Abstract
Intrahepatic cholangiocarcinoma (ICC) comprises approximately 5−30% of primary liver tumors, however it has been increasing over the last several decades. Up to and including the 6th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) edition staging system, ICC was staged the same as hepatocellular carcinoma. In the 7th edition AJCC/UICC manual, the staging system of ICC was revised such that a distinct classification was proposed. Pathologic features for prognosis included vascular invasion, tumor multiplicity, local extension, periductal infiltration and lymph nodal metastasis. Over the last decade, as the incidence of ICC has increased and surgery for this indication has become more common, more data has been published on the prognostic factors associated with long-term survival.
Keywords: Intrahepatic cholangiocarcinoma (ICC), staging, prognostic factors
Introduction
Most malignant tumors identified within the liver are metastatic lesions, however a subset of patients will present with primary liver cancers. Among primary tumors, the incidence of intrahepatic cholangiocarcinoma (ICC) has risen over the last several decades and currently accounts for approximately 5−30% of primary hepatic malignancies (1-3). Concurrent with an increasing incidence, there has been an expanding number of investigative efforts to identify determinants of prognosis, and therefore, more precise staging for ICC (4). Accurate cancer staging serves the purpose of detailing prognostic information, appropriately risk stratifying patients, as well as informing the use of adjuvant therapeutic options (5). Therefore, it is important to accurately identify factors that may influence prognosis and treatment of patients with ICC.
Previously, ICC was staged in an identical manner to hepatocellular carcinoma (HCC). ICC arises, however, from malignant transformation of cholangiocytes (second-order and more peripheral branches) or from progenitor cells, and possesses distinct biologic and prognostic characteristics compared with HCC (6,7). Therefore, in the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging manual, ICC was designated a separate staging system from HCC.
Previously proposed Japanese staging schemes for ICC
Individual staging systems for ICC had previously been proposed by the National Cancer Center of Japan (NCCJ) staging system (Okabayashi) and the Liver Cancer Study Group of Japan (LCSGJ), however these staging classifications were never validated or widely used in Western countries (8-10). When comparing the LCSGJ and NCCJ staging systems with the AJCC/UICC TNM staging scheme, the characterization of regional lymph node involvement (N1 vs. N0) and distant metastases (M1 vs. M0) is largely similar, whereas the T-stage classification varies considerably when comparing the three systems (Tables 1,2). Specifically, the LCSGJ staging system stratifies patients based on tumor size >2 cm, number of tumors and presence of vascular or serosal invasion (9,11). Additionally, the staging system highlights three different morphological subtypes of ICC including the mass-forming, periductal-infiltrating and intraductal-growth type (5,9). In contrast, the AJCC/UICC includes the mass-forming and periductal-infiltrating sub-types as well as the mixed-type, but does not include the intraductal-growth sub-type (8). Similar to the Okabayashi staging system, the AJCC/UICC determines ICC T-stage based on tumor number and presence of vascular invasion, but not on tumor size (5).
Table 1. Comparison of the TNM for intrahepatic cholangiocarcinoma (5).
| Stage classification | AJCC/UICC | Liver cancer study group of Japan | National cancer center in Japan | |||||
|---|---|---|---|---|---|---|---|---|
| Primary tumor (T) | ||||||||
| TX | Primary tumor cannot be assessed | 1 | Tumor size ≤2 cm | T1 | Solitary tumor without vascular invasion | |||
| T0 | No evidence of primary tumor | 2 | Tumor number =1 | |||||
| Tis | Carcinoma in situ (intraductal tumor) | 3 | No portal vein/hepatic vein or serosal | T2 | Solitary tumor with vascular invasion | |||
| T1 | Solitary tumor without vascular invasion | − | ||||||
| T2a | Solitary tumor with vascular invasion | − | − | − | T3 | Multiple tumors with or without vascular invasion | ||
| T2b | Multiple tumors, with or without vascular invasion | T1 | All three criteria | |||||
| T3 | Tumors perforating the visceral peritoneum or involving local hepatic structures by direct invasion | T2 | Two of three criteria | − | − | − | − | |
| T3 | One of three criteria | − | − | − | − | |||
| T4 | Tumor with periductal invasion | T4 | None of three criteria | − | − | − | − | |
| Regional lymph nodes (N) | ||||||||
| Nx | Regional lymph nodes cannot be assessed | N0 | No regional lymph node | N0 | No regional lymph node | |||
| N0 | No regional lymph node metastases | N1 | Regional lymph node metastases present | N1 | Regional lymph node metastases present | |||
| N1 | Regional lymph node metastases present | |||||||
| Distant metastases (M) | ||||||||
| M0 | No distant metastases | M0 | No distant metastases | M0 | No distant metastases | |||
| M1 | Distant metastases present | M1 | Distant metastases | M1 | Distant metastases | |||
AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control.
