Abstract
The aim of present study was to evaluate antioxidant efficacy of pussy willow extract (PWE) and essential oil (PWEO) in stabilization of sunflower oil (SFO) during ambient storage (60 days at 25°C). Initially, total phenolic (TP) and total flavonoid (TF) contents were evaluated. Then, PWE, PWEO, and TBHQ were added to SFO. Peroxide value (PV), carbonyl value (CV), total polar compound (TPC), acid value (AV), and Oxidative stability index (OSI) were measured every 15 days. The results showed that PWE had higher TP and TF than PWEO (TP: 966.72 mg GAE/g and 355.8472 mg GAE/g, respectively; TF: 619.45 mg/100 g and 195.45 mg/100 g, respectively). Furthermore, according to all stabilization parameters, PWE had higher antioxidant efficacy followed by TBHQ, PWEO, and control, respectively. Therefore, PWE has antioxidant activity and it may be recommended as natural strong antioxidants to suppress lipid oxidation.
Keywords: antioxidant activity, oxidative stability, pussy willow, sunflower oil, total phenolic content
Introduction
Sunflower oil (SFO) is an edible oil has been widely used due to its nutritional and medicinal value. sunflower oil is one source of linoleic acid ( 9 cis, 12 cis oxtadecadienoic) which has so many health beneficial effects (Hashemi et al. 2015). Autoxidation or oxidative rancidity is the major cause of quality losses in crude as well as refined oils during storage (Asnaashari et al. 2014a,b). When the crude and refined oils are exposed to factors such as heat, high temperatures, light, trace metals, or oxygen, the problem get more exacerbated (Crapiste et al. 1999; Sikwese and Duodu 2007; Farahmandfar et al. 2015a).
The use of antioxidants is the most appropriate way of stabilizing oils, preventing the autoxidation or oxidative rancidity, and protecting oils from the damages inflicted by free radicals (Farhoosh et al. 2016). The addition of synthetic antioxidants has been one of the most effective and popular method to prevent oxidative rancidity and development of off‐flavors (Eshghi et al. 2014). Investigations have demonstrated that synthetic antioxidants are toxic and carcinogenic for humans (Iqbal et al. 2008). Recently, there has been increased interest in identifying potential sources in order to obtain natural antioxidants (Poiana 2012; Farahmandfar et al. 2015b). Essential oil (EO) and extracts from spices, fruits, herbs, and hull contain effective compounds that prevent from the undesirable oxidative process in foodstuff (Asnaashari et al. 2015a).
Salix aegyptiaca L. (pussy willow) is generally cultivated in some provinces of Iran for hedge and ornamental purposes. The distillate obtained from male inflorescences of plants, with the common local name of “Araghe Bidmeshk” and English name of “Egyptian willow distillate,” in most parts of Iran have long been applied in folk medicine as cardiotonic (Sonboli et al. 2010). The aqueous extract and EO of S. aegyptiaca L. are being used in confectionery and flavorful syrups. In Iranian traditional medicine, S. aegyptiaca has been employed as laxative, cardioprotective, sedative, hypnotic, somnolent, aphrodisiac, orexigenic, carminative, gastroprotectant, anthelmintic, and vermifuge (Karimi et al. 2011). A number of chemical constituents such as flavonoids and volatile substances have been isolated from different parts of the plant (Enayat and Banerjee 2009; Karimi et al. 2011). From current pharmaceutical studies, additional pharmaceutical applications of S. aegyptiaca have revealed antioxidant, anti‐inflammatory, analgesic, anxiolytic, and antihypercholesterolemic effects among others (Hifnawy et al. 2010; Sonboli et al. 2010; Karimi et al. 2011; Rabbani et al. 2011).
Although previous studies have presented the antioxidant properties of pussy willow (PW) extract and EO, there are no reports on the antioxidant efficacy for the stabilization of SFO been shown so far. The purpose of this study was to estimate the antioxidant efficacy of PW flower extract and EO against oil oxidative rancidity and to compare their antioxidant activity with commercially available antioxidant (TBHQ).
