Abstract
Background
Pulmonary regurgitation (PR) is a common residual lesion and major determinant of outcome following surgical repair for tetralogy of Fallot (TOF). We sought to longitudinally study a previously described echocardiographic index as a correlate of PR measured by cardiac magnetic resonance imaging (CMR).
Methods and Results
We conducted a retrospective longitudinal study of patients with baseline and follow up echocardiogram and CMR. The baseline studies were obtained as part of a research protocol, while the follow up studies were performed for clinical purposes. On echocardiogram, the ratio of diastolic and systolic time-velocity integrals (DSTVI) in the main pulmonary artery was calculated. The Wilcoxon matched-pairs signed-rank test was used to test for individual changes in PR on Echocardiogram and CMR. A linear regression of pulmonary valve regurgitant fraction (RF) was fit on DSTVI to identify clinically meaningful cut points of DSTVI.
Thirty-five subjects were included, age at follow up 18.3±3.5 years. The follow-up between consecutive CMRs was a median time of 60 months (interquartile range 46–73). There was a moderate correlation between DSTVI and PR measured as RF by CMR (r=0.62, p=0.0001). A CMR RF of 20% and 40% (the boundaries between mild/moderate and moderate/severe PR) corresponded with DSTVI of 0.52 and 0.79 (95% CI: 0.39; 0.66, and 0.69; 89), respectively. There was no significant change in either DSTVI (p=0.61) or PR (p=0.89) from baseline to follow up.
Conclusions
This study lends further credence to the DSTVI as an accurate reflection of PR. This index might become helpful in the routine echocardiographic assessment of PR. Further studies are needed to determine whether changes in RF by CMR result in changes in DSTVI.
Introduction
Repaired tetralogy of Fallot (TOF) carries excellent results with a significant portion of patients surviving into adulthood1–4. However, surgical repair often requires disruption of the pulmonary valve for adequate relief of obstruction, such that patients are usually left with significant residual pulmonary regurgitation (PR). Long standing PR carries long-term implications, including progressive right ventricular (RV) dilation and dysfunction. In addition, patients with TOF experience a high rate of re-intervention, arrhythmias and a discrete but constant risk of sudden death in adulthood4–7. Objective assessment of PR and RV function by echocardiogram remains challenging. Cardiac magnetic resonance (CMR) is currently the gold standard for evaluating PR and RV performance parameters, and is used to determine the need for re-intervention, such as pulmonary valve replacement to halt disease progression associated with PR8–10. However, echocardiography is more frequently used in the routine outpatient follow up, and therefore attempts have been made to describe objective measures of PR and RV function by this imaging modality. A novel Doppler derived echocardiographic measure, the ratio of the diastolic to systolic time-velocity integral (DSTVI), showed correlation with PR as measured by CMR11. We sought to examine this correlation longitudinally and to determine whether changes in PR by CMR corresponded to changes in DSTVI.
Methods
We conducted a retrospective longitudinal study of subjects enrolled as part of a cross sectional study that subsequently underwent standard of care echocardiograms and CMR performed at most six months apart. Results from the original study are published elsewhere.11,12 This study was approved by The Children’s Hospital of Philadelphia Institutional Review Board for the Protection of Human Subjects.
Study Cohort
We identified subjects with surgically repaired TOF who had previously participated in a cross sectional study protocol and for whom the DSTVI was calculated on the research echocardiogram and the pulmonary regurgitant fraction on the research CMR11. Subjects from the study cohort who subsequently had clinically indicated echocardiogram and CMR between May 05, 2009 and June 7, 2013 were included in this study, resulting in 65 eligible subjects. All types of TOF surgical repair were included (transannular patch, RV-PA conduit, VSD closure and valve-sparing repairs). Exclusion criteria included echocardiogram and CMR performed more than six months apart from each other and patients who underwent interim pulmonary valve replacement, interventions in the RV outflow tract (RVOT) or absence of continuous wave Doppler (CW) interrogation in the main pulmonary artery, resulting in 35 subjects for analysis. Demographic, anatomic and surgical information were available from the original cross sectional study database.