Table 2. Comparison of staging systems for intrahepatic cholangiocarcinoma (5).
| Stage classification | AJCC/UICC | Liver cancer study group of Japan | National cancer center in Japan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 0 | Tis | N0 | M0 | − | − | − | − | − | − |
| Stage I | T1 | N0 | M0 | T1 | N0 | M0 | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 | T2 | N0 | M0 | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 | T3 | N0 | M0 | − | − | − |
| Stage IVA | T4 | N0 | M0 | T4 | N0 | M0 | − | − | − |
| Any T | N1 | M0 | Any T | N1 | M0 | − | − | − | |
| Stage IVB | Any T | Any N | M1 | Any T | Any N | M1 | − | − | − |
| Stage IIIA | − | − | − | − | − | − | T3 | N0 | M0 |
| Stage IIIB | − | − | − | − | − | − | Any T | N1 | M0 |
| Stage IV | − | − | − | − | − | − | Any T | Any N | M1 |
AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control.
In addition to not being well-validated in Western patients, the NCCJ and LCSGJ ICC staging systems have been criticized for a number of other reasons (5). For instance, the Okabayashi ICC staging system was based only on a small population of patients (n=60), all of whom had the mass-forming subtype of ICC and one-third of whom had hepatitis B or C infection (2). In turn, the Okabayshi staging scheme is limited due to its potential lack of generalizability, as well as studies that have suggested a limited prognostic value and applicability of this staging system (12). The proposed LCSGJ staging scheme similarly has exhibited poor correlation among the varied T-stages and survival (Figure 1) (2,5). In particular, the differences in survival among the LCSGJ stages I-III were not significantly different (2,5). Given the shortcomings of the NCCJ and LCSGJ ICC staging systems, as well as the historical lack of formal ICC staging system, the AJCC/UICC proposed a new staging scheme in the 7th edition of the staging manual.
Figure 1.
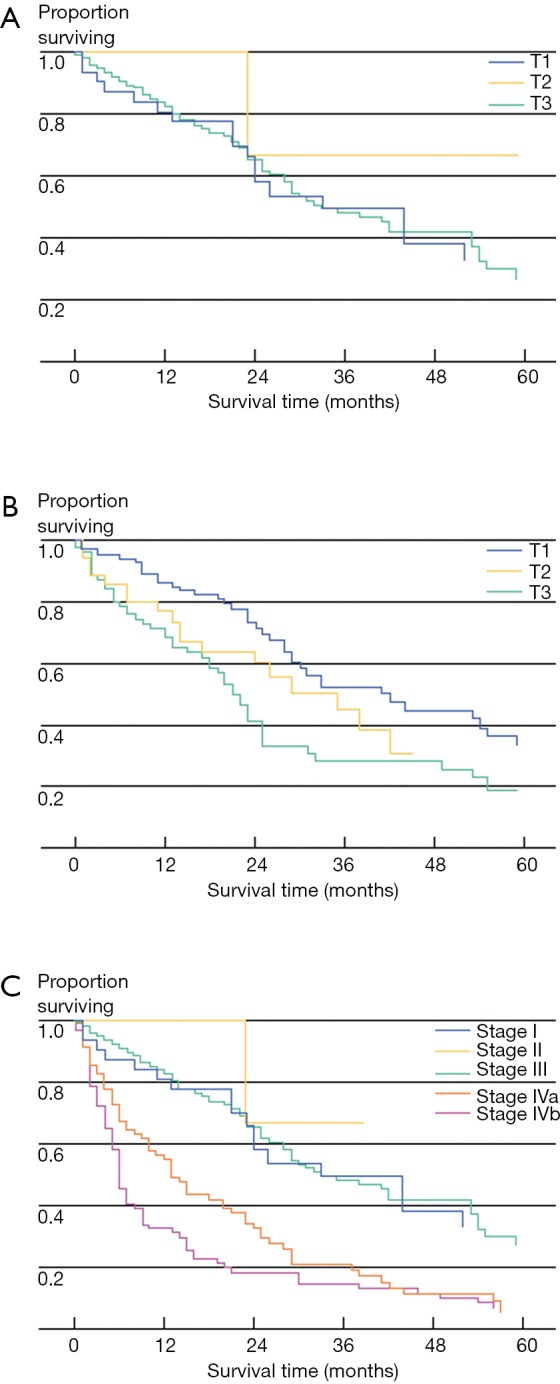
Kaplan-Meier survival curves for patients with intrahepatic cholangiocarcinoma. Patients without nodal or distant disease (N0M0) were stratified according to T-classification using the LCSGJ system (A) and Okabayashi system (B). Additionally, for all patients including those with nodal metastases and distant disease, the LCSGJ staging system (C) is shown. [Used with permission by Nathan et al. A Proposed Staging System for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2009) 16:14-22].
American Joint Committee on Cancer 7th Edition
The 7th edition of the AJCC staging manual transitioned away from the unspecified joint staging system for intrahepatic bile duct tumors and liver tumors that had been present in the previous six editions (8). Contributing to the identification of a need for a new staging system, Nathan et al. investigated predictors of survival among 598 patients undergoing surgery for ICC (12). In this study, the authors demonstrated that the existing 6th edition AJCC/UICC staging system failed to