Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy. Although surgical resection has been a cornerstone in the management of ICC, the efficacies of several non-surgical management strategies have been actively investigated and somewhat preferable outcomes have been reported for ablation therapies in selected cases. Nevertheless, because of the rarity of these tumors and the small number of studies, the clinical significance and actual role of ablation therapies in a multidisciplinary approach for ICC remain unclear. In this article, the reported outcomes of ablation therapies for ICC will be summarized and their potential indications will be discussed.
Keywords: Intrahepatic cholangiocarcinoma (ICC), radiofrequency ablation (RFA), surgery
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a relatively uncommon disease accounting for 5–30% of all primary liver malignancies (1). In the United States, however, the age-adjusted incidence of ICC has increased from 0.32 per 100,000 population in 1975 to 0.85 per 100,000 population in 2000 (2). Although surgical resection is the only curative treatment option, most patients with ICC are not candidate for surgery because most patients already have advanced disease at the time of presentation (3,4). As such, several non-surgical treatments for ICC have been investigated over the years to improve the survival outcomes of patients who are unfit for surgery. However, most previous studies regarding non-surgical treatments have failed to show satisfactory outcomes for ICC. Furthermore, tumor recurrence is common among patients with ICC, occurring in up to 70% even after curative resection (5), and the survival outcomes after recurrence are dismal. Because only a limited number of patients can proceed to repeat resection after recurrence, alternative non-surgical palliative management strategies need to be established.
Among several ablation therapies for solid tumors, radiofrequency ablation (RFA) has been increasingly used in the treatment of liver tumors, and its efficacy in hepatocellular carcinoma has been established (6-8). After an initial report of RFA for ICC (9), several case series have been reported, and a modest efficacy of RFA has been shown for unresectable ICC or recurrent ICC in selected cases (10,11). However, because of the rarity of this tumor, the clinical significance of ablation therapies for ICC remains unclear. In this article, the efficacy of ablation therapies for ICC will be reviewed and their role in multidisciplinary treatment will be discussed based on the reported evidence.
Indication and technical consideration
Given the oncological aggressiveness of ICC compared with hepatocellular carcinoma, surgical resection with an adequate lymphadenectomy remains the cornerstone of therapy for technically resectable disease. Therefore, in most previous studies, the efficacies of ablation therapies were mainly investigated among patients with unresectable ICC or recurrent ICC after surgery (12-21). The reported outcomes regarding ablation therapies for ICC are summarized in Table 1. In most previous reports, RFA was the mainstream treatment and microwave ablation was only used in limited cases. RFA is usually performed under ultrasound guidance, and sufficient ablative margins of at least 0.5 to 1.0 cm surrounding tumors are required to secure the complete ablation of tumor nodules. To achieve an optimal ablative field, a single electrode is used for small lesions (usually measuring up to 2–3 cm in diameter) and multiple or clustered electrodes are used for large lesions (usually greater than 3–3.5 cm in diameter) (11). The reported technical success rate of RFA for ICC ranged from 80% to 100%. However, Giorgio et al. reported that it is difficult to achieve complete ablation during the 1st session when the tumor size exceeds 4 cm, while complete ablation was always achieved for smaller nodules equal to or less than 3.4 cm (16). In multivariate analyses in previous studies, tumor size was identified as the main factor predicting the initial effectiveness of RFA, as well as the survival outcomes after RFA (13,19).
Table 1. Reported clinical outcomes of radiofrequency ablation for intrahepatic cholangiocarcinoma.
| Authors | Country | Study period | Year | N | Size (cm) | Indication | Approach | Mortality (%) | Technical success rate (%) | Technical effectiveness (%) | Major complication (%) | Survival (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | ||||||||||||
| Chiou et al. | Taiwan | 2002–2004 | 2005 | 10 | 1.9–6.8 | Unresectable ICC | Percutaneous RFA | 0 | ND | 80.0 | 0 | ND | – | – |
| Carrafiello et al. | Italy | 2004–2008 | 2010 | 6 | 1.0–5.8 | Unresectable ICC | Percutaneous RFA | 0 | ND | 66.0 | 0 | ND | – | – |