Echocardiogram
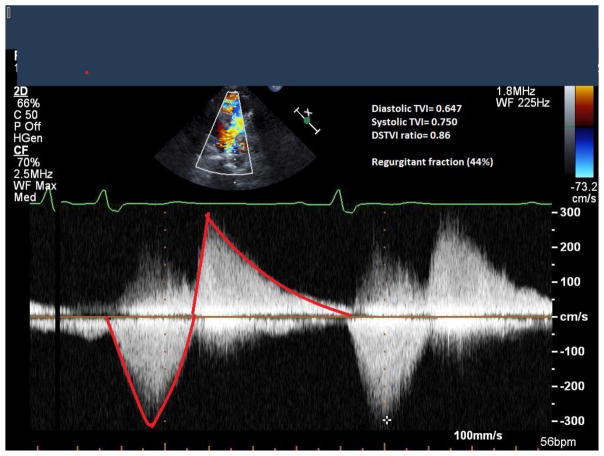
For follow up DSTVI, we reviewed available images from subsequent echocardiograms, performed as part of standard of care according to our institution’s laboratory protocol, using the Phillips IE33 machine (Phillips, Andover, MA, USA). The Doppler-derived time velocity integral has been used as a measure of flow13. From the short axis view, the continuous-wave Doppler tracings from the proximal to mid main pulmonary artery were used to obtain offline measurements of the time velocity integrals of diastolic and systolic flows performed using Syngo Dynamics software (Siemens, Ann Arbor, MI, USA), as previously described.11 Three sets of tracings were obtained and averaged (Figure 1). The ratio between the regurgitant (diastolic) time–velocity integral to the antegrade (systolic) time-velocity integral was called DSTVI. RVOT obstruction was present if the peak velocity was ≥ 3 m/sec on echocardiogram. Those where only pulsed-wave tracings were available were excluded form this analysis.
Figure 1. Diastolic to systolic time-velocity integral ratio obtained from the continuous wave Doppler tracing of the main pulmonary artery.
Pulsed-wave Doppler tracing in the main pulmonary artery. Diastolic flow was divided by the systolic flow to calculate the DSTVI.
CMR
We reviewed results from standard of care CMR studies performed according to our institution’s protocol. CMR variables assessed included pulmonary artery regurgitant fraction (RF), RV end diastolic volume (RVEDVi), RV end systolic volume (RVESVi) and RV ejection fraction (RVEF). Phase contrast velocity mapping with a flow-sensitive gradient-echo sequence was performed in the main pulmonary artery to assess the regurgitant fraction. PR was graded as mild if the RF on CMR was <=20%, moderate if it was between 20% and 40%, and severe if it was >=40%. Published norms for age and sex were used to derive Z scores to grade right ventricular dilation14,15. Follow up CMRs were performed without sedation. This study did not assess the antegrade end-diastolic flow in the main pulmonary artery (so-called “restrictive physiology”) because of its controversial association with pulmonary regurgitation.
Statistical Analysis
Continuous variables were described using mean and SD or median with interquartile ranges when appropriate. Categorical variables were described using count and percentages. Pearson correlation was calculated between DSTVI on echocardiogram and RF on CMR, when these variables were normally distributed. Paired t-test was used to detect any differences in pulmonary valve regurgitation and DSTVI at baseline and follow up. The Wilcoxon matched-pairs signed-ranks test was used to test whether individual changes in baseline to follow up PR (i.e. increase, decrease or unchanged) corresponded to similar changes in baseline to follow up DSTVI. This was used to ensure that any changes pulmonary regurgitation from baseline to follow up were not falsely identified due to wide confidence intervals. A linear regression of RF was fit on DSTVI to identify clinically meaningful cut points of DSTVI. Multivariable regression was used to adjust for potential confounders of the association between RF and DSTVI, such as right ventricular outflow tract obstruction. Statistical significance was reached if P values were <0.05 (2-sided tests). All analyses were performed using STATA statistical software version 11.2, College Station, TX.