stratify patients. In particular, there was no prognostic discrimination among the T2 and T3 categories. Rather, important determinants of outcome included tumor number and presence of vascular invasion, but not tumor size. The French Association of Surgery (AFC) subsequently validated the accuracy of the simplified staging system proposed by Nathan et al. (8). In this study, Farges et al. reported that the staging system proposed by Nathan et al. achieved a more uniform distribution of patients among the varied T-stages than the LCSGJ, Okabayashi or AJCC/UICC 6th edition classification systems (8). The authors noted that the system proposed by Nathan et al. was the only staging system among the four examined that accurately stratified patients by TNM stage. In turn, these data were incorporated into the 7th edition of the AJCC/UICC staging and have been subsequently validated by other authors (4,8).
T–classification
Pathologic variables included in the 7th edition AJCC/UICC T-classification system include a solitary tumor without vascular invasion (T1) or with vascular invasion (T2a), multiple tumors (T2b), tumor penetrating the visceral peritoneum or directly invading adjacent structures (T3), and a periductal infiltrating subtype (T4). In the AJCC/UICC 6th edition manual, which staged HCC and ICC using a unified classification scheme, tumor size (5 cm) was used to differentiate T2 versus T3 disease (1). However, data from the initial study by Nathan and colleagues did not note an association between tumor size and prognosis (12). Consequently, tumor size was omitted as a prognostic factor in the 7th edition ICC staging system (1,12). Other investigations have corroborated the lack of prognostic value of tumor size for ICC (10,13-20) For instance, Lang and colleagues reported on 83 patients undergoing hepatic resection for ICC (14). Whereas gender, disease stage I−II versus III−IV, and negative resection margins (R0) were predictive of survival, tumor size using a 5 cm cut-off value was not associated with long-term outcomes. Similarly, Uenishi et al. reported on 133 patients undergoing hepatic resection for ICC and noted that tumor size did not impact prognosis (13). In this study, the authors reported that only multiple tumors (HR =2.7, P<0.001), lymph node metastasis (HR =2.4, P<0.001) and positive surgical margins (HR =2.0, P=0.009) were associated with poor prognosis. In a separate study, Ribero et al. reported on a multi-institutional study of patients with ICC who had undergone curative intent hepatic resection (16). Elevated CA 19-9 (HR =1.6, P=0.006), multiple tumors (HR =1.5, P=0.009) and lymph node metastasis (HR =2.2, P<0.001) were independently associated with worse outcome, however tumor size was not. Interestingly, while tumor size was associated with a higher risk of lymph node metastasis, multiple tumors, and vascular invasion, tumor size itself was not independently associated with long-term survival on multivariable analysis.
