Abstract
Intrahepatic cholangiocarcinoma (ICC) is a rare disease and carries a poor prognosis with surgery remaining the only curative treatment option. However, due to the late presentation of symptoms and close proximity of the tumors to central hepatic structures, only about 30% of patients are classified eligible to resection. As for palliative approaches, ICC constitutes a possible indication for loco-regional therapies (LRT). As such, intra-arterial therapies (IAT) are reported to be feasible, safe and effective in inducing tumor response in unresectable ICC. The paradigm of IAT is premised on the selective delivery of embolic, chemotherapeutic agents to the tumor via its feeding arteries, thus allowing dose escalation within the carcinoma and reduction of systemic toxicity. Conventional transcatheter arterial chemoembolization (cTACE) so far remains the most commonly used IAT modality. However, drug-eluting beads (DEB)-TACE was initiated with the idea of more selective targeting of the tumor owing to the combined embolizing as well as drug-eluting properties of the microspheres used in this setting. Moreover, radioembolization is performed by intra-arterial administration of very small spheres containing β-emitting yttrium-90 (Y90-RE) to the site of the tumor. Clinical evidence exists in support of survival benefits for IAT in the palliative treatment of ICC compared to surgery and systemic chemotherapy. As for combination regimens, cTACE, DEB-TACE and Y90-RE are reported to achieve conversion of patients to surgery in a sequential treatment planning and simultaneous IAT combinations may provide a therapeutic option for treatment escalation. Regarding the current status of literature, controlled randomized prospective trials to compare different IAT techniques and combination therapies as well as treatment recommendations for different IAT modalities are needed.
Keywords: Transarterial chemoembolization, radioembolization, drug-eluting beads (DEB), intra-arterial therapies (IAT)
Introduction
Cholangiocarcinoma is a rare disease that represents 10% of primary liver malignancies with an incidence of 1–2/100,000 in the U.S. and Europe and higher rates in Asian countries (1,2). Cumulative mortality rates have increased by 39% between 1979 and 2004. This was mainly attributable to rising incidence rates, especially in the group of patients ≥65 years, in which also 72% of cholangiocarcinoma related deaths occurred in 2004 (3). The classification of the disease is based on the anatomic location of the tumor and differentiates between intra- and extrahepatic cholangiocarcinoma. Intrahepatic cholangiocarcinoma (ICC) constitutes no more than 5–15% of all cases (4). As for patient outcome, the prognosis of the disease is dismal and surgical resection is the only curative treatment option with five-year survival rates varying from 14% to 40% (5). Primarily because most patients present at advanced stages due to unspecific clinical symptoms and also because of the oftentimes central localization of the lesions within the liver (6), surgical therapy is only possible in about 30% of the cases (7). As for systemic chemotherapy, regimens containing gemcitabine and cisplatin have been reported as effective in patients with unresectable ICC. However, the median overall survival (OS) rate for such regimen did not exceed one year (8). Over the last decade, the use of image-guided loco-regional therapies (LRT) as a palliative option in unresectable ICC has become increasingly accepted among multidisciplinary teams that manage this subset of liver cancer patients. Of all LRT, catheter-based intra-arterial therapies (IAT) are the most commonly used approaches for the treatment of ICC. In this setting, embolic materials and/or chemotherapeutic agents or internal radiation can be delivered directly to the tumor through the tumor-feeding arteries. Hence, selective payload delivery results in two major benefits of IAT: achievement of high doses of the cytotoxic payload within the tumor tissue while significantly reducing its systemic distribution, thus lowering the risk of adverse events.
While healthy liver tissue predominantly obtains its blood supply from portal vein branches, feeding vessels of liver malignancies mainly derive from the hepatic artery, which constitutes the essential condition for selectively targeted IAT. However, most ICC lesions are hypovascular when diagnosed on contrast-enhanced cross-sectional imaging and may exhibit extensive fibrosis, which can be frequently observed in tumor resectates. Theoretically, these characteristics may reduce the penetration of the tumor with the intra-arterially delivered payload, thus making IAT less effective (9-11). Nevertheless, there is growing evidence for the ability of IAT to achieve high tumor response rates, which might very well result in survival benefits for patients with this liver-dominant disease. The most commonly used techniques of intra-arterial embolotherapy are conventional transcatheter arterial chemoembolization (cTACE), drug-eluting beads (DEB)-TACE and Yttrium-90 radioembolization (Y90-RE) (Figure 1). In this review, we will describe the technical background of the aforementioned techniques and provide an overview of the available clinical evidence for the use of those treatment modalities in the therapy of unresectable ICC.
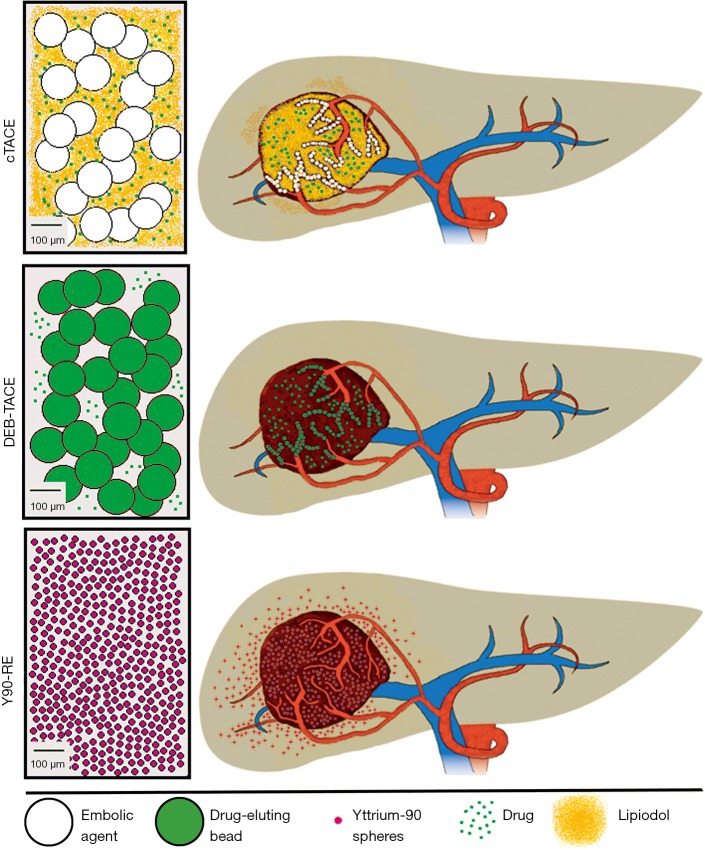
Figure 1.