Materials and Methods
Materials
Refined, bleached, deodorized SFO without any synthetic antioxidants was obtained from Ghoncheh Co. (Sari, Iran). The flowers of PW were purchased from the Ghamsar botanical garden at Kashan, Iran, during March 2014, and authenticated by the Medicinal Plant Incubator Center (Islamic Azad University, Ayatollah Amoli Branch, Amol, Iran). They were washed, air dried in the shade, and then powdered utilizing an electric device and stored in refrigerator (4°C) until use. TBHQ was prepared and purchased from Sigma‐Aldrich (St. Louis, MO). All other chemicals were of analytical grade and purchased from Merck Co., Frankfurt, Germany.
Preparation of PWE and PWEO
The solvent (ethanol) was added to the powdered PW in the ratio of 10:1 (w:v) and the resulting mixture was shaken overnight. After 24 h, for separating the PW particles, the extract was filtered through Whatman No. 42 filter paper. The solvent was completely evaporated in an oven at 40°C. Finally, the obtained extract was stored in a dark container in refrigerator (4°C) until use (Esmaeilzadeh Kenari et al. 2014). EOs were extracted by hydrodistillation from the powdered PW by the Clevenger‐type apparatus, and the obtained oil was stored in a dark container in refrigerator (4°C) until use.
Total phenolic content
The total phenolic (TP) content of PW extracts (PWE) and PWEO was determined according to the Folin–Ciocalteu reagent as described by Pourmorad et al. (2006) and the results were expressed as mg/g gallic acid equivalents (GAE). At a concentration of 1 mg/mL, PWE and PWEO were prepared in their own solvents, and 0.5 mL of each sample was mixed with 2.5 mL of a 10‐fold diluted Folin–Ciocalteu reagent and 2 mL of 7.5% sodium carbonate solution. After the samples were kept for 30 min at room temperature, the absorbance was measured at 760 nm in a UV‐Vis spectrophotometer (PG Instrument, T80, London, England).
Determination of total flavonoid content
The total flavonoid (TF) content of PWE and PWEO was quantified according to the method described by Dewanto et al. (2002) and the results were determined as catechin equivalents (mg/100 g of dry weight). At a concentration of 1 mg/mL, PWE and PWEO were diluted with water (4 mL) in a 10 mL volumetric flask. Initially, 5% NaNO2 solution (0.3 mL) was added to each volumetric flask; at 5 min, 10% AlCl3 (0.3 mL) was added; and at 6 min, 1.0 mol/L NaOH (2 mL) was added. Water (2.4 mL) was then added to the reaction flask and mixed well. Absorbance of the reaction mixture was read at 510 nm.
Preparation of SFO samples for oxidative stability determination
PWE and PWEO were added to SFO at 1200 ppm. Synthetic antioxidant (TBHQ) was employed at a limit of 100 ppm to compare the efficacy of natural antioxidants (Fki et al. 2005). All the samples (120 mL each) were placed in dark brown colored reagent bottles with narrow necks, without stoppers, and stored at ambient conditions (25°C for 60 days) (Anwar et al. 2007). Control samples were also placed under the same storage conditions. Samples (20 g) were removed periodically every 0, 15, 30, 45, and 60 days for analysis. Immediately after storage period, oil samples were withdrawn for triplicate analysis. The oils were sampled for each measurement from separate bottles.
Analytical procedures
Peroxide value (PV) was measured according to the spectrophotometric method of the International Dairy Federation as described by Shantha and Decker (1994). The carbonyl value (CV) of the oils was measured according to the method developed by Endo et al. (2001), using 2‐propanol and 2,4‐decadienal as solvent and standard, respectively. AOCS Official Method Cd 3d‐63 (AOCS 1990) was used to determine the acid value (AV). Total polar compounds (TPC) content was determined according to the economical micromethod developed by Schulte (2004). Oxidative stability index (OSI) is a method whereupon air passes through a lipid under a specific temperature, at which point volatile acids decomposed from lipid peroxidation are driven out by the air and subsequently dissolved in water, thereby increasing its conductivity. The conductivity of the water is constantly measured, and the OSI value is defined as the hours required for the rate of conductivity to reach a predetermined level (Liu et al. 2014).