Results
Patient characteristics
A total of 38 patients had follow up echocardiogram and CMR and met inclusion criteria, of which three were excluded due to pulsed wave Doppler tracings only, therefore 35 subjects contributed to this analysis. The median follow up time from initial to last CMR was 60 months (interquartile range 46; 73). The average time between follow up echocardiogram and CMR was 18±89 days. Most subjects were male (69%) and white (89%). On echocardiogram, most patients had an unobstructed right ventricular outflow tract (Table 1). The follow up group included subjects that had CMR for clinical purposes. Those that underwent VSD closure only or valve-sparing outflow patch were not represented in this cohort, possibly because this group does not require surveillance CMR.
Table 1.
General Patient Characteristics
| Age at follow up CMR, years | 18.3 (±3.5) |
| Time of follow up between CMR, months | 60 (46–73) |
| Sex | |
| Female | 11 (31.4) |
| Male | 24 (68.6) |
| Race | |
| White | 31 (88.9) |
| Black | 4 (11.1) |
| Other | 0 |
| Original pulmonary valve | |
| Stenosis | 21 (60) |
| Atresia | 11 (31.4) |
| Absent pulmonary valve | 3 (8.6) |
| Surgical repair | |
| TAP | 32 (91.4) |
| RV-PA conduit | 3 (8.6) |
| VSD closure only | 0 |
| Non trans-annular patch | 0 |
| Age at surgery, months | 6.1 (±6.2) |
| Time between follow up CMR and echocardiogram, days | 18 (±89) |
| Follow up echocardiogram: | |
| Peak RVOT velocity (m/sec) | 2.5(±0.75) |
| Peak RVOT gradient (mmHg) | 28 (±16) |
| RVOT obstruction (>3m/sec), n | 9 (26) |
| Follow up CMR: | |
| RVEDVi (ml/m2) | 126 (±39) |
| RVEDVi Z-score | 3.2 (±4.7) |
| RVESVi (ml/m2) | 55 (±24) |
| RVESVi Z-score | 4.3 (±4.7) |
| RVEF (%) | 56 (±9) |
| LVEF (%) | 69 (±6) |
CMR indicates Cardiac magnetic resonance; TAP, trans-annular patch; RV-PA right ventricle to pulmonary artery; VSD, ventricular septal defect; RVOT: right ventricular outflow tract; RVEDVi, indexed right ventricular end diastolic volume; RVESVi, indexed right ventricular end systolic volume; RVEF, right ventricular ejection fraction; LVEF, left ventricular ejection fraction.
Data are expressed as mean (±SD), median (interquartile range), or as number (%).
On CMR, there was moderate right ventricular dilation. Both right and left ventricular function was preserved as measured by ejection fraction (Table 1).
