface (18), although surfactants can help the liquid wet the hydrophobic surface (movie S5) (25). AOT is therefore a unique surfactant that has a more pronounced effect than the others in controlling liquid deposition and in reducing unavoidable splash.
DISCUSSION
Compared with the mechanism of liquid deposition enhancement using polymer additives (1), surfactant additives cannot alter viscosity but can reduce the liquid’s surface tension. Although surfactants can decrease the surface tension of the liquid, helping it spread on a hydrophobic surface under a low-speed impact (25), the reduction of surface tension also plays a major role in the increased instability and the enhanced droplet’s splash (20, 21). According to the Kelvin-Helmholtz instability, kmax = 2ρaU2r/3γ, the key to reducing instability is via the retraction velocity Ur, and the brevity of impact contact time should be enough for liquid droplets to wet the superhydrophobic surface (18). In our experiment, local pinning is observed for SDS (Fig. 1F) and TSs (Fig. 1G), and the entire pinning is found for AOT in the peripheral area of maximum spreading (Fig. 1H), where the retraction velocity Ur slows down to a low value, resulting in a small kmax. For the AOT drop, the motion of spreading is greatly confined, leading to extremely low instability and thus retarding the splash (movie S1).
The exceptional molecular structure of AOT distinguishes it from the other two surfactants in reducing splash and in enhancing liquid deposition. Cryo-TEM (transmission electron microscope) imaging was used to prove our assumption, which was achieved by allowing a free-falling surfactant drop to impact the Cu mesh followed by immersion in liquid nitrogen. This mimics the surfactant packing stage during the impact process. The significant differences of the surfactant aggregates are shown in Fig. 2 (A1 to C1), where the multilamellar vesicles were closely packed at the air/water interface for the AOT drop with a mass fraction of 1%, whereas micelles only randomly and loosely existed for the other two surfactant solutions at the same mass fractions. Compared with the sample molecular structures of SDS, TSs, and the previously mentioned surfactants in reducing the liquid bouncing (18, 25), AOT has two alkyl chains and a relatively small hydrophilic head group. This particular molecular structure of AOT is the main reason for its compact and directed alignment and leads to the multilamellar vesicle structure (26). As shown in Fig. 2 (A2 to C2), among the three surfactants, the aqueous solution containing 1% AOT exhibits the lowest DST within a surface age of 80 ms. At the beginning of the bubble pressure measurement of ~10 ms, its DST could decrease to a low value of ~32 mN/m (Fig. 2C2), whereas both 1% SDS and 1% TSs have DSTs that begin in a high value of ~43 mN/m (Fig. 2, A2 and B2). Similar to the property reflected in the diffusion coefficients achieved through 1H nuclear magnetic resonance (NMR) spectrometry (fig. S2) and dynamic contact angles (fig. S3), the DST results indicate that AOT has the fastest diffusion speed to the air/water interface and thus has the strongest ability to reduce the surface tension when there are newly created surfaces.
Fig. 2. The molecular structure, dynamic surface tension, and impact behavior of SDS, TSs, and AOT.
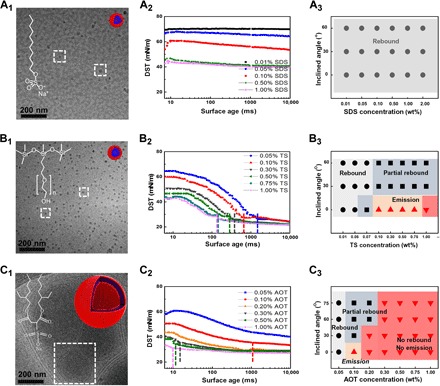
(A1 and B1) Cryo-TEM images of both 1% SDS and 1% TSs show the micelle aggregates at a mass fraction of 1%. (C1) Cryo-TEM image of 1% AOT shows that multilamellar vesicles have formed, indicating the dense aggregates of AOT molecules at the air/water interface. (A2) SDS has a long chain, and its dynamic surface tension (DST) slowly falls in the rapid fall region and cannot reach its equilibrium within a surface age of 100 ms. (A3) The droplets containing SDS are easy to rebound on the superhydrophobic surface even for highly concentrated solution. (B2) TSs have long induction periods in the first tens or hundreds of seconds. (B3) With the increase of the concentration, the induction period is shortened so that the impact behavior turns from bouncing, emission, to no rebound. On the inclined surface, it is still easy to partially rebound. (C2) AOT distinguishes itself from other surfactants in the fastest diffusion speed to form a densely packed molecular layer at the air/water interface. AOT directly approaches low surface tension of around 32 mN/m at the very beginning of the bubble pressure measurement, showing the lowest DST among the three surfactants within a surface age of 80 ms. The DSTs of 1% SDS and 1% TSs begin nearly at the same value of ~43 mN/m. TS quickly decreases to a lower value, and SDS slowly decreases to a higher value. (C3) AOT is the most effective surfactant that inhibits bouncing of the liquid drops on the superhydrophobic surface.