Statistical analysis
All analyses were performed in triplicate and data reported as means ± standard deviation (SD). Statistical analyses were conducted using SPSS (Statistical Program for Social Sciences, SPSS Corporation, Chicago, IL) version 18.0 for Windows. Data were subjected to analysis of variance. Significant differences (P < 0.05) were calculated using Duncan's multiple range tests.
Results and Discussion
TP and TF contents
Phenolic component is one of the major groups of phytochemicals ubiquitously present in the plant kingdom, and they have received much attention in the recent years due to their potential health benefits as an antioxidant and protective agents against cancer and several other diseases (Plumb et al. 1997).
Flavonoids are the most abundant polyphenols in plants, and they have been shown to have potent antioxidant and anticancer activities (Adom and Liu 2002; Dykes and Rooney 2007).
Results of the TP in PWE and PWEO are presented in Table 1. The differences between the content of TP between PWE and PWEO were statistically significant (P < 0.05). The PWE gives higher content of TP (966.72 ± 44.27 mg GAE/g sample) than TP of PWEO (355.84 ± 27.53 mg GAE/g sample). Similar results were also observed related to TF (Table 1). These data suggested that PWE is valuable source of phenolic and flavonoid compounds that might be extracted and used in the food and drug industries.
Table 1.
Total phenolic (TP) and total flavonoid (TF) contents of pussy willow extract and PW essential oil
| Extraction | Total phenolic content(mg GAE/g samples ± SD) | Total flavonoid content(mg/100 g samples ± SD) |
|---|---|---|
| Pussy willow extract | 966.72 ± 44.27a | 619.45 ± 12.2a |
| Pussy willow essential oil | 355.84 ± 27.53b | 195.45 ± 9.12b |
Means ± SD (standard deviation) within a column with the same lowercase letters are not significantly different at p<0.05.
A linear correlation between the antioxidant activity and polyphenolic contents of the plant extracts has been reported by other researchers (Luximon‐Ramma et al. 2002; Karimi et al. 2014). Kamkar et al. (2010), which showed that Mentha pulegium extracts had more antioxidant activity than M. pulegium EO. They stated that the lower antioxidative activity of the Mentha pulegium EO can be due to the lack of some of the different antioxidants in the EO.
Stability of SFOs as affected by the addition of PWE and PWEO
Peroxide value and carbonyl value
PV was used as indicators for the primary oxidation of oil and lipids. Hydroperoxide is the primary oxidation product produced as a result of lipid oxidation (Asnaashari et al. 2015b). The quality of the oil may be decreased if it breaks down into nonvolatile and volatile secondary products (Poiana 2012; Mei et al. 2014). By increasing the storage period time, a continuous increase in PV was observed in all the samples (Fig. 1A). The rise of PV amount was very slow, at the beginning, but it started increasing by 15th day of storage and continued to increase further with the increase in storage period, reaching a maximum value after 60 days storage. A significant difference (P < 0.05) in PV was observed between the control and SFO samples containing PWE and PWEO, which slowed the rate of peroxide formation revealing good antioxidant efficacy in stabilizing oil (Ben‐Ali et al. 2014). Highest PV was observed for control followed by SFO containing PWEO, TBHQ, and PWE, respectively. Such pattern was presented by Kamkar et al. (2010) on the methanol extract and EO of Mentha pulegium compared to the butylated hydroxytoluene (BHT) when added to SFOs.
Figure 1.