Correlation between DSTVI and CMR pulmonary valve regurgitant fraction
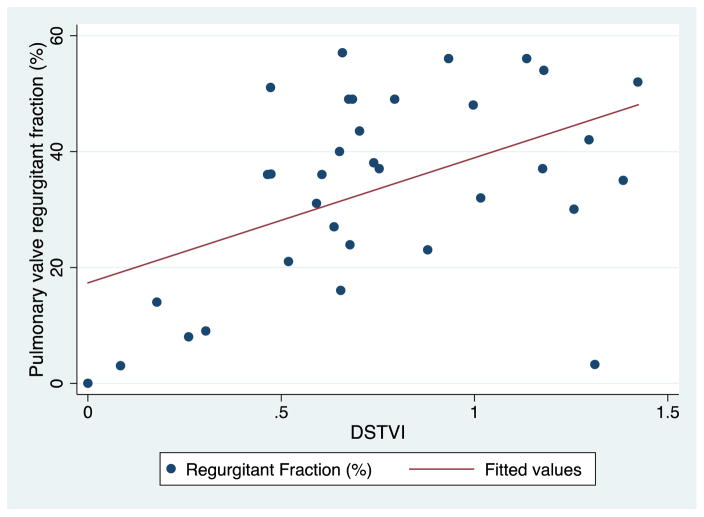
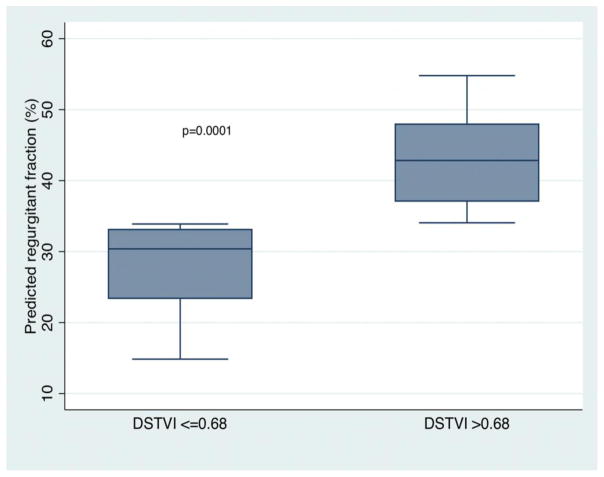
The DSTVI showed moderate correlation with RF by CMR (r=0.62, p= 0.0001, Figure 2). Using the predicted values for DSTVI from linear regression, a CMR RF of 20% and 40% (the boundaries between mild/moderate and moderate/severe PR) corresponded with a DSTVI of 0.52 (95% CI: 0.39–0.66) and 0.79 (95% CI: 0.69–0.89), respectively. Using the median DSTVI of 0.68 as a cutoff, DSTVI of <= 0.68 corresponded to a RF of 28% (20; 37), whereas DSTVI > 0.68 corresponded to a RF of 44% (38; 49), p=0.0001 (Figure 3). Eight patients had a DSTVI greater than 1, all of whom had RF >= 30% (30–56%). The association between DSTVI and RF remained significant after adjusting for the velocity in the RVOT. The velocity in the RVOT was not associated with RF. Mean values for DSVTI and RF are detailed in Table 2.
Figure 2. Correlation between pulmonary regurgitant fraction (CMR) and DSTVI (echocardiogram) at follow up.
Scatter plot of diastolic-systolic time-velocity integral (DSTVI) vs. pulmonary regurgitant fraction (r=0.62, p= 0.0001).
Figure 3. Regression model using DSTVI of <= 0.68 and > 0.68 as predictors of pulmonary regurgitant fraction.
DSTVI of <= 0.68 corresponds to a RF of 27.9% (19.9–36.9), whereas DSTVI > 0.68 corresponds to a RF of 43.8% (38.2–49.4, p=0.0001). On adjusted analysis accounting for the velocity in the RVOT, a DSTVI of <= 0.68 corresponds to a RF of 29.5 % (26–33.3), whereas DSTVI > 0.68 corresponds to a RF of 41% (36–49), p<0.0001).
Table 2.
Change in measures of pulmonary valve regurgitation by CMR and echocardiogram
| Variable | Baseline | Follow up | Change | p |
|---|---|---|---|---|
| DSTVI of MPA (echocardiogram) | 0.75 (±0.36) | 0.80 (±0.46) | 0.04 (±0.50) | 0.61 |
| Pulmonary regurgitant fraction (CMR) | 35.7 (±16.1) | 35.5 (±17.8) | −0.23 (±9.4) | 0.89 |
DSTVI indicates diastolic to systolic time-velocity-integral; MPA, main pulmonary artery; CMR, cardiac magnetic resonant imaging
Data are expressed as mean (±SD). Paired t-test was used to detect differences in pulmonary valve regurgitation and DSTVI at baseline and follow up.