Overview: intra-arterial therapies for intrahepatic cholangiocarcinoma. During conventional transcatheter arterial chemoembolization (cTACE), an emulsion of chemotherapeutic drugs and Lipiodol is delivered selectively to the tumor, followed by embolization of the tumor-feeding arteries. Drug-eluting beads (DEB)-TACE is performed highly selectively using spheres with a dual drug-releasing and embolic potential. The concept of yttrium-90 radioembolization (Y90-RE) consists of the delivery of a β-emitting payload to the tumor in a less selective, lobar fashion. Due to the small size of the microspheres, they have less embolic potential but penetrate deeply into the tumor.
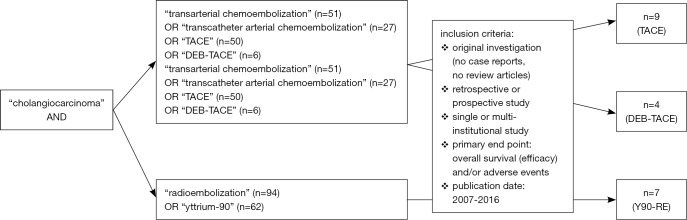
Hence, the bibliographic database of PubMed was screened for prospective and retrospective original articles using the search terms “TRANSARTERIAL” or “TRANSCATHETER ARTERIAL CHEMOEMBOLIZATION”, “DRUG-ELUTING BEADS”, “RADIOEMBOLIZATION”, “YTTRIUM” and according abbreviations in combination with “CHOLANGIOCARCINOMA” as the investigated tumor entity (Figure 2).
Figure 2.
The flow chart illustrates the study selection criteria including the search terms and the most important inclusion criteria.
cTACE—background
Conventional TACE is the most commonly used intra-arterial modality in unresectable ICC. Initially introduced for the therapy of hypervascular hepatocellular carcinoma lesions in the late 1970s, the broad clinical application of cTACE has established this technique as a safe and effective treatment option for several other liver malignancies (12). During cTACE, an emulsion of chemotherapeutics and an oil-based contrast agent (Ethiodol or Lipiodol) is injected into the tumor-supplying branch of the hepatic artery, followed by the administration of an embolizing agent. Due to the predominant central location of ICC within the liver, tumor-feeding vessels may derive from both hepatic arteries. Consequently, separate angiographic evaluation of the right and left hepatic artery is required to ensure selective targeting of the tumor (Figure 3).
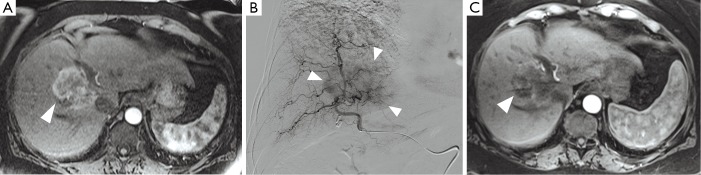
Figure 3.
Transcatheter arterial chemoembolization of intrahepatic cholangiocarcinoma. (A) The baseline contrast-enhanced MRI (ceMRI) scan demonstrates a centrally-located lesion with partial enhancement in the arterial phase (white arrow). Biopsy confirmed intrahepatic cholangiocarcinoma (ICC); (B) during selective angiography of the right hepatic artery, significant tumor blush is observed in the right lobe (white arrows). Subsequently, conventional transcatheter arterial chemoembolization (cTACE) was performed to target the tumor; (C) the ceMRI scan performed one month after cTACE demonstrates central areas of decreased attenuation within the lesion, indicative of tumor necrosis (white arrow).
The most commonly used drug combination in the US and Europe consists of doxorubicin, cisplatin and mitomycin-C, but gemcitabine has also been used (13,14). The drug cocktail is brought into emulsion with Lipiodol, which has a dual function as a drug carrier as well as an embolic agent, that is able to penetrate the tumor vasculature up to the capillaries (12). Upon deposition of the Lipiodol-drug mix, the occlusion of more proximal arterial blood vessels is achieved by the injection of embolic materials such as gelfoam, polyvinyl-alcohol (PVA) particles or trisacryl gelatin (TG) microspheres. This step devascularizes the tumor tissue and is primarily performed in order to prevent the washout of the previously deposited payload (15) (Figure 4). Generally, TACE is tolerated well by the majority of patients without major adverse events. However, abdominal pain, nausea, fever and increase in liver enzymes (limited to 3–4 days without the evidence of sepsis) are frequent transient minor side effects after cTACE procedures and commonly referred to as post-embolization syndrome (16-18).
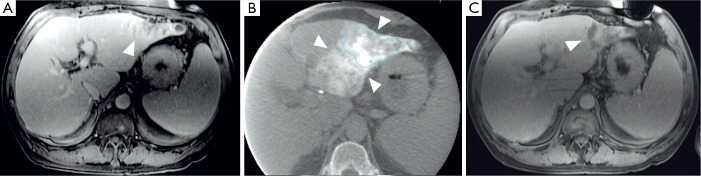
Figure 4.
Transcatheter arterial chemoembolization of intrahepatic cholangiocarcinoma. (A) The baseline contrast-enhanced MRI (ceMRI) determines a mass-forming lesion in the left lobe with marginal enhancement in the portal-venous phase (white arrow). The patient was subjected to conventional transcatheter arterial chemoembolization (cTACE) for tumor treatment afterwards; (B) a cone beam computed tomography (CBCT) scan was performed intra-procedurally during the cTACE procedure. Imaging demonstrates the deposition of Lipiodol (white arrows) within the lesion (blue line); (C) the ceMRI scan performed one month after cTACE reveals hypoenhancing areas within the lesion that indicate necrosis in the portal-venous phase (white arrow). Necrosis was achieved in those areas with the highest Lipiodol deposition on the CBCT scan.
cTACE—clinical evidence
Most studies that investigate clinical outcomes in ICC treated with cTACE are retrospective and do not use a standardized procedure protocol. However, the available literature suggests potential survival benefits in patients with unresectable lesions (19). As such, a retrospective trial included a total of 42 patients who underwent cTACE with different regimens consisting of gemcitabine (2,250 mg/m2) combined with or followed by cisplatin (100–125 mg/m2) and oxaliplatin (85–100 mg/m2). Nineteen patients (45%) were staged with extrahepatic disease. According to the Response Evaluation Criteria in Solid Tumors (RECIST), 20 patients (48%) had stable disease (SD), 15 patients (36%) were found to have progressive disease (PD) and seven patients (17%) could not be evaluated. The median OS for the entire cohort was 9.1 months. Patients with SD showed a median OS of 13.1 months compared to 6.9 months for patients with PD (P=0.017). Moreover, a survival benefit was reported for patients treated with gemcitabine combined with cisplatin over those treated with gemcitabine alone (13.8 vs. 6.3 months, P<0.001) (20).