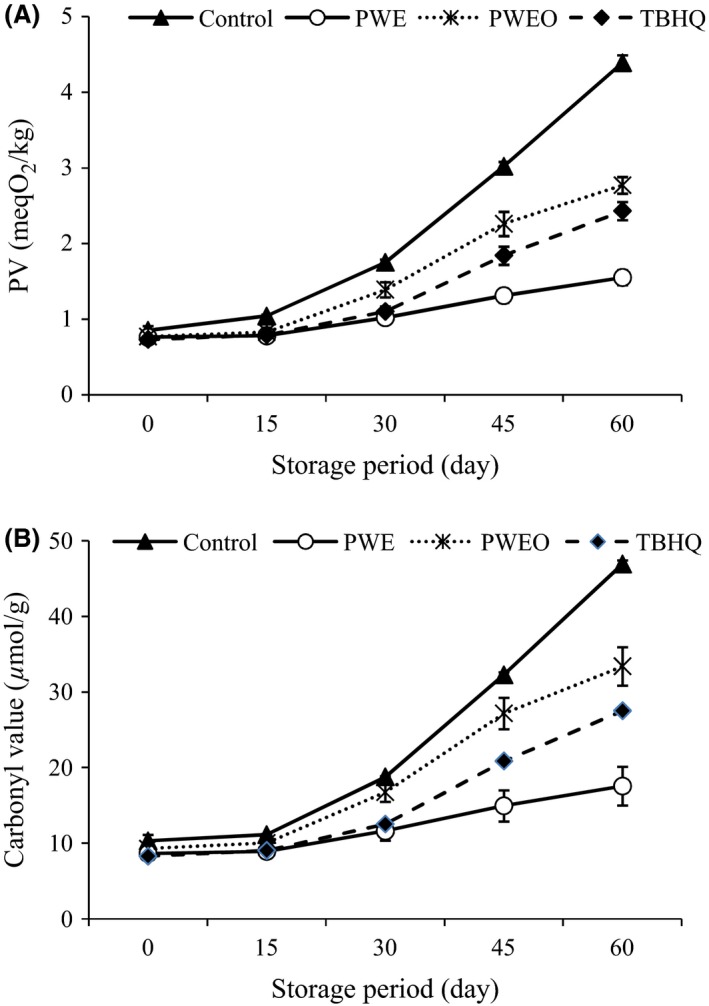
Effect of pussy willow extract (PWE) and PW essential oil (PWEO) on peroxide value (A) and carbonyl value (B) of sunflower oil during ambient storage. Error bars show the variations of three determinations in terms of standard deviation.
According to Woyewoda et al. (1986), primary oxidation products (peroxides) are converted into secondary products that contain carbonyl groups. These compounds are more stable than hydroperoxides and are considered to be a good index of oxidative changes in lipids. A similar trend was also observed about CV in all treatments (Fig. 1B). Thus, the lowest PV and CV were observed in SFO containing PWE. It may be attributed as a higher antioxidant ability of PWE which is related to its polyphenol component. The formation of fatty acid free radicals, which react with or absorb oxygen in the autoxidation process, was prevented by them. This delays the onset of the autoxidative process in oil (Turhan et al. 2009). These results are in agreement with that described previously (Iqbal et al. 2008; Ben‐Ali et al. 2014; Mei et al. 2014).
Acid value
Acid value has become a good indicator of the quality. AV is the number that expresses, in milligrams, the quantity of KOH (in mg) necessary to neutralize free fatty acids in 1 g of oil (Ban and Kang 2014). AV contents of the SFO as affected by the TBHQ, PWE, and PWEO during ambient condition are given in Figure 2. By increasing the storage period, a continuous increase in AV was observed in all the samples. The steady increase in the AV can be attributed partly to hydrolysis of triacylglycerols and partly to the component carboxyl groups present in autoxidative process (Petukhov et al. 1999; Nasirullah 2001). Control exhibited the highest AV at all the stages of analyses during storage. There was no statistically significant difference between SFOs that contain PWEO and TBHQ (P > 0.05). The oil samples stabilized with PWE showed lowest levels of AV compared to the other, which suggests the superiority of the PWE over the other antioxidants, such as PWEO and TBHQ, in delaying oxidation in SFO.
Figure 2.