Besides reducing splashing on natural superhydrophobic leaves, AOT is the most effective surfactant that inhibits the bouncing and splashing of the liquid drops on the artificial superhydrophobic surface at both low (~1.2 m s−1) and high (~2.5 m s−1) impact speeds. The artificial superhydrophobic surfaces include a microstructured/nanostructured superhydrophobic surface composed of 20-nm hydrophobic SiO2 nanoparticle composites with a typical size and spacing of around 200 nm and a CuO nanosheet structured superhydrophobic surface with a typical size of about 3 to 6 μm in length and 200 to 600 nm in width. The water contact angles of these artificial superhydrophobic surfaces are 161.3 ± 0.5° and 159.1 ± 1.7°, respectively, which are much higher than those of the natural B. oleracea L. leaf. A fluorinated glass slide with a water contact angle of 112.8 ± 1.1° is also used for comparison. As shown in movie S5, although the droplets containing SDS can properly deposit on a smooth hydrophobic surface, it is difficult to reduce the rebound on a superhydrophobic surface, no matter how low or how high the impact speed is, even for highly concentrated solutions. For TSs, with the increase in concentration, the induction period is shortened so that the low-speed impact behavior turns to emission from bouncing, whereas it is still easy to partially rebound after a high-speed impact. In contrast, for AOT, the liquid droplets can deposit on the hydrophobic surface at a low concentration of 0.1% and at any superhydrophobic surfaces with a concentration of 0.3% (movie S5).
A diagram is shown in Fig. 3 to explain how the receding splash can be substantially depressed by AOT. Driven by the inertia, the impacting liquid first spreads to a maximum diameter (27). As shown in the spreading state of the diagram, the liquid droplet experiences a large surface deformation during the high-speed impact, and the curved edge of the spreading drop is completely out of equilibrium when it reaches its maximum diameter. Then, surface tension acts on the liquid to retract the flow above the substrate. For water, the drop would break up into multiple droplets during the receding state and would splash in the final state (Fig. 1E). For the surfactant drops, if the surfactant molecules cannot replenish the newly created air/water interface in time, typically with high DST, then the surface tension of the deformed drop could not be uniform, where nonuniform receding behavior occurs (Fig. 3B). Examples can be found for the SDS drop (Fig. 1F), the TS drop (Fig. 1G), and the AOT drop in the micelle region (fig. S4). In contrast, if the surfactant with a low beginning DST can effectively saturate the newly created surface within ~1.8 ms (corresponding to the spreading time) and maintain the homogeneous low surface tension at the air/liquid/solid interface, then the liquid can uniformly deposit on the superhydrophobic surface (Fig. 3C). As shown in Fig. 1H and fig. S4, a gentle and uniform receding contact line will be obtained similar to AOT in the vesicle region. These results provide direct evidence for the role of AOT in controlling the receding splash.
Fig. 3. Schematic illustration for splash inhibition on the superhydrophobic surface by surfactant additives.