Changes in pulmonary valve regurgitant fraction
In this cohort, there was no significant progression in PR over time, measured as DSTVI (p=0.61) or as RF by CMR (p=0.89). Signed rank test showed that individual changes in RF corresponded with changes in the same direction in DSTVI (p=0.75). Moreover, the confidence intervals for baseline and follow up DSTVI and regurgitant fraction were similar, indicating that the lack of interval change was not due to wide and variable confidence intervals (Table 2).
Association with right ventricular outflow tract obstruction
Subjects with RVOT obstruction had lower DSTVI ratios as compared to those without RVOT obstruction (0.46±0.25 vs.0.87±0.34, p= 0.0011). This difference was driven by the higher systolic TVI in those with RVOT obstruction as compared to those without (0.90±0.14 vs. 0.53±0.13, p>0.0001), while the diastolic TVI was comparable (0.41±0.21 vs. 0.44±0.17, p=0.62, respectively). There was no difference in regurgitant fraction between the groups (32±20% vs. 34.5±14%, respectively, p=0.51). On multivariable analysis, DSTVI was independently associated with regurgitant fraction when adjusted for RVOT peak velocity, or peak RVOT gradient. Moreover, the peak velocity across the RVOT was not a confounder of this association (multivariable analysis p values for DSTVI ratio 0.03, peak RVOT velocity 0.91, respectively. R2=0.22).
Discussion
Residual PR following repair of TOF is central to the pathogenesis of RV dilation and dysfunction and thereby late morbidity in TOF. Continued attempts are made to identify echocardiographic-derived quantitative measures of RV function and pulmonary valve regurgitation. In this study, we sought to examine the DSTVI longitudinally as a quantitative measure of PR in TOF. We found that there was no significant change in PR over time by either imaging modality. Finally, the DSTVI is moderately correlated with PR on CMR at follow up, similarly to our previous results.
Other studies have attempted to identify measures of pulmonary valve regurgitation by echocardiography. These include: 1) presence of diastolic flow reversal in the branch pulmonary arteries, which lacks a quantitative capacity; 2) width of vena contracta, which may not be present in the most severe cases of PR and has no standards for the pulmonary valve; 3) the PR index by M-mode echocardiography (PRIME), which relies on good imaging of branch pulmonary arteries, and 4) the Pulmonary Regurgitation index, using PR duration divided by diastolic time, which has not yet been validated.16–18 Our group previously described a moderate correlation of the ratio of the diastolic to systolic time velocity integral of the main pulmonary artery (DSTVI) with pulmonary regurgitant fraction by CMR.11 In this study we demonstrated this correlation longitudinally. We showed that lower values of DSTVI correlated with mild PR and higher values of DSTVI corresponded with more severe PR. The lack of overlap between confidence intervals supports the ability of the DSTVI to discriminate between mild and moderate PR, thereby potentially making this index of clinical utility. We found similar results in our previous study11.
Subjects that had DSTVI >1 had at least moderate PR by CMR. Most patients who have undergone TOF repair, in particular those with a trans-annular patch, experience disruption of the pulmonary valve with a similar area of the regurgitant jet as the effective orifice of the pulmonary valve. In some, there may be some retained competency of the valve, or there may be dynamic changes in the size of the main pulmonary artery, causing the DSTVI ratio to be >1.
While we acknowledge that we have not answered whether DSTVI will progress should RF increase, we were reassured by the fact that the DSTVI tracked with the RF. Nonetheless, there was no significant change in PR as measured by RF or DSTVI over the relatively short time period of our study. Similar to our findings, other investigators have not found a progression in pulmonary RF over an average follow-up time of four years.19,20 However, individual changes in RF tracked with changes in DSTVI, demonstrating the internal consistency of the DSTVI. In this age group, and with a median follow up of 5 years, there was no progression of pulmonary valve regurgitation.