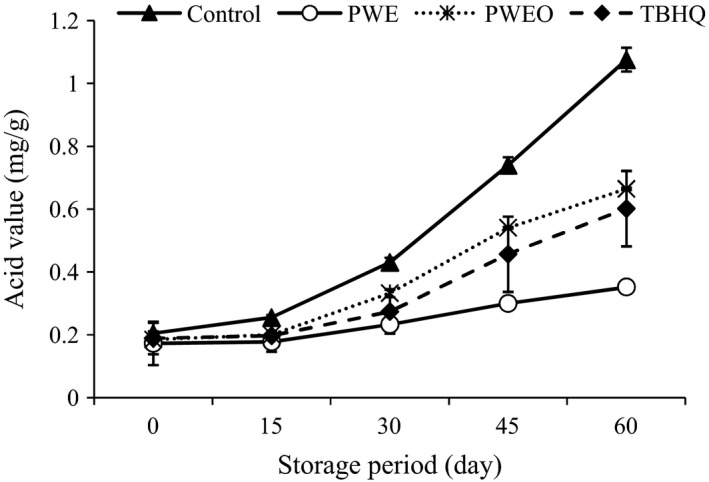
Effect of pussy willow extract (PWE) and PW essential oil (PWEO) on acid value of sunflower oil during ambient storage.
Total polar compounds
Polar compounds is an important indicator of oil quality of used oils, giving information of the total amount of newly formed compounds having higher polarity than that of triacylglycerols (Li et al. 2015). The TPC of treatments during the ambient storage are presented in Figure 3. The TPC content increased until the end of the storage period (in all samples). The TPC content of SFO contain antioxidants significantly (P < 0.05) lower than the control samples during the storage time. Literature has shown that the fractions of TPC isolated from oxidized oils are toxic to laboratory animals (Pantzaris 1998). Therefore, it has been purposed that frying oils containing more than 24–27% of TPC content should be discarded (Firestone 1993). Only the control among all the samples reached the discarding range of TPC content during storage condition.
Figure 3.
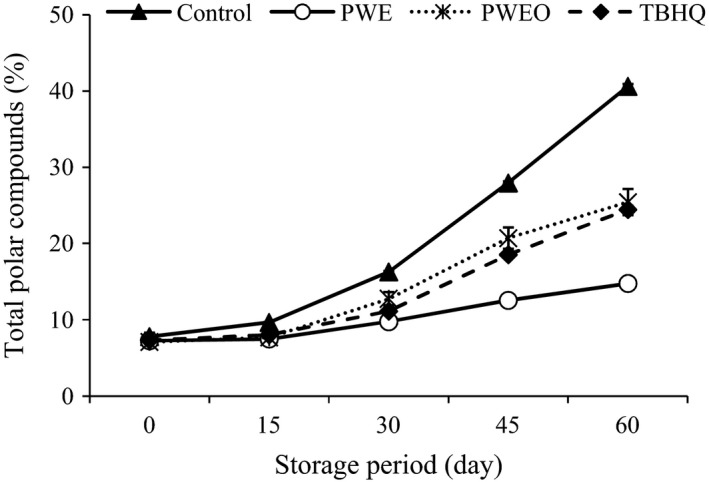
Effect of pussy willow extract (PWE) and PW essential oil (PWEO) on total polar compounds of sunflower oil during ambient storage.
The lowest TPC content at the end of storage period was observed in SFO containing PWE. Farhoosh and Kenari (2009) stated that canola oils containing sesame and rice bran oils were more stable than the canola oils without additives during frying, based on TPC formation.
Oxidative stability index
The OSI is an American Oil Chemists' Society approved method that determines the relative resistance of oils to oxidation. Oxidative stability instrument can be employed to determine OSI of oil samples and it is the commercial piece of equipment for the automated determination of oxidative resistance as well as it is well‐known by the fats and oils industries (Shahidi and Zhong 2005; Senanayake 2013). Changes in the OSI of the SFO affected by the PWE, PWEO, and TBHQ during the ambient storage are shown in Figure 4. A continued decrease in OSI with the increase in storage period was observed in all the samples. But in control, the rate of decrease was higher than others. There was no statistically significant difference between SFOs containing PWE and TBHQ (P > 0.05). The rate of decrease in OSI throughout the storage period indicates higher efficiency of PWE for the stabilization of SFO than PWEO.
Figure 4.