Another chemotherapeutic agent that is commonly used for cTACE is mitomycin-c. With regards to this, a retrospective survival analysis included 15 patients for the palliative treatment with cTACE using mitomycin-c (10 mg) for 59 treatment sessions over a period of six years. The patients were diagnosed with inoperable ICC with a mean tumor diameter of 10.8±4.6 cm and multifocal disease in seven patients. Previous treatments were reported for seven patients including liver resection (n=1, 6.7%), RFA (n=2, 13.3%) and systemic chemotherapy (n=4, 26.7%). One patient (6.7%) had liver cirrhosis, however, Child Pugh score was A in 14 (93.3%) and B in one patient (6.7%). The authors reported SD in nine patients (59.9%) for best interim response to cTACE and Kaplan Meier analysis revealed a median OS of 16.3 months (95% CI, 9.4–32.5 months). One patient (6.7%) showed PR and four had PD (26.7%). As for severe adverse events, one patient (6.7%) developed anaphylactic reaction to iodine containing contrast agent and another patient (6.7%) presented with gastric ulcera due to Lipiodol displacement (21).
A more recent retrospective study was conducted to analyze survival benefits among all available therapeutic options for ICC. Out of 273 patients with ICC, 130 (47.6%) underwent surgical resection, 111 (40.7%) received systemic chemotherapy/best supportive care and a total of 32 (11.7%) underwent TACE with mitomycin-c (10 mg; n=29) or doxorubicin-eluting beads (n=3). Patients with extrahepatic disease were excluded. The median OS of surgical patients was 28 months, with significant variations between patients with positive lymph node status (N1; 9 months) or resection margin (R+; 11 months) compared to N0 (37 months, P<0.001) and R0 resection (37 months, P<0.001). The median OS for patients, who underwent TACE was 11 months. The authors concluded that surgery did not show a significant survival benefit for patients with R+ or N1 when compared to those treated with TACE (22).
Park et al. have also compared cTACE (n=72) with symptomatic supportive therapy (n=83) in the palliative treatment of 155 patients with unresectable ICC. Extrahepatic disease was found in 39 patients (54%) of the TACE cohort and in 50 patients (60%) of the supportive care group. After TACE with cisplatin at a dose of 2 mg/kg BW, PR was achieved as best tumor response to treatment in 15 patients (23%), SD was present in 44 patients (66%) and PD in seven patients (11%) according to RECIST at the 1-month follow-up (mean 1.1±0.34 months). Survival analyses demonstrated significant prolongation of the median OS in the TACE group (12.2 months) compared to the supportive treatment group (3.3 months; P<0.001). Moreover, responders to TACE showed significantly higher median OS (22 months) compared to nonresponders (10.9 months; P=0.0001) according to RECIST (23).
The use of cTACE as an adjuvant therapy after radical surgery has been explored in a retrospective analysis in a larger cohort of 125 patients. Fifty-three (42%) out of 125 patients received cTACE with a variety of drug combinations [5-FU (500 mg), carboplatin (100 mg), epirubicin (20 mg), hydrocamptothecin and gemcitabine (1,000 mg)] and outcome was compared with the surgical control group. Patients treated with cTACE showed prolonged survival compared to the control group (1-, 3- and 5-year OS of 69.8%, 37.7% and 28.3% vs. 54.2%, 25.0% and 20.8%). The median OS in the adjuvant cTACE group was twelve months and surgery only resulted in a median OS of five months. However, cTACE did not delay the recurrence of the disease in this setting (14).
Recently, Yang et al. retrospectively analyzed the efficacy of TACE [gemcitabine (600–1,000 mg) and oxaliplatin (50–100 mg)] with simultaneous microwave ablation therapy in 26 patients with advanced ICC of whom 20 (76.9%) were newly diagnosed and 5 (19.2%) had recurrent tumors after initial resection. Patients with extrahepatic disease or previous systemic or radiation therapy were excluded from the analysis. Complete ablation was achieved in 36 of 39 lesions (92.3%) and residual tumor (R+) was identified at the 1-month follow-up in two patients with three tumors (7.7%). No major complications occurred and after a mean follow-up of 19.2±6.3 months (range, 6–30 months), a promising median OS of 19.5 months and PFS of 6.2 months (range, 3–12 months) was reported. Thus, the authors claimed the combination therapy to be a safe and feasible alternative for patients with a maximum ECOG being 2. However, no matched pair analysis with a control group was performed (24).
One of the few available prospective trials included 115 patients with unresectable ICC who received cTACE with different combinations of mitomycin-c (8 mg/m2), gemcitabine (1,000 mg/m2) and cisplatin (35 mg/m2). The median OS was 13 months from initial embolization and no statistically significant differences were observed between regimens (18). A smaller study included 17 patients with unresectable ICC, who underwent a median of two cTACE sessions per patient. The regimen consisted of 50 mg doxorubicin, 100 mg cisplatin and 10 g mitomycin-c followed by embolization with PVA or Embosphere particles (Biosphere Medical, Rockland, MA). Six patients (35%) had received other palliative treatments prior to cTACE. The procedure was well-tolerated by a majority of patients (82%) and no major adverse events were reported. The median OS was 23 months from the time of diagnosis and two patients became surgically resectable following the cTACE procedure (9). In another prospective study, 62 patients with either ICC (n=37) or intrahepatic adenocarcinoma of unknown primary (n=25) were treated with a median of 2.7 cTACE sessions per patient using doxorubicin (50 mg), cisplatin (100 mg), mitomycin-c (10 mg) and PVA particles. Eighteen patients (29%) had previously received systemic chemotherapy and seven patients (11%) had prior liver resection. The median OS was 20 months from diagnosis and 15 months from first cTACE. Patients with prior chemotherapy had significantly prolonged survival compared to those who had received cTACE only (28 vs. 16 months; P=0.02) (13).