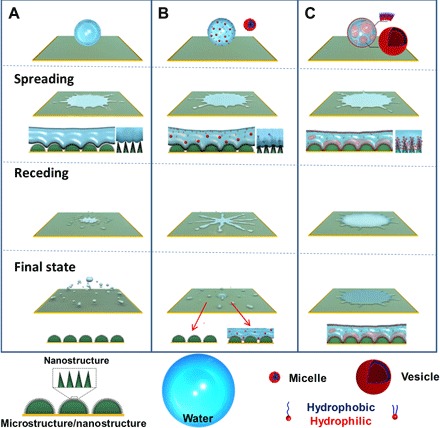
(A) The impacting water drop mainly breaks up in the receding stage after spreading to the maximum lamellar liquid. The upward increased capillary force induced by the squeezed air entrapment in the nanostructure easily makes the water drop take off the surface. (B) For the surfactants in the micelle region, the surfactant molecules cannot replenish the newly created air/water interface (high DST), and the surface tension of the deformed drop is not uniform, explaining the nonuniform receding behavior. The nonuniform surface tension leads to partial wettability transition, and several scattered fragments stick on the substrate as SDS, TSs, and AOT in the micelle region. The reduced surface tension makes the entry of the impacting drop in the nanostructure much easier because of the reverse of capillary force. (C) Because of the lowest DST and dense aggregates at the air/water interface, AOT in the vesicle region effectively saturates the newly created surface within a short time and maintains a homogeneous low surface tension at the air/liquid/solid interface so that it can change the surface wettability as long as the drop contacts the surface, thus leading to hardly receding behavior and a large wetting area.
The underlying mechanism for the abovementioned transient knockdown of the receding velocity at the pinning area can be ascribed to the wettability transition in the spreading phase. At the peripheral area of maximum spreading, the impacting water drop slides over air cushions that are trapped on or beneath the superhydrophobic surface, and it is difficult for the water to enter the nanostructures (Fig. 3A). The upward increased capillary force induced by the squeezed air entrapment in the nanostructures easily makes the water drop take off the surface (28). Similar behavior is observed for the micelle surfactant drops (Fig. 3B). The final “floating” state of the micelle surfactant drop, the 0.1% AOT drop, indicates that micelle surfactant drops could not properly reverse surface wettability, which can be seen from the cryo-SEM image in fig. S5. In contrast, the reduction of surface tension induced by the vesicle surfactant leads to the dropdown, reverses the capillary force, and makes an easier and deeper entry of the impacting drop in the nanostructure (the side view in spreading state in Fig. 3C). Cryo-SEM was used to prove the reverse of surface wettability during the impact, where the vesicle surfactant drop (1% AOT drop) is trapped between the gaps of nanoneedles and fully wets the nanostructured superhydrophobic surface (fig. S5). The outward hydrophobic tails of the surfactants at the air/liquid interface act as bridges to connect the drop and the nanostructure by hydrophobic force and to change the wettability of the superhydrophobic surface. Through this process, the surfactant droplets can be pinned and thus reduce the receding velocity via the wettability transition at the peripheral area of maximum spreading. As a result, the high-speed impacting AOT drops can firmly and quickly deposit on the superhydrophobic surface.
The wettability transition at the central contact point is easier than at the peripheral area because capillary forces are overcome by inertial effects (29) at a high Weber number regime (We > 200). Both the water drop and the surfactant drop tend to become convex in the nanostructure of the superhydrophobic surface because of the downward hammer pressure and the dynamic pressure (8). However, the water repellency of structure chemistry and the huge upward Laplace pressure induced by the squeezed air entrapment in the nanostructure rebound the water drop, as shown in Fig. 1E.
AOT also manifests itself in inhibiting rebound and splash on tilted superhydrophobic surfaces. Superhydrophobic surfaces with tilted angles of 30o, 60o, and 75o are used. In the experiment, the SDS shows little effect on the liquid deposition within the tested concentration region (Fig. 2A3), although it is a good choice to inhibit rebound on the hydrophobic surface, as shown in movie S5 (15, 16, 23). The TS drop shows the impact behavior from bouncing, emission, to no rebound as the concentration increases from 0.01 to 1%, but it still rebounds partially on the oblique superhydrophobic surface (Fig. 2B3). The percentage of liquid that bounces off the surface can be quantitatively measured through an analytical microbalance, and the impact processes of surfactant drops on the horizontal and tilted superhydrophobic surfaces are shown in movie S3. Only AOT can suppress the rebound of aqueous droplets on both horizontal and oblique surfaces at a low mass fraction of 0.3% at any inclined angles (Fig. 2C3).
Figure S1 depicts the impact behaviors of aqueous drops containing the AOT additive in three regions. At concentrations lower than the critical micelle concentration, complete bouncing occurs along with complete receding behavior. At the micelle region, partial receding of the contact line is accompanied with partial splashing, partial rebound, and no rebound. Scarce receding of the contact line takes place only at the vesicle region, indicating that both the rebound and receding splashes have been greatly inhibited.