More recently, several other authors have suggested that tumor size may indeed be prognostically important (3,21-24). For example, in a systematic review of ICC that examined 57 studies including 4,756 patients, Mavros et al. reported that large tumor size was associated with a worse long-term outcome (3). Of note, while statistically significant, the hazard of death associated with incremental increases in tumor size were somewhat modest (HR =1.1, P<0.001). In a separate study, Hyder et al. examined over 500 patients who underwent resection of ICC and proposed a nomogram to predict long-term survival after curative intent surgical resection (23). Certain factors included in the 7th edition AJCC/UICC staging system were associated with worse overall survival (OS) including multiple tumors (HR =1.58, P<0.001), lymph node metastasis (HR =1.78, P=0.01), and vascular invasion (HR =2.1, P<0.001). In addition, tumor size was noted to be associated with increased risk of death (HR =1.5, P<0.001). Interestingly, the authors noted that tumor size up to 7 cm was associated with progressively worse survival after which long-term outcomes tended to plateau. The nomogram had good predictive accuracy (c-statistic =0.71) and seemed to perform better than the 7th edition AJCC/UICC staging system (c-statistic =0.59). In a study examining 367 ICC patients from China, Wang and colleagues developed a separate predictive nomogram (25). Similar to the one proposed by Hyder et al. determinants of survival included tumor number (2−3 nodules: HR =1.6, P=0.045; >3 nodule: HR =6.1, P<0.001), lymph node metastases (HR =2.1, P<0.001), vascular invasion (HR =1.6, P=0.005), as well as tumor diameter (HR =1.1, P<0.001). In a validation cohort of 82 patients, the nomogram demonstrated reliable capacity to predict 3-year survival (c-statistic=0.75). In a study that examined a mixed-cure model of 584 patients from multiple institutions undergoing hepatic resection for ICC, Spolverato et al. similarly noted that vascular invasion, multifocal disease, lymph node metastasis, periductal invasion, poor-grade ICC, and tumor size were all prognostic of long-term outcomes (6).
In the AJCC/UICC 7th edition staging system, tumor multiplicity is another important factor associated with prognosis. Specifically, tumor number (single vs. multiple) with or without vascular invasion designate the T2-classification of ICC (T2a vs. T2b). Tumor number and vascular invasion have been well-established adverse factors for survival following resection of ICC (5,6,16,23,25-27). For example, Weber et al. noted that vascular invasion was the factor most strongly associated with worse overall survival (28). Similarly, Guglielmi and colleagues identified vascular invasion (HR =4.11, P=0.01) as one of the most important determinants of outcome following liver resection for ICC (29). In addition, Igami and colleagues reported that tumor multiplicity was one of the most robust prognostic factors among patients with ICC after curative-intent hepatectomy (19). In the Hyder et al. nomogram, tumor number (HR =1.58, P<0.001) and vascular invasion (HR =2.1, P<0.001) were both notable risk factors of poor outcome following curative intent resection of ICC (23). Similarly, the Wang et al. nomogram also identified tumor number and vascular invasion as important in determining survival (25). In fact, the authors noted that increasing tumor number was strongly associated with long-term outcomes (2−3 nodules: HR =1.6, P=0.045; >3 nodule: HR =6.1, P<0.001) (25).