A recent prospective trial compared TACE with Y90-RE and radiofrequency ablation (RFA), inter alia. Fifty-five patients with unresectable ICC and good performance status (median ECOG 1 and Karnofsky-Index 70%) received single (n=37, 67.3%) or a combination of local therapies (n=18, 32.7%) including TACE [doxorubicin (2.5 mg/mL) and cisplatin (2.5 mg/mL); n=2] and Y90-RE (resin microspheres; n=5), high-dose rate brachytherapy (HDR-BT) + Y90-RE (n=3), TACE + intra-venous (i.v.) chemotherapy (n=1), Y90-RE + intra-arterial (i.a.) chemotherapy (n=1), HDR-BT + i.v. chemotherapy + Y90-RE (n=2) (rest: chemotherapy, HDR-BT-based combination therapies and RFA only). Y90-RE was preferably performed in multinodular disease whereas TACE was the favorable treatment modality in single lesions without portal vein thrombosis (PVT). Biologically aggressive tumors required additional systemic chemotherapy. As for local tumor control, TACE achieved PD and Y90-RE partial response (PR) for best tumor response and the median OS of all patients was 16 months from first local therapy and 33.1 months from diagnosis. The objective tumor response (liver only) was identified as one of the independent factors influencing OS with 29.8 months for complete response (CR) and PR and 9.3 months for SD and PD (P=0.005). No grade III or IV complications were observed but 43 patients died within the period of follow-up (median, 11.7 months; range, 0.9–52.2 months). Additionally, the authors identified the serum tumor markers CA19-9 and CEA, RECIST and the number of lesions as independent prognostic factors, whereas extrahepatic disease showed no correlation with patient survival (25) (Table 1). Similarly, ongoing prospective studies include the first randomized controlled trial to compare the efficacy of Y90-RE and cTACE in terms of radiographic response on contrast-enhanced MRI (ceMRI) scans in 24 patients with unresectable ICC (NCT01798147) (26).
Table 1. Current level of evidence for the treatment of intrahepatic cholangiocarcinoma with conventional transcatheter arterial chemoembolization (cTACE).
| Investigators [year] | Study design | Anticancer agents (cTACE) | Control groups | Study cohort (n) | ECOG status | Extrahepatic lesions | Previous systemic chemotherapy | Adverse events (grade III/IV) | Median OS (mo from first cTACE) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Gusani et al. [2008] | Retrospective | Gemcitabine, cisplatin, oxaliplatin | No | 42 | 0–1 | n=19 (45%) | N/A | Myocardial infarction (n=1), hepatic abscess (n=1), hyperbili-rubinemia (n=2), thrombocyto-penia (n=2), over sedation (n=1) | 9.1 | (20) |
| Herber et al. [2007] | Retrospective | Mitomycin-c | No | 15 | N/A | N/A | n=4 (27%) | Anaphylactic shock (7%), gastric ulceration (7%) | 16.3 | (21) |
| Scheuermann et al. [2013] | Retrospective | Mitomycin-c | Ctace (n=32) vs. surgery (n=130) vs. systemic chemotherapy (n=111) | 273 | N/A | No | N/A | Massive ascites (n=1), dissection or occlusion of the hepatic artery (n=2) | 11 | (22) |
| Park et al. [2011] | Retrospective | Cisplatin | cTACE (n=72) vs. supportive care (n=83) | 155 | 0–2 | n=39 (54%) in TACE group; n=50 (60%) in supportive care group | No | Haematological toxicity events (n=9, 13%), non-haematological toxicity events (n=17, 24%) | 12.2 | (23) |
| Shen et al. [2011] | Retrospective | Adjuvant: 5-FU, epirubicin, hydrocamptothecin, gemcitabine, carboplatin | Hepatic resection only (n=72) vs. adjuvant cTACE (n=53) | 125 | 0–1 | No | No | No | 12 | (14) |
| Yang et al. [2015] | Retrospective | Gemcitabine, oxaliplatin (ctace in combination with microwave ablation) | No | 26 | 0–2 | No | No | No | 19.5 | (24) |
| Vogl et al. [2012] | Prospective | Gemcitabine, mitomycin-c, cisplatin | No | 115 | N/A | N/A | N/A | No | 13 | (18) |
| Burger et al. [2005] | Prospective | Doxorubicin, cisplatin, mitomycin-c | No | 17 | 0–3 | n=5 (29%) | n=6 (35%) | Treatment-related mortality (6%), acute cholecystitis (n=1, 6%), ascites (n=1, 6%) | N/A (23 from diagnosis) | (9) |
| Kiefer et al. [2011] | Prospective | Doxorubicin, cisplatin, mitomycin-c | No | 62 | 0–2 | n=19 (31%) | n=18 (29%) | Pulmonary edema and elevated cardiac enzymes (n=1), pulmonary infarct (n=1), severe postembolization syndrome (n=1), hyper-glycemia (n=1), acute renal failure and dehydration postprocedure (n=1) | 15 | (13) |
OS, overall survival; mo, months; N/A, not available.
As evidence in support of the efficacy of cTACE for unresectable ICC is continuously growing, Schernthaner et al. have recently introduced cone-beam (CB) CT as an innovative technique with high accuracy in the detectability of ICC lesions and accurate radiographic response evaluation after LRT. The retrospective analysis included 17 patients who underwent CBCT, digital subtraction angiography (DSA) and ceMRI directly prior to cTACE intervention. Interestingly, only 45.9% of the lesions were depicted by DSA whereas CBCT detected 73.8% (early phase) and 93.4% (late phase), suggesting the integration of CBCT for intra-procedural tumor detection and treatment planning (27) (Figure 4).
DEB-TACE—background
As the process of emulsification between Lipiodol and drugs is very unstable and the two components are likely to separate after intra-arterial injection, DEB were launched with the aim of reducing the risk of systemic distribution and increasing intra-tumoral drug concentrations. DEB-microspheres have a dual embolizing and drug-eluting potential and can be loaded with specific chemotherapeutic agents. Administered in the same manner as cTACE, the drug-eluting properties of the microspheres increase the exposure of the tumor to the drug. This is achieved by a local, controlled release of the chemotherapeutic agent for a prolonged period. Hence, this new modality was reported to optimize selective drug delivery to the tumor and to reduce systemic toxicity (28).
Currently, there are two different types of microspheres in use: PVA-based microspheres (LC Beads for the U.S., DC Beads for Europe; Biocompatibles, BTG, UK), which are usually loaded with doxorubicin (DEBDOX) or irinoctecan (DEBIRI) (29,30) and superabsorbent polymer (SAP) microspheres (QuadraSpheres for the U.S., HepasSpheres for Europe; Merit Medical, US) that can be loaded with a variety of drugs, such as irinotecan, anthracyclin antibiotics and platin-based chemotherapeutics (31,32).
DEB-TACE—clinical evidence
The use of oxaliplatin-preloaded (50 mg) microspheres (HepaSpheres, Biosphere Medical, France) combined with systemic chemotherapy (oxaliplatin and gemcitabine) in the treatment of hepatic malignancies (seven ICC) was examined in a small retrospective comparative study including nine patients. The cohort that received DEB-TACE was compared to a retrospectively acquired group of eleven patients, who were treated with chemotherapy (FOLFOX) only. With one exception, Child Pugh class B and C as well as extrahepatic disease were exclusion factors in both groups. According to RECIST criteria, four patients (44%) in the DEB-TACE group achieved PR and five patients (56%) showed SD. The median OS after DEB-TACE and chemotherapy was 30 months compared to 12.7 months for chemotherapy alone (33).