Finally, DSTVI was lower in patients with RVOT obstruction by echocardiogram, a finding driven by the higher systolic TVI in those with RVOT obstruction. However, RVOT obstruction was not a confounder of the association between DSTVI and regurgitant fraction. Similarly to our findings, a previous study in patients with repaired TOF found no differences in regurgitant fraction between those with and without RVOT obstruction.21
Limitations
This was a retrospective study, which may introduce a selection bias as patients with more severe disease may be more likely to undergo repeat testing as part of standard of care. However, the initial study cohort was ascertained as part of a cross sectional study, and the purpose of this investigation was not to evaluate outcomes, but rather to evaluate the utility of a measure that strives to quantify disease severity.
We did not find a progression in pulmonary regurgitation by CMR or ECHO within the follow-up time and therefore we do not know whether pulmonary regurgitation does not progress after a certain degree or time from surgery, or whether the follow-up time did not allow us to identify changes if such occur. Nonetheless, there was no progression detected on both imaging modalities. Other investigators have also demonstrated a lack of pulmonary regurgitation progression at similar follow up time.19,20,22,23
There was a time lag between echocardiogram and CMR, and therefore there could be changes in hemodynamic status, however by inclusion criteria, the average time between echocardiogram and CMR in our study was less than one month, during which time significant changes in hemodynamic status are unlikely to occur. We did not assess the presence of restrictive physiology, since this was not the goal of the study. Because both echocardiographic and CMR measurements of flow did not account for restrictive physiology, we do not believe that the lack of this assessment affects our study findings. Moreover, we chose not to compare the DSTVI to other previously published measures of pulmonary regurgitation by echocardiography because the main goal of our study was to validate this parameter longitudinally. Finally, RVOT obstruction is not a confounder of the association between DSTVI and regurgitant fraction in a group of subjects without significant RVOT obstruction,
Conclusions
We confirmed the association between DSTVI on echocardiogram and PR by CMR in patients following repair of TOF. This index has potential utility in quantifying pulmonary regurgitation and perhaps identifying those in need of early screening by CMR. Further studies are needed to determine whether DSTVI tracks with changes in RF by CMR particularly when in conjunction with RVOT obstruction. If future studies demonstrated changes in DSTVI with changes in RF, then this measure would be of clinical utility in following patients longitudinally.
Acknowledgments
This work was in part supported by the National Institutes of Health P50-HL74731 (EG), U01HL098153 (EG, LMR), UL1TR000003 (EG), and K01HL125521 (LMR). The funding sources had no role in in study design, the collection, analysis and interpretation of data, writing of the report or in the decision to submit the article for publication.
References
- 1.Sarris GE, Comas JV, Tobota Z, Maruszewski B. Results of reparative surgery for tetralogy of Fallot: data from the European Association for Cardio-Thoracic Surgery Congenital Database. Eur J Cardiothorac Surg. 2012;42:766–74. doi: 10.1093/ejcts/ezs478. [DOI] [PubMed] [Google Scholar]
- 2.Alexiou C, Mahmoud H, Al-Khaddour A, et al. Outcome after repair of tetralogy of Fallot in the first year of life. Ann Thorac Surg. 2001;71:494–500. doi: 10.1016/s0003-4975(00)02444-9. [DOI] [PubMed] [Google Scholar]
- 3.Bacha EA, Scheule AM, Zurakowski D, et al. Long-term results after early primary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;122:154–61. doi: 10.1067/mtc.2001.115156. [DOI] [PubMed] [Google Scholar]