Spolverato and colleagues specifically examined the outcome of patients with ICC who had large (>7 cm) or multifocal tumors following liver resection (22). Patients with unifocal and smaller (<7 cm) tumors had an improved median disease free-survival and overall survival compared with patients who had larger tumors or multifocal disease (disease-free survival: 14.1 vs. 9.5 months, P<0.001; overall survival: 32.0 vs. 21.1 months, P=0.003). Of note, the incidence of R0 resection was no different among patients with large or multifocal tumors versus those with smaller, unifocal ICC (86.5% vs. 81.1%: P=0.1). Patients with >3 tumor nodules (HR =1.56, P=0.02), nodal metastasis (HR =1.47, P=0.02) and poor tumor differentiation (HR =1.48, P=0.01) had the worse prognosis after hepatic resection. In fact, patients with concurrent multifocal tumors and lymph node metastases had a 5-year survival of only 3.2%, which was worse than patients with either risk factor alone (12.8%) or patients with unifocal disease and no lymph node metastasis (28.8%). Sakamoto and colleagues also have reported that tumor multiplicity was a poor prognostic feature, even among patients with lymph node metastasis (11). In a study of 419 patients undergoing hepatic resection for ICC, tumor number, lymph node metastasis and distant metastasis were all parameters associated with long-term survival. Interestingly, vascular invasion impacted overall survival among the 267 patients without nodal disease (N0M0), but lost its prognostic significance among patients who had nodal metastasis. de Jong and colleagues similarly reported that vascular invasion was associated with prognosis among patients with N0 nodal status (HR =2.11, P=0.003), but not patients with N1 status (HR =1.22, P=0.75) (18).
Visceral peritoneal exposure or invasion into adjacent organs (T3) has also been identified as a prognostic factor among patients with ICC. In fact, in the AFC-IHCC-2009 Study Group investigation, patients who had local extrahepatic spread demonstrated particularly poor outcomes (8). Tumor extension into adjacent organs was associated with survival equivalent to patients who had metastatic disease. In a single institution study of 370 patients, Zhou and colleagues noted that local extrahepatic extension was associated with survival even after controlling for competing risk factors on multivariable analysis (HR =1.5, P=0.008) (30). In the nomogram by Wang and colleagues direct invasion or local extrahepatic spread was also noted to be predictive of survival (HR =1.6, P=0.025) (25).
The AJCC/UICC 7th edition staging includes periductal growth pattern and categorizes patients with this risk factor as T4 disease (and therefore stage IVA). Shimada and colleagues noted that the periductal growth type of ICC was more commonly associated with vascular invasion and lymph node metastases compared with the mass-forming type ICC, indicating periductal tumors may be more often discovered at an advanced stage (27). A recent investigation by Sakamoto and colleagues of 756 patients undergoing surgical resection for confirmed mass-forming or periductal-infiltrating ICC similarly noted that presence of periductal invasion was associated with a worse long-term outcome (11). In this study, major biliary invasion (defined as invasion of a first-order biliary branch) was correlated with periductal invasion (defined as tumor that extends mainly longitudinally along the bile duct) (9). Biliary invasion was strongly associated with prognosis among N0M0 patients (HR =2.9, P=0.001). However, as the authors pointed out, patients with multiple tumors (T2b) consistently demonstrate a worse outcome than patients with periductal invasion (T4). As such, these data, as well as data from other studies, have questioned the impact of periductal infiltration on survival (15,19). Igami et al. recently sought to examine the clinical utility of the AJCC/UICC 7th edition (19). When evaluating the T-stage categories, the authors noted that periductal invasion (T4 category) did not impact survival. In fact, patients with periductal invasion had a 5-year survival of 50% vs. 30% among patients without periductal invasion (P=0.676).