A prospectively designed multi-institutional review included 24 patients with unresectable ICC and a mean number of two target lesions (range, 1–5) who were treated with total of 42 DEB-TACE sessions. Ten patients (41.7%) presented with recurrent ICC after resection (n=7) or RFA (n=2) and 20 patients (83.3%) had received chemotherapy before. The DEB-TACE regimen using DC/LC Beads (Biocompatibles, Farnham, UK) consisted of doxorubicin (150 mg) and irinotecan (75 mg; range, 40–100 mg) and was combined with systemic chemotherapy in eight patients (33.3%). The mean tumor diameter was relatively large with 11.5 cm (range, 4–33.3 cm) and ten patients (41.7%) presented with extrahepatic disease. As for treatment efficacy, the median OS was 17.5 months and three patients (12.5%) were converted to surgical resection postprocedurally. However, grade III complications occurred in four patients (16.7%) including hepatorenal syndrome (n=1), sepsis (n=1) and liver failure (n=2) (34).
An early prospective trial reported eleven patients with unresectable ICC, who underwent a median of three DEB-TACE sessions using DC Beads (Biocompatibles, BTG, UK) preloaded with doxorubicin (75 mg/2 mL). All patients had previously received systemic chemotherapy and/or hepatic resection. The median OS was 13 months and tumor response was 100% according to RECIST (29). Another prospective series was designed to compare DEBIRI (irinotecan 200 mg; DC/LC Beads, Biocompatibles/BTG, UK; n=26) with cTACE (mitomycin-c 15 mg; gelfoam; n=10) and systemic chemotherapy (gemcitabine and oxaliplatin; n=31). Seven patients (26.9%) in the DEBIRI group had received prior chemotherapy. Compared to cTACE and systemic chemotherapy, DEBIRI revealed prolonged median OS (5.7 vs. 11 vs. 11.7 months) and was well-tolerated with only few reports on post-embolization syndrome (35) (Table 2).
Table 2. Current level of evidence for the treatment of intrahepatic cholangiocarcinoma with drug-eluting beads-transcatheter arterial chemotherapy (DEB-TACE).
| Investigators [year] | Study design | Anticancer agents (DEB-TACE) | Control groups | Study cohort (n) | Performance status | Extrahepatic lesions | Previous systemic chemotherapy | Adverse events (grade III/IV) | Median OS (mo from first DEB-TACE) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Poggi et al. [2009] | Retrospective | Oxaliplatin | Systemic chemotherapy (FOLFOX) | 9 | ECOG 0–1 | N/A | n=9 (100%) | No | 30 | (33) |
| Schiffman et al. [2011] | Prospective | Doxorubicin, irinotecan | No (systemic chemotherapy as literature review) | 24 | ≥60% Kanowsky-Index | n=10 | n=20 (83%) | Hepatorenal death (n=1), port sepsis (n=1), hepatic insufficiency (n=2) | 17.5 | (34) |
| Aliberti et al. [2008] | Prospective | Doxorubicin | No | 11 | ≥60% Kanowsky-Index | N/A | n=11 (100%) | No | 13 | (29) |
| Kuhlmann et al. [2012] | Prospective | Irinotecan | DEB-TACE vs. cTACE vs. systemic chemotherapy | 67 | ≥70% Kanowsky-Index | n=43 (n=11 in DEB-TACE group) | n=7 (10%) (n=5 in DEB-TACE group) | Abdominal pain (n=7), hepatic abscess (n=1), pleural empyema due to biliary leakage (n=1), death due to cholangitis (n=1) | 11.7 | (35) |
OS, overall survival; mo, months; N/A, not available.
Y90-RE—background
Y90-RE is a form of selective internal radiation therapy (SIRT). The concept consists of the intra-arterial delivery of small embolic particles (20–40 µm) containing the radionucleotide Y90, that emits β-radiation (36). Usually, a target dose of 120 Gy is delivered, attaining much higher local doses compared to external-beam radiation. Moreover, external-beam radiation has had limited use in the therapy of liver malignancies because liver tissue is extremely radiation-sensitive and maximal tolerable doses remain far below tumoricidal levels (37). Hence, Y90-RE allows maximization of treatment efficacy while sparing the healthy liver parenchyma from radiation-induced injury.
Currently, two major devices are available: glass-based microspheres (TheraSphere, MDS, Nordion, Ottawa, Ontario, Canada) and resin-based microspheres (SIR-Sphere, Sirtex, New South Wales, Australia) (38). Due to the relatively small size of the microspheres, they only have limited embolizing properties but may contribute to a better penetration of the tumor. In further contrast to TACE, Y90-microspheres are commonly administered in a less selective, lobar fashion that provides better reproducibility over TACE procedures (39).
Given the small size and the severe radiation potency of Y90-particles, complications may derive from unintended extrahepatic deployment of the payload. One major complication is the development of gastroduodenal ulcera secondary to non-target Y90 administration, with varying incidences from 3% to 24% after Y90-RE (40). An unrecognized right gastric artery, proximal administrations and those resulting in stasis of flow present increased risk for ulceration refractory to medical management (41,42). Hence, the absence of angiographically concealed arterial shunts has to be approved prior to treatment. In particular, all patients must be subjected to shunt evaluation using technetium-99 macroagglutinated albumin (Tc-MAA), SPECT and angiographic imaging (39,43).
In 1999, Canada was the first country to approve Y90-RE for palliative treatment of hepatic malignancies. In the U.S., the procedure is FDA-approved for the use in HCC only. Application in patients with other entities of liver cancer requires individual institutional review board approval (44).
Y90-RE—clinical evidence
Efficacy and safety of selective Y90-RE (SIR-Spheres) were retrospectively investigated in an adjuvant setting including 33 patients with cholangiocarcinoma. Prior treatment included hepatic resection in twelve patients (36%), systemic chemotherapy in 27 patients (79%) and TACE or RFA in five patients (15%). The study revealed a median OS of 22 months from the first procedure and time-to-progression was 9.8 months. Association of good ECOG performance status with prolongation of survival after Y90-RE was observed (ECOG 0: 29.4, ECOG 1: 10.0, ECOG 2: 5.1 months median OS; P<0.001) (39). As for further prognostic factors, FDG-negative and small tumors and lower tumor load were reported to correlate with prolonged OS after Y90-RE (SIR-Spheres) in other studies (45).