CONCLUSION
In conclusion, although we have mainly focused on a specific microstructured/nanostructured superhydrophobic surface with varying tilted angles to elucidate the role of the vesicle surfactant (AOT) in inhibiting the receding splash, the scarce or gentle receding behavior can also be generalized to apply to other artificially fabricated superhydrophobic surfaces and other single-drop impact and spray processes at varied impact velocities (Fig. 4 and movies S6 to S9). In addition, AOT is shown to be a stable surfactant molecule (fig. S6). This work helps advance our understanding of how to control liquid deposition on superhydrophobic surfaces. Therefore, this approach can potentially be used to improve the efficiency of pesticide spraying and to reduce environmental pollution.
Fig. 4. The splash inhibition of vesicle AOT generalizes to other superhydrophobic surfaces.
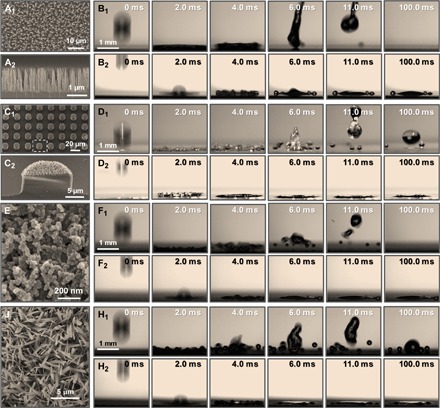
(A1 and A2) SEM images of silicon nanowire–arrayed surface from top and side views. (C1 and C2) SEM images of patterned superhydrophobic surfaces with arrayed silicon micropillars and nanowires from top and cross-sectional views. (E and G) SEM images of the microstructured/nanostructured superhydrophobic SiO2 surface and the superhydrophobic CuO surface with nanoneedles. (B1, D1, F1, and H1) The impacting water drop makes a big splash on superhydrophobic surfaces. (B2, D2, F2, and H2) The receding splash is greatly inhibited by 1% AOT on these superhydrophobic surfaces. The impact velocity of each impacting drop is 2.53 ± 0.11 m s−1.
MATERIALS AND METHODS
Superhydrophobic silicon nanowire structures
A silicon wafer was cleaned with acetone, ethanol, and deionized water before it was immersed in 5 weight % hydrofluoric acid solution for 1 min to remove the oxidation layer. Then, it was put in a mixed solution of 4.8 M hydrofluoric acid and 0.5 mM silver nitrate for 1 min to deposit silver seed on the substrate. It was successively immersed in a solution of 4.8 M hydrofluoric acid and 0.15 M hydrogen peroxide for 30 min to realize metal-assisted etching of silicon, which resulted in the acquisition of silicon nanowire structures. The typical length of the nanowire was ~1.2 μm, and the space between nanowires was about 50 nm. The as-prepared silicon plate was O2 plasma–treated at 150 W for 30 s and then put in a sealed container together with a piece of glass coated with 0.5 ml of (heptadecafluoro-1,1,2,2-tetradecyl)trimethoxysilane. The container was evacuated with a vacuum pump. After 3 hours at 80°C, the plate showed surface superhydrophobicity with a contact angle of 154.5 ± 3.2°.
Microstructured/nanostructured SiO2 surface
The commercial glass plates were cleaned with acetone, ethanol, and deionized water. In accordance with our previous research (30), the polymer-particle dispensed solution was prepared by adding 1 ml of Capstone ST-200 (DuPont Co.) solution and 1 g of hydrophobic fumed silica nanoparticles (average particle size of 14 nm; Evonik Degussa Co.) in 5 ml of acetone and 20 ml of ethanol. The solution was mixed and stirred for 30 min in a closed bottle. Precleaned glass plates were dipped in this solution at a speed of 80 mm s−1 and pulled out from the solution at a speed of 100 mm s−1. Owing to the rapid evaporation of the solvent, the semitransparent membrane quickly transformed into a white coating with extremely high water repellency. From an SEM observation, the aggregate of SiO2 nanoparticles had random features of typical size and spacing of around 200 nm.
Superhydrophobic CuO nanosheets
The copper plate was first cleaned with acetone, ethanol, and purified water before modification. It was then immersed in a solution of 0.15 M ammonium persulfate and 2.5 M sodium hydroxide for 20 min and subsequently immersed in a 0.1 M perfluorodecanoic acid solution for 1 hour. The prepared CuO nanosheets were about 3 to 6 μm in length and 200 to 600 nm in width. After the copper plate was rinsed with distilled water and dried with N2, it showed a high water repellency with a contact angle of 159.1 ± 1.7°.