- 4.Park CS, Lee JR, Lim HG, Kim WH, Kim YJ. The long-term result of total repair for tetralogy of Fallot. Eur J Cardiothorac Surg. 2010;38:311–7. doi: 10.1016/j.ejcts.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–81. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 6.Knauth AL, Gauvreau K, Powell AJ, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–6. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 7.Oechslin EN, Harrison DA, Harris L, et al. Reoperation in adults with repair of tetralogy of fallot: indications and outcomes. J Thorac Cardiovasc Surg. 1999;118:245–51. doi: 10.1016/S0022-5223(99)70214-X. [DOI] [PubMed] [Google Scholar]
- 8.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–82. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation. 2013;128:1855–7. doi: 10.1161/CIRCULATIONAHA.113.005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer-Rosa L, Yang W, Kutty S, Rychik J, Fogel M, Goldmuntz E. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: a comparative analysis of echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:637–43. doi: 10.1161/CIRCIMAGING.112.972588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercer-Rosa L, Paridon SM, Fogel MA, et al. 22q11. 2 deletion status and disease burden in children and adolescents with tetralogy of Fallot. Circ Cardiovasc Genet. 2015;8:74–81. doi: 10.1161/CIRCGENETICS.114.000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA. Noninvasive Doppler determination of cardiac output in man. Clinical validation Circulation. 1983;67:593–602. doi: 10.1161/01.cir.67.3.593. [DOI] [PubMed] [Google Scholar]
- 14.Sarikouch S, Peters B, Gutberlet M, et al. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging. 2010;3:65–76. doi: 10.1161/CIRCIMAGING.109.859074. [DOI] [PubMed] [Google Scholar]
- 15.Tandri H, Daya SK, Nasir K, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–4. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Renella P, Aboulhosn J, Lohan DG, et al. Two-dimensional and Doppler echocardiography reliably predict severe pulmonary regurgitation as quantified by cardiac magnetic resonance. J Am Soc Echocardiogr. 2010;23:880–6. doi: 10.1016/j.echo.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Festa P, Ait-Ali L, Minichilli F, Kristo I, Deiana M, Picano E. A new simple method to estimate pulmonary regurgitation by echocardiography in operated fallot: comparison with magnetic resonance imaging and performance test evaluation. J Am Soc Echocardiogr. 2010;23:496–503. doi: 10.1016/j.echo.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Davlouros PA, Kilner PJ, et al. Doppler-echocardiographic assessment of pulmonary regurgitation in adults with repaired tetralogy of Fallot: comparison with cardiovascular magnetic resonance imaging. Am Heart J. 2004;147:165–72. doi: 10.1016/s0002-8703(03)00527-1. [DOI] [PubMed] [Google Scholar]
- 19.Wijesekera VA, Raju R, Precious B, et al. Sequential Right and Left Ventricular Assessment in Posttetralogy of Fallot Patients with Significant Pulmonary Regurgitation. Congenit Heart Dis. 2016 doi: 10.1111/chd.12354. [DOI] [PubMed] [Google Scholar]
- 20.Shin YR, Jung JW, Kim NK, et al. Factors associated with progression of right ventricular enlargement and dysfunction after repair of tetralogy of Fallot based on serial cardiac magnetic resonance imaging. Eur J Cardiothorac Surg. 2016;50:464–9. doi: 10.1093/ejcts/ezw049. [DOI] [PubMed] [Google Scholar]
- 21.Spiewak M, Biernacka EK, Malek LA, et al. Right ventricular outflow tract obstruction as a confounding factor in the assessment of the impact of pulmonary regurgitation on the right ventricular size and function in patients after repair of tetralogy of Fallot. J Magn Reson Imaging. 2011;33:1040–6. doi: 10.1002/jmri.22532. [DOI] [PubMed] [Google Scholar]
- 22.Wald RM, Valente AM, Gauvreau K, et al. Cardiac magnetic resonance markers of progressive RV dilation and dysfunction after tetralogy of Fallot repair. Heart. 2015;101:1724–30. doi: 10.1136/heartjnl-2015-308014. [DOI] [PubMed] [Google Scholar]
- 23.Buddhe S, Shah A, Lai WW. Progression of right ventricular dilation in repaired tetralogy of Fallot. J Magn Reson Imaging. 2015;41:730–7. doi: 10.1002/jmri.24610. [DOI] [PubMed] [Google Scholar]