| Kamphues et al. | Germany | 2002–2008 | 2010 | 13 | ND | Recurrent ICC | Stereotactic RFA | 0 | ND | ND | ND | 92 | 52 | – |
| Kim et al. | Korea | 2000–2009 | 2011 | 13 | 0.8–8.0 | Unresectable ICC | Percutaneous RFA | 0 | 88.0 | 88.0 | 8.0 | 85 | 51 | 15 |
| Giorgio et al. | Italy | 2003–2010 | 2011 | 10 | 2.4–5.5 | Unresectable ICC | Percutaneous RFA | 0 | ND | ND | 0 | 100 | 83 | 83 |
| Kim et al. | Korea | 1999–2009 | 2011 | 20 | 0.7–4.4 | Recurrent ICC | Percutaneous RFA | 0 | ND | 95.0 | 10.0 | 70 | 21 | – |
| Yu et al. | China | 2006–2010 | 2011 | 15 | 1.3–9.9 | Unresectable ICC | Percutaneous microwave | 0 | 91.7 | 87.5 | 13.0 | 60 | – | – |
| Xu et al. | China | 1998–2010 | 2012 | 18 | 1.4–6.9 | Unresectable ICC/recurrent ICC | Percutaneous RFA/microwave | 0 | 92.0 | 92.0 | 5.0 | 36 | 30 | 30 |
| Fu et al. | China | 2000–2010 | 2012 | 17 | 2.1–6.8 | Unresectable ICC/recurrent ICC | Percutaneous RFA | 0 | 96.2 | 96.2 | 6.0 | 85 | 43 | 29 |
| Haidu et al. | Austria | 2004–2010 | 2012 | 11 | 2.0–10.0 | Unresectable ICC/recurrent ICC | Stereotactic RFA | 0 | 100.0 | 92.0 | 27.0 | 91 | 71 | – |
| Zhang et al. | China | 2007–2011 | 2013 | 77 | ND | Recurrent ICC | Percutaneous RFA/microwave | 0 | 100.0 | 94.7 | 3.9 | 70 | 21 | – |
| Butros et al. | USA | 1998–2011 | 2014 | 7 | 1.3–3.3 | Unresectable ICC/recurrent ICC | Percutaneous RFA | 0 | 100.0 | 89.0 | 0 | 100 | 60 | 20 |
ICC, intrahepatic cholangiocarcinoma; RFA, radiofrequency ablation; ND, no data.
Effectiveness of RFA
The technical effectiveness (i.e., complete ablation without local progression for at least 1 month), which was defined by the Society of Interventional Radiology reporting standards (22), has been reported to be 80% to 100% in previous studies. However, the local tumor progression rate after RFA was relatively high, ranging from 8% to 50% (12-15,17,19,20), and the pooled rate in a meta-analysis was reported to be 21% (95% CI, 13–30%) (11). The rate of major complication observed after RFA was 8% in the evaluable population, as shown in Table 1.
The pooled 1-, 3-, and 5-year survival rates in the meta-analysis were 82% (95% CI, 72–90%), 47% (95% CI, 28–65%), and 24% (95% CI, 11–40%), respectively (11). These data were compatible with the clinical outcomes recently estimated using the SEER database (23). Amini et al. reported that in reviewing 1,232 patients selected from the SEER database, only 64 (5.2%) patients underwent ablation therapy alone. Interestingly, they noted that the median survival of patients treated with ablation therapy was 20 months, which was worse than that of patients treated with resection but better than that of patients treated with radiation therapy alone (23). Although these outcomes are likely to be influenced by the differences in the baseline characteristics of the patients in each group, for a selected group of patients, RFA might confer a modest survival advantage, compared with other non-surgical treatment options including radiation therapy or chemotherapy.
Prognostic impact and role of RFA in multidisciplinary treatment
A multivariate analysis in the study using the SEER database (23) revealed that ablation therapy may have a preferable prognostic impact, compared with the best supportive care (BSC), with a hazard ratio (HR) of 0.57 (95% CI, 0.40–0.83), while surgery alone (HR, 0.46; 95% CI, 0.39–0.54) or surgery + radiotherapy (HR, 0.46; 95% CI, 0.35–0.60) were strongly correlated with a better survival outcome.
Given the separate indication in actual clinical settings between surgery and RFA, it is difficult to compare the efficacy of surgery and RFA directly for primary tumors. However, for patients with recurrent ICC after surgery, it has been reported that ablation therapy (RFA or microwave ablation) have been reported to have an overall efficacy similar to that of repeated hepatic resection especially in patients with tumors up to 3 cm in diameter (24). This observation is consistent with previous reports examining the use of ablation therapies for ICC, and suggests that although ablation therapy might be effective in selected patients with recurrent ICC, its indication should be limited according to tumor size (14,16).
Compared with surgery, a clear advantage of RFA is its lower invasiveness and lower morbidity rate. In the largest series comparing ablation therapies with repeated resection for recurrent ICC, the major complication rate was 3.9% after ablation therapies, while it was 46.9% after repeated resection (24). Therefore, in a selected population with small recurrent tumors, ablation therapies could be a first-line treatment comparable with surgical resection.