The systematic review by Al-Adra et al. summarizes current clinical evidence available for the efficacy and toxicity of Y90-RE. Median OS and radiologic response were investigated as primary outcome and morbidity/mortality and the ability to convert unresectable to resectable ICC as secondary outcome. Briefly, a total of twelve studies (n=5 retrospective; n=7 prospective) was included amounting to a total of 298 patients. Weighted median OS was 15.5 months (range, 7–22.2 months), based on eleven included studies. For radiologic response analysis, RECIST or mRECIST (n=7), WHO (n=1) and PERCIST (n=1) were use. A pooled analysis revealed mean PR in 28% and SD in 54% of patients at a 3-month follow-up. Additionally, three studies reported successful downstaging of patients by Y90-RE with subsequent surgery in seven patients. The most common types of morbidity following Y90-RE were fatigue (33%), abdominal pain (28%) and nausea (25%) (46).
As surgery remains the only curative therapy for ICC, a larger retrospective trial investigated the ability of Y90-RE combined with systemic chemotherapy to convert patients with advanced stage ICC to resection. A total of 45 patients with unresectable ICC received the abovementioned combination therapy and was compared to a total of 54 patients who underwent primary resection. Four patients (9%) were treated for tumor recurrence and 27 (60%) patients were classified as palliative candidates with multilocular disease. However, in eight of the remaining patients, ICC had developed in non-cirrhotic livers and was eventually treated with surgery curative intent. Prior to resection, these patients had received chemotherapy (5FU, platin, gemcitabine) in combination with Y90-RE (TheraSpheres). No grade III/IV complications were observed. As for downstaging of the tumors, significant volume reduction [295 (range, 90–1,250) vs. 168 (range, 46–535) mL; P=0.02] was observed during the median follow-up of 15.6 months (range, 4–40.7 months). Due to the tumor shrinkage, proximity of the central liver structures decreased in seven cases and the tumors became resectable. Six of these patients (85.7%) survived the postoperative period and one (14.3%) died 6.5 months after surgery at the age of 80 years with a median overall recurrence-free survival of 19.1 months (47).
In the first prospective series to report Y90-RE, 24 patients diagnosed with unresectable ICC were treated with glass-based Y90 particles (TheraSpheres) in one or two procedures each. Seven patients (29%) had prior chemotherapy and bilobar and extrahepatic disease were present in 16 (67%) and eight patients (33%), respectively. The median OS was 14.9 months and patients naive to systemic chemotherapy demonstrated a survival benefit compared to the previously treated group (31.8 vs. 4.4 months) (44). Another prospective study included 25 patients treated with resin-based Y90-RE (SIR-Spheres) for unresectable ICC. A total of 17 patients (68%) had received systemic chemotherapy and twelve patients (48%) were staged with extrahepatic metastases. The median OS was 9.3 months from the first RE and 1-, 2- and 3-year survival rates of 40%, 27% and 13%, respectively, were reported (48). A further prospective trial was conducted to examine feasibility and safety of resin-based Y90-RE (SIR-Spheres). Nineteen patients refractory to systemic chemotherapy were included and four (21.1%) had received additional DEB-TACE before. After a median of 1.6 procedures per patient, RECIST revealed SD in 68%, PR in 11% and PD in 21%. The 1-year survival rate was 56% and median OS was 11.5 months after the first Y90-RE (49). In a larger prospective series, 46 patients with local or infiltrative ICC underwent 92 Y90-RE procedures using glass-based TheraSpheres for comparison of survival rates. The median OS for patients with solitary tumors was 14.3 months and infiltrative disease revealed a median OS of 6.1 months. Five patients (10.9%) were deemed to curative resection after RE. The procedures were well-tolerated. However, abdominal pain was the most frequently reported side effect and present in 54% of patients (41). A prospective correlate study was conducted to assess effectiveness and tumor response to Y90-RE (SIR-Spheres) in a total of 21 patients with ICC and refractory to systemic chemotherapy. Extrahepatic disease was considered as an exclusion factor. Overall response rate (CR/PR) calculated by RECIST was 4.7% compared to 38% according to modified RECIST (mRECIST). The median OS was 16.3 months from first Y90-RE. There were no relevant toxicities reported (50).
As for imaging-based tumor response evaluation, newer studies have investigated the prognostic value of FDG-PET and PERCIST criteria in the setting of LRT. Haug et al. defined response to Y90-RE (SIR-Spheres) as a decrease of the SUV ≥30% compared to baseline imaging. Hence, 19 (74%) of the 26 included patients were classified as responders in FDG-PET imaging at a 3-month follow-up. The results corresponded well with the median OS being 22.3 months for responders and 6.9 months for nonresponder (P<0.05). However, the PET-based results showed good correlation with EASL criteria (PR/CR in n=20, 78%) but not with RECIST (available in n=23; PR in n=5, 22%; no CR) (51). More recently, FDG-PET and concomitant PERCIST criteria were used to evaluate response of ICC in 18 patients six weeks after Y90-RE with SIR-Spheres. PR was achieved in 14 patients (82.3%) and SD in three patients (17.6%). Similar to the aforementioned trial, imaging-based response evaluation corresponded well with the mean OS being 18.2 and 9.9 months for responders (SUV decrease ≥50%) and nonresponders, respectively (52).
A recent phase I trial investigated the concept of chemoradiation by using the radiosensitizing agent capecitabine in combination with Y90-RE. Its impact on the maximum tolerated Y90-dose (MTD-Y90) in glass-based RE (TheraSpheres) was evaluated in an escalating study design that included 17 patients with ICC or liver metastases. An MTD-Y90 >170 Gy was reported and only two patients experienced dose-limiting toxicity. These results suggest radiosensitizing to be an option for Y90-dose escalation and subsequent improvement of technical efficacy (53).
A retrospective series from 2013 was designed to compare efficacy, morbidity and survival after different IAT procedures. A total of 198 patients with advanced ICC, who were primarily treated with a total of 464 IAT procedures in five major hepatobiliary institutions in the US, were included. Patients were treated with cTACE (n=128, 64.7%; gemcitabine and cisplatin or doxorubicin, cisplatin and mitomycin-c), transarterial embolization (TAE) (n=13, 6.6%), DEB-TACE (n=11, 5.6%) or Y90-RE (n=46, 42.3%) and 30 patients (15%) had simultaneously received systemic chemotherapy. Generally, procedures were well-tolerated and only 16 patients (8.1%) reported major adverse events such as acute renal or hepatic failure. The median OS in the entire cohort was 13.2 months and no significant differences were observed stratified by the type of IAT (cTACE 13.4 vs. TAE 14.3 vs. DEB-TACE 10.5 vs. Y90-RE 11.3 months; P=0.46). According to mRECIST, 41 patients (25.5%) had CR or PR whereas 99 patients (61.5%) had SD and 21 patients (13%) revealed PD. Tumor response was associated with survival prolongation (CR/PR 32.4 months vs. PD 6.4 months; P<0.05) (19). Similarly, a recently published meta-analysis compared hepatic artery-based therapies with median OS as the primary outcome and tumor response to therapy and toxicity as secondary endpoints. Overall, 20 articles including a total of 657 patients were analyzed. The highest median OS for i.a. chemoinfusion was reported to be 22.8 months (range, 9.8–35.8 months, 95% CI), 13.9 months (range, 9.5–18.3 months) for Y90-RE, 12.4 months (range, 10.9–13.9 months) for cTACE and 12.3 months (range, 11–13.5 months) for DEB-TACE. Response to therapy as defined by CR and PR according to RECIST was highest in i.a. chemoinfusion>Y90-RE>cTACE>DEB-TACE. However, grade III/IV complication rate was highest in i.a. chemoinfusion>cTACE>DEB-TACE (54) (Table 3).