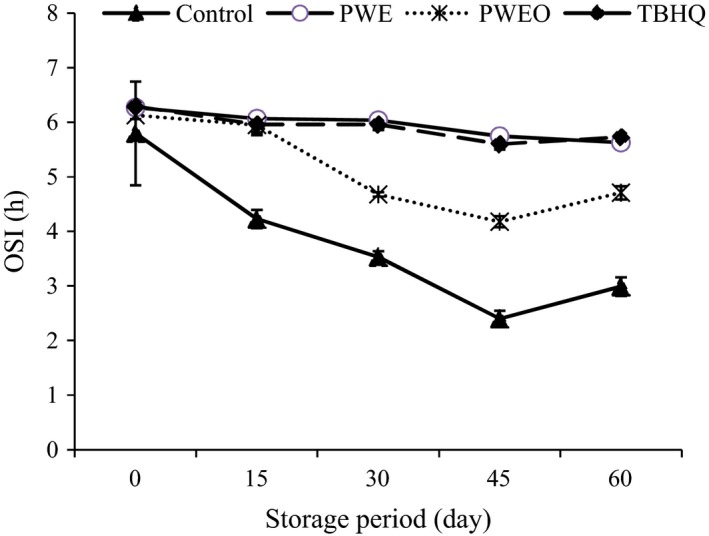
Effect of pussy willow extract (PWE) and PW essential oil (PWEO) on oxidative stability index of sunflower oil during ambient storage.
Conclusion
The results of the present study apparently demonstrated that the PWE had high TP and TF contents. Moreover, PWE exhibited strong antioxidant activity in stabilizing SFO during ambient storage, which was almost more effective than the antioxidant activity of synthetic antioxidants (TBHQ) as well as PWEO. Therefore, it is suggested that the PWE could be safely used for the stabilization of food systems instead of synthetic antioxidants.
Conflict of Interest
None declared.
References
- Adom, K. K. , and Liu R. H.. 2002. Antioxidant activity of grains. J. Agric. Food Chem. 50:6182–6187. [DOI] [PubMed] [Google Scholar]
- Anwar, F. , Siddiq A., Iqbal S., and Asi M. R.. 2007. Stabilization of sunflower oil with Moringa oleifera leaves under ambient storage. J. Food Lipids 14:35–49. [Google Scholar]
- Aocs, O. 1990. Tentative methods of the American Oil Chemists' Society. American Oil Chemists Society Press, Champaign. [Google Scholar]
- Asnaashari, M. , Farhoosh R., and Sharif A.. 2014a. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil‐in‐water emulsion. Food Chem. 159:439–444. [DOI] [PubMed] [Google Scholar]
- Asnaashari, M. , Tajik R., and Khodaparast M. H. H.. 2014b. Antioxidant activity of raspberry (Rubus fruticosus) leaves extract and its effect on oxidative stability of sunflower oil. J. Food Sci. Technol. 52:5180–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaashari, E. , Asnaashari M., Ehtiati A., and Farahmandfar R.. 2015a. Comparison of adaptive neuro‐fuzzy inference system and artificial neural networks (MLP and RBF) for estimation of oxidation parameters of soybean oil added with curcumin. J. Food Meas. Charact. 9:215–224. [Google Scholar]
- Asnaashari, M. , Hashemi B., Mohammad S., Mehr H. M., and Asadi Yousefabad S. H.. 2015b. Kolkhoung (Pistacia khinjuk) Hull oil and Kernel oil as antioxidative vegetable oils with high oxidative stability and nutritional value. Food Technol. Biotechnol. 53:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban, G.‐H. , and Kang D.‐H.. 2014. Effects of gamma irradiation for inactivating Salmonella Typhimurium in peanut butter product during storage. Int. J. Food Microbiol. 171:48–53. [DOI] [PubMed] [Google Scholar]
- Ben‐Ali, M. , Dhouib K., Damak M., and Allouche N.. 2014. Stabilization of sunflower oil during accelerated storage: use of basil extract as a potential alternative to synthetic antioxidants. Int. J. Food Prop. 17:1547–1559. [Google Scholar]
- Crapiste, G. H. , Brevedan M. I., and Carelli A. A.. 1999. Oxidation of sunflower oil during storage. J. Am. Oil Chem. Soc. 76:1437–1443. [Google Scholar]
- Dewanto, V. , Wu X., Adom K. K., and Liu R. H.. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50:3010–3014. [DOI] [PubMed] [Google Scholar]
- Dykes, L. , and Rooney L.. 2007. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 52:105–111. [Google Scholar]
- Enayat, S. , and Banerjee S.. 2009. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 116:23–28. [Google Scholar]
- Endo, Y. , Li C. M., Tagiri‐Endo M., and Fujimoto K.. 2001. A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2‐propanol as a solvent. J. Am. Oil Chem. Soc. 78:1021–1024. [Google Scholar]