N-classification
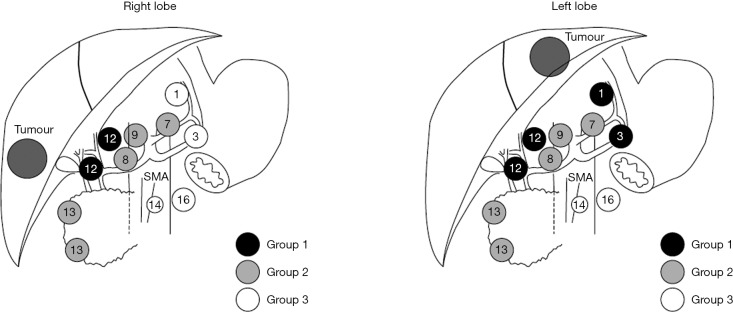
Regional lymph node metastasis is designated as N1 disease and includes involvement of hilar (hepatoduodenal), periduodenal and peripancreatic nodes (1). Currently, the National Comprehensive Cancer Network guidelines for ICC suggest lymph node dissection should be considered at time of hepatectomy, but lymphadenectomy is not a formal recommendation (31). A multi-center analysis demonstrated that only approximately 55% of patients in Western institutions undergo lymph node dissection (18,32). In comparison, lymph node dissection is often a standard component to hepatectomy for ICC in Japanese centers (18). Whether to evaluate the nodal basin is an important topic as lymph node metastases can be present in up to 25−50% of patients who undergo hepatic resection for ICC (33,34). Among patients with ICC who have regional nodal disease, the hepatoduodenal lymph nodes are most likely to be involved. As such, the LCSGJ recommends regional lymph node dissection of groups 1 and 2 lymph node basins, which depending on whether the ICC tumor is located on the right or left side of the liver (Figure 2) (35). Many investigators have advocated for routine lymphadenectomy at the time of hepatic resection for ICC given the prognostic importance of nodal disease status (2,32,36). In fact, lymph node metastasis is one of the strongest factors that portends a poor prognosis (6,11,13,15-19,23,25,34,37).
Figure 2.
Grouping of regional lymph nodes according to the Classification of Primary Liver Cancer by the Liver Cancer Study Group of Japan. 1, Lymph nodes in the right cardial region; 3, lymph nodes along the lesser curvature of the stomach; 7, lymph nodes along the left gastric artery; 8, lymph nodes along the common hepatic artery; 9, lymph nodes along the celiac artery; 12, lymph nodes in the hepatoduodenal ligament; 13, lymph nodes on the posterior surface of the pancreatic head; 14, lymph nodes at the root of the mesentery; 16, para-aortic lymph nodes. (Used with permission by Shimada at el. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. BJS 2001;88,1463-66).
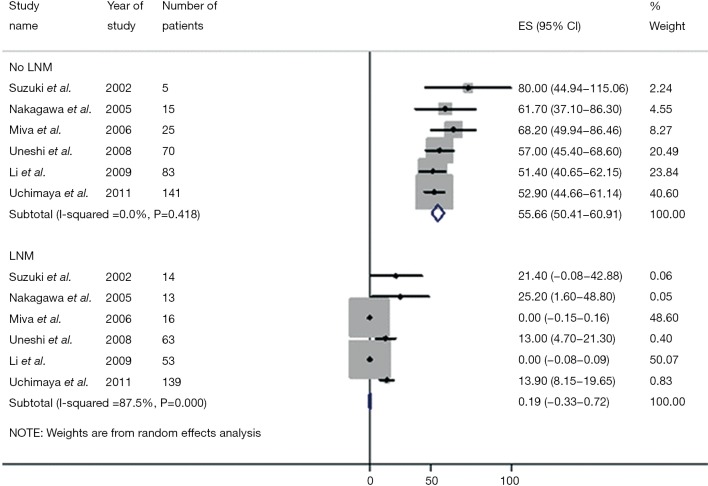
de Jong and colleagues determined the impact of lymph node status on outcome (18). In this large multi-institutional study, only approximately one-half of patients underwent a lymphadenectomy with a median nodal count of three. Among patients who underwent lymph node dissection, approximately 30% had lymph node metastasis with the median number of positive nodes being one. Of note, patients with N1 disease fared considerably worse than patients without lymph node metastases. In a separate study, Choi and colleagues similarly noted that lymph node metastasis was strongly associated with overall survival (HR =3.317, P=0.021) (15). In a study by the Japanese Society of Hepatic, Pancreatic and Biliary Surgery, 341 patients from 9 institutions were examined after hepatectomy for ICC (17). Among patients undergoing lymphadenectomy, patients who were node negative had a much better survival compared with patients who had lymph node metastasis (5-year survival: 46.4% vs. 7.0%; P<0.001)—with lymph node status being the most important factor associated with prognosis. Consistent with these data, Amini and colleagues published a systematic review that noted a significant disparity between the survival of patients who did and did not have lymph node metastases (3-year survival: 0.2% vs. 55.6%) (Figure 3) (33). The incidence of lymph node metastasis among the 1,800 patients who had a lymphadenectomy was 45%—again suggesting that many patients with ICC harbor lymph node disease and that routine lymph node dissection should be strongly considered for ICC.
