Abstract
Controversy persists about whether snoring can affect atherosclerotic changes in adjacent vessels, independent of obstructive sleep apnea and other cardiovascular risk factors. This study examined the independent association between snoring and carotid artery intima-media thickness (IMT) in nonapneic snorers and nonsnorers. We studied 180 nonapneic snorers and nonsnorers undergoing in a full-night home-based sleep study. Snoring sound was objectively measured by a microphone. Based on snoring time across the night, participants were classified as nonsnorers (snoring time: 0%), mild snorers (1–25%), and moderate to heavy snorers (≥ 25%). We measured IMT on both common carotid arteries. The three groups were matched by age, body-mass index, cholesterol, blood pressure, and glucose levels, using weights from generalized boosted-propensity score models. Mean carotid IMT increased with increased snoring time across the night in women: nonsnorers (0.707 mm), mild (0.718 mm), and moderate to heavy snorers (0.774 mm), but not in men. Snoring during at least one fourth of a night’s sleep is independently associated with subclinical changes in carotid IMT in women only.
Keywords: atherosclerosis, carotid intima-media thickness, sleep-related breathing disorders, snoring
INTRODUCTION
There is growing interest in the potential role of snoring in cardiovascular health. Whether snoring impacts cardiovascular disease, independent of obstructive sleep apnea (OSA) and other cardiovascular risk factors, remains controversial. One debate concerns possible effects of snoring on carotid atherosclerosis. Lee and colleagues reported that heavy snoring (> 50% snoring time during sleeping) with mild OSA correlated with carotid atherosclerosis, but not with femoral atherosclerosis, suggesting a possible local impact of snoring (Lee et al., 2008). Two epidemiological studies have reported conflicting results (Li et al., 2012, Ramos-Sepulveda et al., 2010). A gender difference emerged in the relationship between self-reported snoring and subclinical changes in carotid atherosclerosis in a large Korean cohort of middle-aged and older adults (Kim et al., 2014), assessing only self-reported snoring. Lack of information on objective measurement of snoring has impeded the elucidation of the impact of snoring. We applied an objective snoring measurement using a microphone with a subset of participants enrolled in our previous study (Kim et al., 2014), thereby examining whether objectively measured snoring time is independently associated with carotid artery intima-media thickness (IMT) in non-apneic snorers.
METHODS
Participants
We studied participants enrolled in the Korean Genome and Epidemiology Study—an ongoing cohort study of Korean middle-aged and older adults (Kim et al., 2007). We analyzed data from a subset of participants (n = 314) who consecutively participated in a home-based sleep study with snoring measures between 2012 and 2013. All participants were free of known cardiovascular disease and stroke. We excluded 12 participants due to recording errors and a short sleep duration (< 3 hours) during the study, and those with an apnea/hypopnea index (AHI, the frequency of apnea and hypopnea events per hour of sleep) greater than 10 (n = 122), to minimize confounding effects of OSA. Our final samples consisted of 125 male and 55 female participants. The study was approved by an institutional review committee at Korea University Ansan Hospital and the University of Pennsylvania; all participants provided written consent.
Measurements
Snoring and OSA
We performed a full-night home-based sleep study using a T3 device (Noxmedical®, Iceland) to measure snoring and apnea-hypopnea index (AHI) (Arnardottir et al., 2015, Cairns et al., 2014). Embedded microphones recorded snoring sound (8kHz sampling). Automatic analysis of snoring was performed using Noxturnal software. Briefly, by the adaptive threshold method, snoring episodes were detected when they met a relative threshold (four times higher than the background noise of the signal) and duration (up to 3s). Other techniques that increased the specificity of detection of snoring included determination of synchronization with inspiration below a maximal frequency level (500Hz) and exclusion of any noise resulting from movement. In addition, all the snoring records were manually reviewed and corrected by a trained sleep technician. To calculate snoring time during sleep, we identified all snoring episodes continuing without interruption across the night, summed their duration, then divided by total time in bed. Apnea and hypopnea were defined according to the American Association of Sleep Medicine scoring manual (Berry et al., 2012).
Carotid artery intima-media thickness
We measured IMT in the distal far and near walls ≈1 cm proximal to bifurcation on both common carotid arteries using B-mode ultrasound, as previously described (Kim et al., 2014). Mean values of IMT were the averages of mean IMTs obtained from the four segments.
Covariates
Study participants completed interviewer-administered questionnaires regarding age, current smoking, alcohol intake (at least once a month), current medications, and snoring. We calculated body-mass index (BMI) as weight (kg)/height (m)2 and performed measurements of blood pressure twice in a sitting position using mercury sphygmomanometers after at least a 5-minute rest period. To assay levels of lipids and glucose, we drew blood from a vein in the morning, after an 8-hour fasting period and did assessments by a ADVIA 1650 system (Bayer®, Germany).