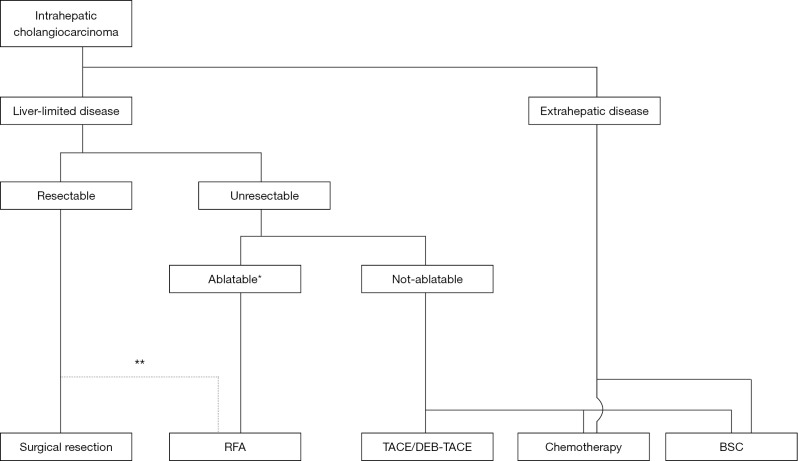
Although only limited evidence from a small number of studies has been reported because of the rarity of this tumor, ablation therapies (mainly RFA) could be a treatment of choice for selected cases of ICC (Figure 1). The indications for RFA should be determined based on the local expertise and availability of the equipment required for ablative therapies. Further studies involving a large cohort of patients are needed to refine the current indication criteria and our knowledge of ablative therapies for ICC.
Figure 1.
Algorithm for the selection of treatment for intrahepatic cholangiocarcinoma at Toranomon Hospital. *, possibility of complete ablation should be determined according to the local expertise and equipment; **, RFA can be used as a second choice for tumors up to 3 cm in diameter. RFA, radiofrequency ablation; TACE, transarterial chemoembolization; DEB-TACE, transarterial chemoembolization with drug-eluting beads; BSC, best supportive care.
Conclusions
Although surgical resection is the first choice of treatment for patients with resectable ICC, ablative therapies, mainly RFA, may have a modest prognostic impact for patients who are unfit for surgical resection at presentation or those with recurrence after surgery. Given the available data on the survival outcomes after RFA for ICC, ablation therapies could be a treatment of choice, especially for patients with small lesions up to 3 cm in diameter.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Kaczynski J, Hansson G, Wallerstedt S. Incidence, etiologic aspects and clinicopathologic features in intrahepatic cholangiocellular carcinoma--a study of 51 cases from a low-endemicity area. Acta Oncol 1998;37:77-83. 10.1080/028418698423212 [DOI] [PubMed] [Google Scholar]
- 2.Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. 10.1016/j.jhep.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 3.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. 10.1097/00000658-199610000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. 10.1055/s-2004-828889 [DOI] [PubMed] [Google Scholar]
- 5.Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol 2016;23:235-43. 10.1245/s10434-015-4642-9 [DOI] [PubMed] [Google Scholar]
- 6.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569-77; quiz 578. 10.1038/ajg.2011.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lencioni R, Crocetti L. Image-guided ablation for hepatocellular carcinoma. Recent Results Cancer Res 2013;190:181-94. 10.1007/978-3-642-16037-0_12 [DOI] [PubMed] [Google Scholar]
- 8.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210-24. 10.1002/bjs.7669 [DOI] [PubMed] [Google Scholar]
- 9.Slakey DP. Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg 2002;68:395-7. [PubMed] [Google Scholar]
- 10.Simo KA, Halpin LE, McBrier NM, et al. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J Surg Oncol 2016;113:62-83. 10.1002/jso.24093 [DOI] [PubMed] [Google Scholar]
- 11.Han K, Ko HK, Kim KW, et al. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol 2015;26:943-8. 10.1016/j.jvir.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 12.Butros SR, Shenoy-Bhangle A, Mueller PR, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging 2014;38:490-4. 10.1016/j.clinimag.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 13.Carrafiello G, Laganà D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol 2010;33:835-9. 10.1007/s00270-010-9849-3 [DOI] [PubMed] [Google Scholar]
- 14.Chiou YY, Hwang JI, Chou YH, et al. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci 2005;21:304-9. 10.1016/S1607-551X(09)70125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2012;23:642-9. 10.1016/j.jvir.2012.01.081 [DOI] [PubMed] [Google Scholar]
- 16.Giorgio A, Calisti G, DE, Stefano G, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res 2011;31:4575-80. [PubMed] [Google Scholar]
- 17.Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35:1074-82. 10.1007/s00270-011-0288-6 [DOI] [PubMed] [Google Scholar]
- 18.Kamphues C, Seehofer D, Eisele RM, et al. Recurrent intrahepatic cholangiocarcinoma: single-center experience using repeated hepatectomy and radiofrequency ablation. J Hepatobiliary Pancreat Sci 2010;17:509-15. 10.1007/s00534-009-0256-6 [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. 10.2214/AJR.10.4937 [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2011;80:e221-5. 10.1016/j.ejrad.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 21.Xu HX, Wang Y, Lu MD, et al. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol 2012;85:1078-84. 10.1259/bjr/24563774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S377-90. 10.1016/j.jvir.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Amini N, Ejaz A, Spolverato G, et al. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014;110:163-70. 10.1002/jso.23605 [DOI] [PubMed] [Google Scholar]
- 24.Zhang SJ, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:3596-602. 10.1245/s10434-013-3035-1 [DOI] [PubMed] [Google Scholar]