Figure 3.
Meta-analysis of 3-year survival among patients with intrahepatic cholangiocarcinoma stratified by lymph node status. Patients without lymph node involvement experienced an approximate 55.7% 3-year survival compared to 0.2% 3-year survival in those possessing lymph node metastases. (Used with permission by Amini et al. Management of Lymph Nodes During Resection of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: A Systematic Review. J Gastrointest Surg 2014;18:2136-48).
Currently, there is no discrimination among tumor volume in the regional (hepatoduodenal, periduodenal and peripancreatic) lymph node basin as any metastatic disease is considered N1 classification. Guglielmi et al. reported that among patients with lymph node metastasis, there was uniform involvement of the hepatoduodenal lymph node station (34). Patients with <3 lymph node metastases had a superior overall survival compared with patients who had ≥3 lymph node metastases (52 vs. 12 months; P=0.02). Kim and colleagues similarly noted that patients with ≥3 lymph node metastases had a worse disease-specific survival compared with patients who had only one lymph node metastasis (HR =1.47, P=0.03) (38). Interestingly, among patients who had N0 disease, there was an incremental survival benefit associated with each negative node up to three lymph nodes. In a different study, Igami et al. reported that location of the metastatic lymph node also influenced overall survival (19). Specifically, survival was worse among patients with gastrohepatic lymph node metastasis compared with patients who had lymph node metastasis located in the hepatoduodenal, peripancreatic or periduodenal area. As such, both number and location of involved lymph nodes may allow further stratification of patients in the future.
M classification
Currently, nodal involvement of common hepatic, celiac, periaortic or caval lymph nodes represent M1 disease in the AJCC/UICC 7th edition staging system (1,4).
Some investigators have noted, however, that patients with lymph node metastases had an improved survival compared with patients who had M1 disease, raising the question as to whether lymph node metastases should be considered stage IV disease (26). Patients with extrahepatic disease in other locations beyond the nodal basins are also considered to have M1 disease.
Conclusions
Since the AJCC/UICC 7th edition staging for ICC was adopted, an increasing volume of literature has been published on the various prognostic factors associated with long-term survival. While many studies have validated the existing 7th edition staging system, future staging systems will need to incorporate additional modifications to help better refine the prognostic stratification of patients with ICC.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2010;26:269-73. 10.1097/MOG.0b013e328337c899 [DOI] [PubMed] [Google Scholar]
- 2.Bartella I, Dufour JF. Clinical Diagnosis and Staging of Intrahepatic Cholangiocarcinoma. J Gastrointestin Liver Dis 2015;24:481-9. [DOI] [PubMed] [Google Scholar]
- 3.Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 4.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 5.Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22. 10.1038/nrgastro.2011.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer 2015;121:3998-4006. 10.1002/cncr.29619 [DOI] [PubMed] [Google Scholar]
- 7.Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene 2013;32:4861-70. 10.1038/onc.2012.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. 10.1007/s00534-002-0732-8 [DOI] [PubMed] [Google Scholar]
- 10.Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 2001;92:2374-83. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto Y, Kokudo N1, Matsuyama Y, et aL. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer 2016;122:61-70. 10.1002/cncr.29686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:14-22. 10.1245/s10434-008-0180-z [DOI] [PubMed] [Google Scholar]