Statistical Analysis
The analytic strategy for this study was to create propensity score (PS) matched snoring groups (none vs mild vs moderate to heavy) for males and females on the basis of ten observed covariates (see Table 1), followed by a statistical comparison of the matched groups in terms of carotid IMT. The estimation of the PS weights used for outcome modeling relied on generalized boosted modeling and the TWANG package in R. Ten covariates were matched using the weighted PS in men and women, respectively, within a small (<0.2) to moderate (<0.4) absolute standardized mean difference (Cohen, 1992). Outcome modeling of the carotid IMT was accomplished using weighted general linear modeling, stratified by gender.
Table 1.
Comparison of unweighted and weighted characteristics among three groups with different amount of snoring by gender
| Characteristics | Snoring time
|
Snoring time
|
||||
|---|---|---|---|---|---|---|
| None | Mild (snoring<25%) | Moderate to heavy (25% or more) | None | Mild (snoring<25%) | Moderate to heavy (25% or more) | |
| In men, unweighted | In men, weighted | |||||
| (n=37) | (n=82) | (n=94) | ||||
| Age (years) | 59.2 | 61.7 | 59.9 | 59.6 | 60.9 | 60.3 |
| BMI (kg/m2) | 23.4 | 24.1 | 25.0 | 23.8 | 24.2 | 24.7 |
| Systolic BP (mmHg) | 114 | 119 | 122 | 118 | 119 | 121 |
| Diastolic BP (mmHg) | 76 | 78 | 80 | 77 | 78 | 79 |
| Fasting glucose (mg/dl) | 94 | 99 | 103 | 96 | 100 | 102 |
| Total cholesterol (mg/dl) | 184 | 182 | 193 | 186 | 187 | 191 |
| Triglyceride (mg/dl) | 132 | 132 | 161 | 145 | 135 | 156 |
| HDL (mg/dl) | 53 | 49 | 50 | 52 | 50 | 50 |
| Drinking (%) | 13.8 | 34.1 | 52.2 | 14.6 | 41.1 | 44.4 |
| Smoking (%) | 8.6 | 34.3 | 57.1 | 12.4 | 42.4 | 45.3 |
|
| ||||||
| In women, unweighted | In women, weighted | |||||
| (n=17) | (n=43) | (n=31) | ||||
| Age (years) | 57.7 | 61.4 | 61.4 | 58.3 | 61.1 | 61.4 |
| BMI (kg/m2) | 23.6 | 24.2 | 25.2 | 23.2 | 24.2 | 24.9 |
| Systolic BP (mmHg) | 119 | 116 | 116 | 117 | 116 | 116 |
| Diastolic BP (mmHg) | 76 | 73 | 73 | 76 | 73 | 73 |
| Fasting glucose (mg/dl) | 93 | 93 | 97 | 92 | 94 | 96 |
| Total cholesterol (mg/dl) | 196 | 200 | 191 | 197 | 200 | 192 |
| Triglyceride (mg/dl) | 120 | 126 | 122 | 115 | 126 | 124 |
| HDL (mg/dl) | 60 | 57 | 57 | 61 | 57 | 58 |
| Drinking (%) | 30.8 | 19.2 | 50.0 | 19.7 | 46.1 | 34.3 |
| Smoking (%) | 3.5 | 0.0 | 0.0 | 3.5 | 0.00 | 0.00 |
Note. Ten covariates in this table were matched among three snoring groups using weighted propensity score.
RESULTS
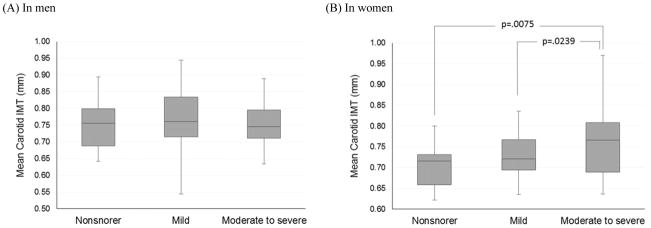
Table 1 shows a comparison of unweighted and weighted baseline characteristics among three groups with different snoring times by gender. Figures 1(A) and 1(B) show differences in mean IMT among three snoring groups by gender. In women only, mean IMT progressively increased (p = .0267) with increased snoring time (Figure 1B): nonsnorers (0.707 mm), mild (0.718 mm), and moderate to heavy snorers (0.774 mm). Moderate to heavy snorers had significantly higher IMT than nonsnorers (p = 0.0075) and mild snorers (p = 0.0239). However, no significant difference emerged in mean carotid IMT between snorers and nonsnorers in men (Figure 1A).
Figure 1.