- Eshghi, N. , Asnaashari M., Haddad Khodaparast M. H., and Hosseini F.. 2014. Evaluating the potential of natural curcumin for oxidative stability of soybean oil. Nat. Prod. Res. 28:1375–1378. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh Kenari, R. , Mohsenzadeh F., and Amiri Z. R.. 2014. Antioxidant activity and total phenolic compounds of Dezful sesame cake extracts obtained by classical and ultrasound‐assisted extraction methods. Food Sci. Nutr. 2:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmandfar, R. , Asnaashari M., and Sayyad R.. 2015a. Comparison antioxidant activity of Tarom Mahali rice bran extracted from different extraction methods and its effect on canola oil stabilization. J. Food Sci. Technol. 52:6385–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmandfar, R. , Safari R., Ahmadi Vavsari F., and T. Bakhshandeh . 2015b. The effect of Ajwain (Trachyspermum ammi) extracted by ultrasound‐assisted solvent on quality properties of silver carp (Hypophthalmichthys molitrix) surimi stored at 4C. J. Food Process. Preserv. 40:291–297. [Google Scholar]
- Farhoosh, R. , and Kenari R. E.. 2009. Anti‐rancidity effects of sesame and rice bran oils on canola oil during deep frying. J. Am. Oil Chem. Soc. 86:539–544. [Google Scholar]
- Farhoosh, R. , Johnny S., Asnaashari M., Molaahmadibahraseman N., and Sharif A.. 2016. Structure–antioxidant activity relationships of o‐hydroxyl, o‐methoxy, and alkyl ester derivatives of p‐hydroxybenzoic acid. Food Chem. 194:128–134. [DOI] [PubMed] [Google Scholar]
- Firestone, D. 1993. Worldwide regulation of frying fats and oils. Inform‐Int. News Fats Oils Related Mater. 4:1366–1386. [Google Scholar]
- Fki, I. , Allouche N., and Sayadi S.. 2005. The use of polyphenolic extract, purified hydroxytyrosol and 3, 4‐dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: a potential alternative to synthetic antioxidants. Food Chem. 93:197–204. [Google Scholar]
- Hashemi, S. M. B. , Khaneghah A. M., Tavakolpour Y., Asnaashari M., and Mehr H. M.. 2015. Effects of ultrasound treatment, UV irradiation and Avishan‐e‐Denaei essential oil on oxidative stability of sunflower oil. J. Essent. Oil Bearing Plants 18:1083–1092. [Google Scholar]
- Hifnawy, M. S. , Ammar N. M., and Karawya M. S.. 2010. Phytochemical study and evaluation of the anti‐inflammatory activity of some medicinal plants growing in Egypt. Med. J. Islamic World Acad. Sci. 18:139–150. [Google Scholar]
- Iqbal, S. , Haleem S., Akhtar M., Zia‐Ul‐haq M., and Akbar J.. 2008. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Res. Int. 41:194–200. [Google Scholar]
- Kamkar, A. , Javan A. J., Asadi F., and Kamalinejad M.. 2010. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food Chem. Toxicol. 48:1796–1800. [DOI] [PubMed] [Google Scholar]
- Karimi, I. , Hayatgheybi H., Kamalak A., Pooyanmehr M., and Marandi Y.. 2011. Chemical composition and effect of an essential oil of Salix aegyptiaca L., Salicaceae, (musk willow) in hypercholesterolemic rabbit model. Revista Brasileira de Farmacognosia 21:407–414. [Google Scholar]
- Karimi, E. , Mehrabanjoubani P., Keshavarzian M., Oskoueian E., Jaafar H. Z., and Abdolzadeh A.. 2014. Identification and quantification of phenolic and flavonoid components in straw and seed husk of some rice varieties (Oryza sativa L.) and their antioxidant properties. J. Sci. Food Agric. 94:2324–2330. [DOI] [PubMed] [Google Scholar]
- Li, J. , Cai W., Sun D., and Liu Y.. 2015. A quick method for determining total polar compounds of frying oils using electric conductivity. Food Anal. Methods 9:1444–1450. [Google Scholar]