- 13.Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg 2008;15:417-22. 10.1007/s00534-007-1315-5 [DOI] [PubMed] [Google Scholar]
- 14.Lang H, Sotiropoulos GC, Sgourakis G, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg 2009;208:218-28. 10.1016/j.jamcollsurg.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 15.Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. 10.1245/s10434-009-0631-1 [DOI] [PubMed] [Google Scholar]
- 16.Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg 2012;147:1107-13. 10.1001/archsurg.2012.1962 [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:443-52. 10.1007/s00534-010-0349-2 [DOI] [PubMed] [Google Scholar]
- 18.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 19.Igami T, Ebata T, Yokoyama Y, et al. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World J Surg 2011;35:2501-9. 10.1007/s00268-011-1242-0 [DOI] [PubMed] [Google Scholar]
- 20.Yedibela S, Demir R, Zhang W, et al. Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol 2009;16:404-12. 10.1245/s10434-008-0227-1 [DOI] [PubMed] [Google Scholar]
- 21.Miwa S, Miyagawa S, Kobayashi A, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol 2006;41:893-900. 10.1007/s00535-006-1877-z [DOI] [PubMed] [Google Scholar]
- 22.Spolverato G, Kim Y, Alexandrescu S, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol 2015;22:2218-25. 10.1245/s10434-014-4223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. 10.1001/jamasurg.2013.5168 [DOI] [PubMed] [Google Scholar]
- 24.Shen WF, Zhong W, Xu F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol 2009;15:5976-82. 10.3748/wjg.15.5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka M, Ito H, Kimura F, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 2002;89:1525-31. 10.1046/j.1365-2168.2002.02268.x [DOI] [PubMed] [Google Scholar]
- 27.Shimada K, Sano T, Sakamoto Y, et al. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J Surg 2007;31:2016-22. 10.1007/s00268-007-9194-0 [DOI] [PubMed] [Google Scholar]
- 28.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. 10.1016/S1072-7515(01)01016-X [DOI] [PubMed] [Google Scholar]
- 29.Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-54. 10.1007/s00268-009-9970-0 [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Jiang X, Li Q, et al. A simple and effective prognostic staging system based on clinicopathologic features of intrahepatic cholangiocarcinoma. Am J Cancer Res 2015;5:1831-43. [PMC free article] [PubMed] [Google Scholar]
- 31.Benson AB, 3rd, D'Angelica MI, Abrams TA, et al. Hepatobiliary cancers, version 2.2014. J Natl Compr Canc Netw 2014;12:1152-82. [DOI] [PubMed] [Google Scholar]
- 32.Uenishi T, Yamamoto T, Takemura S, et al. Surgical treatment for intrahepatic cholangiocarcinoma. Clin J Gastroenterol 2014;7:87-93. 10.1007/s12328-014-0460-z [DOI] [PubMed] [Google Scholar]
- 33.Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg 2014;18:2136-48. 10.1007/s11605-014-2667-1 [DOI] [PubMed] [Google Scholar]
- 34.Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013;17:1917-28. 10.1007/s11605-013-2331-1 [DOI] [PubMed] [Google Scholar]
- 35.Shimada M, Yamashita Y, Aishima S, et al. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg 2001;88:1463-6. 10.1046/j.0007-1323.2001.01879.x [DOI] [PubMed] [Google Scholar]
- 36.Uenishi T, Yamazaki O, Yamamoto T, et al. Serosal invasion in TNM staging of mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2005;12:479-83. 10.1007/s00534-005-1026-8 [DOI] [PubMed] [Google Scholar]
- 37.Shirabe K, Mano Y, Taketomi A, et al. Clinicopathological prognostic factors after hepatectomy for patients with mass-forming type intrahepatic cholangiocarcinoma: relevance of the lymphatic invasion index. Ann Surg Oncol 2010;17:1816-22. 10.1245/s10434-010-0929-z [DOI] [PubMed] [Google Scholar]
- 38.Kim Y, Spolverato G, Amini N, et al. Surgical Management of Intrahepatic Cholangiocarcinoma: Defining an Optimal Prognostic Lymph Node Stratification Schema. Ann Surg Oncol 2015;22:2772-8. 10.1245/s10434-015-4419-1 [DOI] [PubMed] [Google Scholar]