Patterned pillar-structured silicon substrate
Silicon wafers (n-type doped with phosphorus, <100>-oriented, 525 μm thick) were patterned using standard photolithography techniques. A thin layer of positive resist was spray-coated onto the silicon wafer at a rotational speed of 3000 rpm, which was followed by an ultraviolet (UV) exposure process (Karl Suss MA6). Then, the UV-exposed Si wafer was immersed in the resist developer to remove the unexposed photoresist. Subsequently, deep reactive ion etching was performed. Micropillar has a diameter of 10 μm, a space of 10 μm, and a height of 20 μm. After the substrates were resist-stripped (Microposit Remover 1165), they were cleaned with ethanol and acetone before the chemical modification process was performed. The as-prepared silicon plate was O2 plasma–treated and then put in a sealed container together with a piece of glass coated with 0.5 ml of (heptadecafluoro-1,1,2,2-tetradecyl)trimethoxysilane for 2 hours at 80°C.
Characterization
Analysis of the droplet deposition on the B. oleracea L. leaf surface and superhydrophobic substrates was recorded with i-SPEED 3 (Olympus) high-speed cameras from the oblique view and a FASTCAM Mini UX100 (Photron) from the side view, respectively. SEM images were obtained using a field-emission SEM at 10 kV (Hitachi S-4800). The images of cryogenic electron microscopy were carried out using a field-emission SEM (Hitachi S-4300) equipped with extra low–temperature equipment at 3 kV (cryo-electron microscope, Leica). Cryo-TEM images were obtained with FEI Tecnai Spirit BioTwin TEMs. Cryo-transfer holders were used to ensure low-temperature transfer and observation of frozen hydrated specimens. Contact angles were measured using a contact angle measurement device (OCA 20, DataPhysics), with droplets of 3 μl to be removed dynamically. Each reported contact angle was an average of at least five independent measurements. The diffusion rates were determined with a Bruker AVANCE 600 NMR spectrometer. The DSTs were carried out with an automatic maximum bubble pressure tensiometer (Krüss BP100), which measures the behavior of a surfactant over a wide speed range as part of a single, fully automatic measuring process and determines surface tension as a function of surface age. The measured range in the time window is from 10 ms to 10 s. The capillary diameter is 0.210 mm.
Supplementary Material
Acknowledgments
The high-speed imaging, artificial superhydrophobic surface fabrication, and most of the work were performed in the Key Laboratory of Bio-inspired Materials and Interfacial Science, Technical Institute of Physics and Chemistry. The DSTs of surfactant drop, 1H NMR, and SEM (cryo-SEM) measurements were acquired in the Key Laboratory of Colloid and Interface Science, Institute of Chemistry. The B. oleracea L. leaf was provided by Henan Agricultural University. Cryo-TEM was performed in Tsinghua University. Funding: This work was financially funded by the National Research Fund for Fundamental Key Projects (grant 2014CB93220), the National Natural Science Foundation of China (NSFC) (grants 21121001 and 91127025), and the Key Research Program of the Chinese Academy of Sciences (grant KJZD-EW-M01). This work was also supported by NSFC (grants 51473172, 51473173, 51173190, and 21121001) and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (grant XDA09020000). M.S. was funded by the Fundamental Key Projects, 2014CB93220, in 2014. Author contributions: L.J. and Y.W. conceived and designed the experiments. M.S. and Z.D. performed the experiments. Z.D. fabricated artificial superhydrophobic surfaces. M.S., Y.W., and Z.D. analyzed the data. M.S. and Z.D. wrote the original manuscript, and L.J., Z.D., and Y.W. revised it. All authors discussed the results and commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/3/e1602188/DC1
fig. S1. Scarce receding of drop-substrate contact line for the AOT drop.
fig. S2. Diffusion coefficient (diffusion speed) of SDS, TSs, and AOT.
fig. S3. The dynamic spread (wetting) state of contact angle as a function of time.
fig. S4. Different liquid impact behaviors in the micelle and vesicle regions.
fig. S5. Cryo-SEM of the final wetting state of micelle and vesicle surfactant drops.
fig. S6. NMR characterization of the stability of the AOT surfactant over time.