- Liu, P. , Kerr B., Chen C., Weber T., Johnston L., and Shurson G.. 2014. Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J. Anim. Sci. 92:2950–2959. [DOI] [PubMed] [Google Scholar]
- Luximon‐Ramma, A. , Bahorun T., Soobrattee M. A., and Aruoma O. I.. 2002. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 50:5042–5047. [DOI] [PubMed] [Google Scholar]
- Mei, W. S. C. , Ismail A., Esa N. M., Akowuah G. A., Wai H. C., and Seng Y. H.. 2014. The effectiveness of rambutan (Nephelium lappaceum L.) extract in stabilization of sunflower oil under accelerated conditions. Antioxidants 3:371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirullah. 2001. Development of deep frying edible vegetable oils. J. Food Lipids 8:295–304. [Google Scholar]
- Pantzaris, T. 1998. Comparison of monounsaturated and polyunsaturated oils in continuous frying. Grasas Aceites 49:319–325. [Google Scholar]
- Petukhov, I. , Malcolmson L., Przybylski R., and Armstrong L.. 1999. Frying performance of genetically modified canola oils. J. Am. Oil Chem. Soc. 76:627–632. [Google Scholar]
- Plumb, G. W. , Price K. R., Modes M. J., and Williamson G.. 1997. Antioxidant properties of the major polyphenolic compounds in broccoli. Free Radic. Res. 27:429–435. [DOI] [PubMed] [Google Scholar]
- Poiana, M.‐A. 2012. Enhancing oxidative stability of sunflower oil during convective and microwave heating using grape seed extract. Int. J. Mol. Sci. 13:9240–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmorad, F. , Hosseinimehr S., and Shahabimajd N.. 2006. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 5:12–15. [Google Scholar]
- Rabbani, M. , Vaseghi G., Sajjadi S., and Amin B.. 2011. Persian herbal medicines with anxiolytic properties. Journal of Medicinal Plants. 10:7–11. [Google Scholar]
- Schulte, E. 2004. Economical micromethod for determination of polar components in frying fats. Eur. J. Lipid Sci. Technol. 106:772–776. [Google Scholar]
- Senanayake, S. N. 2013. Green tea extract: chemistry, antioxidant properties and food applications–a review. J. Funct. Foods 5:1529–1541. [Google Scholar]
- Shahidi, F. , and Zhong Y.. 2005. Lipid oxidation: measurement methods. Bailey's Ind. Oil Fat Prod. John Wiley & Sons, Inc; 357–385. [Google Scholar]
- Shantha, N. C. , and Decker E. A.. 1994. Rapid, sensitive, iron‐based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 77:421–424. [PubMed] [Google Scholar]
- Sikwese, F. , and Duodu K. G.. 2007. Antioxidant effect of a crude phenolic extract from sorghum bran in sunflower oil in the presence of ferric ions. Food Chem. 104:324–331. [Google Scholar]
- Sonboli, A. , Mojarrad M., Ebrahimi S. N., and Enayat S.. 2010. Free radical scavenging activity and total phenolic content of methanolic extracts from male inflorescence of Salix aegyptiaca grown in Iran. Iran. J. Pharm. Res. 9:293. [PMC free article] [PubMed] [Google Scholar]
- Turhan, S. , Sagir I., and Temiz H.. 2009. Oxidative stability of brined anchovies (Engraulis encrasicholus) with plant extracts. Int. J. Food Sci. Technol. 44:386–393. [Google Scholar]
- Woyewoda, A. , Shaw S., Ke P., and Burns B.. 1986. 143 Recommended laboratory methods for assessment of fish quality, numeral 1448: canadian technical report of fisheries and aquatic sciences. Minister of Supply and Services Canada, Halifax, Canada. [Google Scholar]