Table 3. Current level of evidence for the treatment of intrahepatic cholangiocarcinoma with Yttrium-90 radioembolization (Y90-RE).
| Investigators [year] | Study design | Spheres (Y90-RE) | Study cohort (n) | ECOG status | Extrahepatic lesions | Previous systemic chemotherapy | Adverse events (grade III/IV) | Median OS (months from first Y90-RE) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Hoffmann et al. [2012] | Retrospective | SIR-Spheres | 33 | 0–2 | n=8 (24%) | n=27 (79%) | No | 22 | (39) |
| Rayar et al. [2015] | Retrospective | TheraSpheres | 45 | 0–2 | n=27 (60%) | Yes | No | N/A | (47) |
| Ibrahim et al. [2008] | Prospective | TheraSpheres | 24 | 0–2 | n=8 (33%) | n=7 (29%) | Albumin toxicities (n=4, 17%), bilirubin toxicity (n=1, 4%), refractory gastroduodenal ulcer (n=1, 4%); (clinical toxicity grading N/A) | 14.9 | (44) |
| Saxena et al. [2010] | Prospective | SIR-Spheres | 25 | 0–2 | n=12 (48%) | n=17 (68%) | Albumin and bilirubin toxicity (n=2, 8%), alkaline phosphatase toxicity (n=1, 4%); (clinical toxicity grading N/A) | 9.3 | (48) |
| Rafi et al. [2013] | Prospective | SIR-Spheres | 19 | 0–2 | No | n=19 (100%) | Bilirubin toxicity (n=1, 5%), thrombocytopenia (n=1, 5%) | 11.5 | (49) |
| Mouli et al. [2013] | Prospective | TheraSpheres | 46 | 0–2 | No | No | Albumin toxicity (n=4, 9%), bilirubin toxicity (n=3, 7%), refractory gastroduodenal ulcer (n=1, 2%) | 6.1 | (41) |
| Camacho et al. [2014] | Prospective | TheraSpheres | 21 | 0–2 | No | n=21 (100%) | N/A | 16.3 | (50) |
OS, overall survival; mo, months; N/A, not available.
Conclusions
In summary, despite the lack of randomized controlled trials, current literature indicates evidence in support of the use of LRT for patients with unresectable ICC. In particular, IAT have proven feasible, safe and effective in inducing local tumor response. Current clinical evidence suggests prolonged survival without severe impairment of the quality of life of patients in a palliative setting. Additionally, some studies report survival benefits for IAT over systemic chemotherapy and the ability of downstaging tumors until eligible to resection. Besides minimal invasiveness, the main advantages of IAT result from selective targeting of the tumor: locally increased drug concentrations in the tumor whilst avoiding systemic toxicity. Although Y90-RE is increasingly accepted, cTACE remains the most frequently used IAT procedure in the palliative therapy of ICC. Except for minor toxicities subject to the post-embolization syndrome, embolization procedures are predominantly well-tolerated. However, prospective randomized trials and meta-analyses are needed to definitely establish the impact of IAT on patient survival. To broaden the scope of application, the outcomes of different IAT modalities ought to be standardized and compared and indications have to be clearly defined.
Acknowledgements
Funding: JF Geschwind reports grants from the National Institutes of Health [NIH/NCI R01 CA160771, P30 CA0069730] and Philips Medical, during the conduct of the study; personal fees from Consultant to Nordion; personal fees from Consultant to Biocompatibles/BTG; personal fees from Consultant to Bayer HealthCare; grants from DOD; grants from Biocompatibles/BTG; grants from Bayer HealthCare; grants from Nordion; grants from Context Vision; grants from SIR; grants from RSNA; and grants from Guerbet, outside the submitted work. JF Geschwind is the founder and CEO of Prescience Labs, LLC. J Chapiro reports grants from the German-Israeli Foundation for Scientific Research and Development, the Berlin Institute of Health/Charité Foundation, Philips Healthcare and the Rolf W. Günther Foundation for Radiological Science during the study. LJ Savic reports scholarships from the German National Academic Foundation and the Medical Excellence Initiative by the Manfred Lautenschläger Foundation outside the submitted work.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. 10.1016/S0140-6736(05)67530-7 [DOI] [PubMed] [Google Scholar]
- 2.Hong K, Geschwind JF. Locoregional intra-arterial therapies for unresectable intrahepatic cholangiocarcinoma. Semin Oncol 2010;37:110-7. 10.1053/j.seminoncol.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134-44. 10.1053/j.gastro.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 4.Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. 10.1634/theoncologist.9-1-43 [DOI] [PubMed] [Google Scholar]
- 5.Poultsides GA, Zhu AX, Choti MA, et al. Intrahepatic cholangiocarcinoma. Surg Clin North Am 2010;90:817-37. 10.1016/j.suc.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Sasaki A, Aramaki M, Kawano K, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. Br J Surg 1998;85:1206-9. 10.1046/j.1365-2168.1998.00815.x [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. 10.1245/s10434-007-9627-x [DOI] [PubMed] [Google Scholar]
- 8.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. 10.1093/annonc/mdt540 [DOI] [PubMed] [Google Scholar]
- 9.Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61. 10.1097/01.RVI.0000143768.60751.78 [DOI] [PubMed] [Google Scholar]
- 10.Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736-750.e4. 10.1016/j.jamcollsurg.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Lee JI, Campbell JS. Role of desmoplasia in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol 2014;61:432-4. 10.1016/j.jhep.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 12.Yamada R, Nakatsuka H, Nakamura K, et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J 1980;26:81-96. [PubMed] [Google Scholar]
- 13.Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer 2011;117:1498-505. 10.1002/cncr.25625 [DOI] [PubMed] [Google Scholar]
- 14.Shen WF, Zhong W, Liu Q, et al. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg 2011;35:2083-91. 10.1007/s00268-011-1171-y [DOI] [PubMed] [Google Scholar]
- 15.Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S425-34. 10.1016/j.jvir.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 16.Cohen MJ, Bloom AI, Barak O, et al. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol 2013;19:2521-8. 10.3748/wjg.v19.i16.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pomoni M, Malagari K, Moschouris H, et al. Post embolization syndrome in doxorubicin eluting chemoembolization with DC bead. Hepatogastroenterology 2012;59:820-5. [DOI] [PubMed] [Google Scholar]