movie S1. Surfactant drop impact on the B. oleracea L. leaf.
movie S2. Influence from surface morphology and impact speed.
movie S3. Surfactant drop impact on microstructured/nanostructured superhydrophobic surface with different types, with varied concentrations, and at different tilted angles.
movie S4. Controlling liquid splash on superhydrophobic surfaces by a vesicle surfactant: The comparison of the water drop’s splashing and the 1% AOT drop’s deposition on superhydrophobic surfaces with a long recording time.
movie S5. Surfactant drops’ impact on hydrophobic surfaces, microstructured/nanostructured superhydrophobic surfaces, and nanostructured superhydrophobic surfaces at low and high speeds.
movie S6. Water spray impact on horizontal superhydrophobic surface.
movie S7. Water spray impact on tilted superhydrophobic surface.
movie S8. AOT (1%) spray impact on horizontal superhydrophobic surface.
movie S9. AOT (1%) spray impact on tilted superhydrophobic surface.
REFERENCES AND NOTES
- 1.Bergeron V., Bonn D., Martin J. Y., Vovelle L., Controlling droplet deposition with polymer additives. Nature 405, 772–775 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Galliker P., Schneider J., Eghlidi H., Kress S., Sandoghdar V., Poulikakos D., Direct printing of nanostructures by electrostatic autofocussing of ink nanodroplets. Nat. Commun. 3, 890 (2012). [DOI] [PubMed] [Google Scholar]
- 3.de Ruiter J., Lagraauw R., van den Ende D., Mugele F., Wettability-independent bouncing on flat surfaces mediated by thin air films. Nat. Phys. 11, 48–53 (2014). [Google Scholar]
- 4.Boukhalfa H. H., Massinon M., Belhamra M., Lebeau F., Contribution of spray droplet pinning fragmentation to canopy retention. Crop Prot. 56, 91–97 (2014). [Google Scholar]
- 5.Liu Y. H., Moevius L., Xu X., Qian T., Yeomans J. M., Wang Z., Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 10, 515–519 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird J. C., Dhiman R., Kwon H.-M., Varanasi K. K., Reducing the contact time of a bouncing drop. Nature 503, 385–388 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Xiao Z., Chan P. C. H., Lee Y.-K., Li Z., A comparative study of droplet impact dynamics on a dual-scaled superhydrophobic surface and lotus leaf. Appl. Surf. Sci. 257, 8857–8863 (2011). [Google Scholar]
- 8.Lu Y., Sathasivam S., Song J., Crick C. R., Carmalt C. J., Parkin I. P., Robust self-cleaning surfaces that function when exposed to either air or oil. Science 347, 1132–1135 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kwon H.-M., Paxson A.-T., Varanasi K.-K., Patankar N. A., Rapid deceleration-driven wetting transition during pendant drop deposition on superhydrophobic surfaces. Phys. Rev. Lett. 106, 036102 (2011). [DOI] [PubMed] [Google Scholar]
- 10.McCarthy M., Gerasopoulos K., Enright R., Culver J. N., Ghodssi R., Wang E. N., Biotemplated hierarchical surfaces and the role of dual length scales on the repellency of impacting droplets. Appl. Phys. Lett. 100, 263701 (2012). [Google Scholar]
- 11.Bartolo D., Josserand C., Bonn D., Retraction dynamics of aqueous drops upon impact on non-wetting surfaces. J. Fluid Mech. 545, 329–338 (2005). [Google Scholar]
- 12.Liu T., Kim C.-J., Turning a surface superrepellent even to completely wetting liquids. Science 346, 1096–1100 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Marengo M., Antonini C., Roisman I. V., Tropea C., Drop collisions with simple and complex surfaces. Curr. Opin. Colloid Interface Sci. 16, 292–302 (2011). [Google Scholar]
- 14.Tsai P., Hendrix M. H. W., Dijkstra R. R. M., Shui L., Lohse D., Microscopic structure influencing macroscopic splash at high weber number. Soft Matter 7, 11325–11333 (2011). [Google Scholar]
- 15.Kim H., Park U., Lee C., Kim H., Hwan M. K., Kim J., Drop splashing on a rough surface: How surface morphology affects splashing threshold. Appl. Phys. Lett. 104, 161608 (2014). [Google Scholar]
- 16.Hao P., Lv C., Niu F., Yu Y., Water droplet impact on superhydrophobic surfaces with microstructures and hierarchical roughness. Sci. China Phys. Mech. Astron. 57, 1376–1381 (2014). [Google Scholar]
- 17.Gauthier A., Symon S., Clanet C., Quéré D., Water impacting on superhydrophobic macrotextures. Nat. Commun. 6, 8001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard D., Clanet C., Quéré D., Surface phenomena: Contact time of a bouncing drop. Nature 417, 811 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Damak M., Mahmoudi S. R., Hyder M. N., Varanasi K. K., Enhancing droplet deposition through in-situ precipitation. Nat. Commun. 7, 12560 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon S. S., Jepsen R. A., Nissen M. R., O’Hern T. J., Experimental investigation on splashing and nonlinear fingerlike instability of large water drops. J. Fluid Struct. 23, 101–115 (2007). [Google Scholar]
- 21.Xu L., Zhang W. W., Nagel S. R., Drop splashing on a dry smooth surface. Phys. Rev. Lett. 94, 184505 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Castrejón-Pita A. A., Castrejón-Pita J. R., Hutchings I. M., Breakup of liquid filaments. Phys. Rev. Lett. 108, 074506 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Huh H. K., Jung S., Seo K. W., Lee S. J., Role of polymer concentration and molecular weight on the rebounding behaviors of polymer solution droplet impacting on hydrophobic surfaces. Microfluid. Nanofluid. 18, 1221–1232 (2014). [Google Scholar]
- 24.Duez C., Ybert C., Clanet C., Bocquet L., Making a splash with water repellency. Nat. Phys. 3, 180–183 (2007). [Google Scholar]
- 25.Hao C., Zhou Y., Zhou X., Che L., Chu B., Wang Z., Dynamic control of droplet jumping by tailoring nanoparticle concentrations. Appl. Phys. Lett. 109, 021601 (2016). [Google Scholar]
- 26.Fan Y., Cao M., Yuan G., Wang Y., Yan H., Han C. C., Aggregation behavior in mixed system of double-chained anionic surfactant with single-chained nonionic surfactant in aqueous solution. J. Colloid Interface Sci. 299, 928–937 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Reyssat M., Richard D., Clanet C., Quéré D., Dynamical superhydrophobicity. Faraday Discuss. 146, 19–33 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Wang Q. B., Yao X., Liu H., Quéré D., Jiang L., Self-removal of condensed water on the legs of water striders. Proc. Natl. Acad. Sci. U.S.A. 112, 9247–9252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonini C., Amirfazli A., Marengo M., Drop impact and wettability: From hydrophilic to superhydrophobic surfaces. Phys. Fluids 24, 102104 (2012). [Google Scholar]
- 30.Dong Z. C., Ma J., Jiang L., Manipulating and dispensing micro/nanoliter droplets by superhydrophobic needle nozzles. ACS Nano 7, 10371–10379 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/3/e1602188/DC1
fig. S1. Scarce receding of drop-substrate contact line for the AOT drop.
fig. S2. Diffusion coefficient (diffusion speed) of SDS, TSs, and AOT.
fig. S3. The dynamic spread (wetting) state of contact angle as a function of time.
fig. S4. Different liquid impact behaviors in the micelle and vesicle regions.
fig. S5. Cryo-SEM of the final wetting state of micelle and vesicle surfactant drops.
fig. S6. NMR characterization of the stability of the AOT surfactant over time.
movie S1. Surfactant drop impact on the B. oleracea L. leaf.
movie S2. Influence from surface morphology and impact speed.
movie S3. Surfactant drop impact on microstructured/nanostructured superhydrophobic surface with different types, with varied concentrations, and at different tilted angles.
movie S4. Controlling liquid splash on superhydrophobic surfaces by a vesicle surfactant: The comparison of the water drop’s splashing and the 1% AOT drop’s deposition on superhydrophobic surfaces with a long recording time.
movie S5. Surfactant drops’ impact on hydrophobic surfaces, microstructured/nanostructured superhydrophobic surfaces, and nanostructured superhydrophobic surfaces at low and high speeds.
movie S6. Water spray impact on horizontal superhydrophobic surface.
movie S7. Water spray impact on tilted superhydrophobic surface.
movie S8. AOT (1%) spray impact on horizontal superhydrophobic surface.
movie S9. AOT (1%) spray impact on tilted superhydrophobic surface.