- 18.Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer 2012;131:733-40. 10.1002/ijc.26407 [DOI] [PubMed] [Google Scholar]
- 19.Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86. 10.1245/s10434-013-3127-y [DOI] [PubMed] [Google Scholar]
- 20.Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg 2008;12:129-37. 10.1007/s11605-007-0312-y [DOI] [PubMed] [Google Scholar]
- 21.Herber S, Otto G, Schneider J, et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol 2007;30:1156-65. 10.1007/s00270-007-9032-7 [DOI] [PubMed] [Google Scholar]
- 22.Scheuermann U, Kaths JM, Heise M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol 2013;39:593-600. 10.1016/j.ejso.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Kim JH, Yoon HJ, et al. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 2011;66:322-8. 10.1016/j.crad.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Yang GW, Zhao Q, Qian S, et al. Percutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinoma. Onco Targets Ther 2015;8:1245-50. 10.2147/OTT.S84764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidensticker R, Seidensticker M, Doegen K, et al. Extensive Use of Interventional Therapies Improves Survival in Unresectable or Recurrent Intrahepatic Cholangiocarcinoma. Gastroenterol Res Pract 2016;2016:8732521. 10.1155/2016/8732521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloeckner R, Ruckes C, Kronfeld K, et al. Selective internal radiotherapy (SIRT) versus transarterial chemoembolization (TACE) for the treatment of intrahepatic cholangiocellular carcinoma (CCC): study protocol for a randomized controlled trial. Trials 2014;15:311. 10.1186/1745-6215-15-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schernthaner RE, Lin M, Duran R, et al. Delayed-Phase Cone-Beam CT Improves Detectability of Intrahepatic Cholangiocarcinoma During Conventional Transarterial Chemoembolization. Cardiovasc Intervent Radiol 2015;38:929-36. 10.1007/s00270-014-1026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong K, Khwaja A, Liapi E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006;12:2563-7. 10.1158/1078-0432.CCR-05-2225 [DOI] [PubMed] [Google Scholar]
- 29.Aliberti C, Benea G, Tilli M, et al. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol 2008;31:883-8. 10.1007/s00270-008-9336-2 [DOI] [PubMed] [Google Scholar]
- 30.Constantin M, Fundueanu G, Bortolotti F, et al. Preparation and characterisation of poly(vinyl alcohol)/cyclodextrin microspheres as matrix for inclusion and separation of drugs. Int J Pharm 2004;285:87-96. 10.1016/j.ijpharm.2004.07.025 [DOI] [PubMed] [Google Scholar]
- 31.Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol 2014;37:154-64. 10.1007/s00270-013-0632-0 [DOI] [PubMed] [Google Scholar]
- 32.Seki A, Hori S, Kobayashi K, et al. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol 2011;34:557-65. 10.1007/s00270-010-9975-y [DOI] [PubMed] [Google Scholar]
- 33.Poggi G, Amatu A, Montagna B, et al. OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol 2009;32:1187-92. 10.1007/s00270-009-9694-4 [DOI] [PubMed] [Google Scholar]
- 34.Schiffman SC, Metzger T, Dubel G, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol 2011;18:431-8. 10.1245/s10434-010-1333-4 [DOI] [PubMed] [Google Scholar]
- 35.Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarty R, Dash A, Pillai MR. Availability of yttrium-90 from strontium-90: a nuclear medicine perspective. Cancer Biother Radiopharm 2012;27:621-41. 10.1089/cbr.2012.1285 [DOI] [PubMed] [Google Scholar]
- 37.Veeze-Kuijpers B, Meerwaldt JH, Lameris JS, et al. The role of radiotherapy in the treatment of bile duct carcinoma. Int J Radiat Oncol Biol Phys 1990;18:63-7. 10.1016/0360-3016(90)90268-O [DOI] [PubMed] [Google Scholar]
- 38.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-78. 10.1097/01.RVI.0000233785.75257.9A [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. 10.1007/s00270-011-0142-x [DOI] [PubMed] [Google Scholar]
- 40.Konda A, Savin MA, Cappell MS, et al. Radiation microsphere-induced GI ulcers after selective internal radiation therapy for hepatic tumors: an underrecognized clinical entity. Gastrointest Endosc 2009;70:561-7. 10.1016/j.gie.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 41.Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. 10.1016/j.jvir.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam MG, Banerjee S, Louie JD, et al. Root cause analysis of gastroduodenal ulceration after yttrium-90 radioembolization. Cardiovasc Intervent Radiol 2013;36:1536-47. 10.1007/s00270-013-0579-1 [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Lago I, Carretero C, Herráiz M, et al. Long-term follow-up study of gastroduodenal lesions after radioembolization of hepatic tumors. World J Gastroenterol 2013;19:2935-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 2008;113:2119-28. 10.1002/cncr.23818 [DOI] [PubMed] [Google Scholar]
- 45.Soydal C, Kucuk ON, Bilgic S, et al. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med 2016;30:29-34. 10.1007/s12149-015-1026-y [DOI] [PubMed] [Google Scholar]
- 46.Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 2015;41:120-7. 10.1016/j.ejso.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol 2015;22:3102-8. 10.1245/s10434-014-4365-3 [DOI] [PubMed] [Google Scholar]
- 48.Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. 10.1245/s10434-009-0777-x [DOI] [PubMed] [Google Scholar]
- 49.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. 10.1007/s00270-012-0463-4 [DOI] [PubMed] [Google Scholar]
- 50.Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25:256-65. 10.1016/j.jvir.2013.10.056 [DOI] [PubMed] [Google Scholar]
- 51.Haug AR, Heinemann V, Bruns CJ, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging 2011;38:1037-45. 10.1007/s00259-011-1736-x [DOI] [PubMed] [Google Scholar]
- 52.Filippi L, Pelle G, Cianni R, et al. Change in total lesion glycolysis and clinical outcome after (90)Y radioembolization in intrahepatic cholangiocarcinoma. Nucl Med Biol 2015;42:59-64. 10.1016/j.nucmedbio.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 53.Hickey R, Mulcahy MF, Lewandowski RJ, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys 2014;88:1025-31. 10.1016/j.ijrobp.2013.12.040 [DOI] [PubMed] [Google Scholar]
- 54.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 2015;111:213-20. 10.1002/jso.23781 [DOI] [PubMed] [Google Scholar]