Difference in mean intima-media thickness on carotid arteries with increasing amount of snoring time by gender in subjects without obstructive sleep apnea
Note. IMT: intima-media thickness; Box plots represent distribution of IMT data in each group. The boxes represent the median (black middle line) limited by the 25th and 75th percentiles. The whiskers are the upper and lower adjacent values; Moderate to heavy snorers had significantly higher IMT than nonsnorers (p = 0.0075) and mild snorers (p = 0.0239) in women (B). There was no significant difference emerged in mean carotid IMT between snorers and nonsnorers in men (A).
DISCUSSION
Using an objective measurement of snoring, we confirmed our previous findings based on self-report that an independent association between snoring and subclinical manifestation of carotid atherosclerosis exists in women only. Despite the small sample size, carotid IMT increased with objective snoring time in nonapneic women. Animal studies using a rabbit model have suggested that snoring-induced vibration can transmit to adjacent tissues and vessels that may cause endothelial dysfunction and exacerbate atherosclerosis and plaque rupture on carotid arteries (Almendros et al., 2007, Amatoury et al., 2006). Others have reported that snoring-like vibration can lead to increased local inflammatory response in upper-airway tissues (Almendros et al., 2007).
We previously speculated that morphological differences between men and women, such as fat distribution in neck and soft tissue volumes in the upper airway could cause gender differences as a consequence of snoring. Women with similar AHI have smaller neck girth than men (Resta et al., 2005). Also, fat distribution in neck and BMI were the most significant risk factors for OSA in men, whereas abdominal fat and neck-to-waist ratio accounted for 37% of variance in apneic events in women (Simpson et al., 2010). Despite lacking precise knowledge of potential mechanisms to explain whether anatomical differences between genders could lead to different consequences of snoring on carotid blood vessels, we hypothesize that neck-area fat deposition may dampen effects of snoring-induced vibration on the carotid artery in men. In addition, it is plausible that men have a larger distance between their upper airway and carotid arteries than women, which may attenuate the possible mechanical stimulus of snoring-induced vibration on the adjacent vessels.
In conclusion, our larger epidemiological study with snoring questionnaires and now a study with an objective snoring measure both support a significant association between snoring and carotid IMT in women, independent of OSA but not in men. However, relatively small sample size limits the generalizability of the present study. Further research with a larger sample is needed to establish causality, to identify mechanisms to explain the gender differences and the potential clinical implications of our observations.
Acknowledgments
This research was supported by a research fund (2012-E71002-00, 2013-E71001-00) from Korea Centers for Disease Control and Prevention, the National Institute of Health/National Institute of Nursing Research (K99/R00-NR013177) in the United States, and a program project grant from the U.S. National Institutes of Health (P01-HL094307).
Footnotes
There is no conflict of interest.
- Conception and design: Kim J, Shin C, Pack A, Riegel B
- Acquisition and interpretation of data: Kim J, Shin C, Pack A, Riegel B, Chirinos J
- Statistical analysis of the data: Kim J, Hanlon A, Lee SK
- Drafting of manuscript or critical revision of major intellectual content: All authors
- Final approval of the version to be published: All authors
References
- Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farre R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–7. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- Amatoury J, Howitt L, Wheatley JR, Avolio AP, Amis TC. Snoring-related energy transmission to the carotid artery in rabbits. Journal of applied physiology (Bethesda, Md: 1985) 2006;100:1547–53. doi: 10.1152/japplphysiol.01439.2005. [DOI] [PubMed] [Google Scholar]
- Arnardottir ES, Isleifsson B, Agustsson JS, et al. How to measure snoring? A comparison of the microphone, cannula and piezoelectric sensor. Journal of sleep research. 2015 doi: 10.1111/jsr.12356. [DOI] [PubMed] [Google Scholar]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns A, Wickwire E, Schaefer E, Nyanjom D. A pilot validation study for the NOX T3(TM) portable monitor for the detection of OSA. Sleep & breathing = Schlaf & Atmung. 2014;18:609–14. doi: 10.1007/s11325-013-0924-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. A Power Primer. Psychological Bulletin. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Kim J, Pack A, Maislin G, Lee SK, Kim SH, Shin C. Prospective observation on the association of snoring with subclinical changes in carotid atherosclerosis over four years. Sleep medicine. 2014;15:769–75. doi: 10.1016/j.sleep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yi H, Shin KR, Kim JH, Jung KH, Shin C. Snoring as an independent risk factor for hypertension in the nonobese population: the Korean Health and Genome Study. American journal of hypertension. 2007;20:819–24. doi: 10.1016/j.amjhyper.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu J, Wang W, et al. Association of self-reported snoring with carotid artery intima-media thickness and plaque. Journal of sleep research. 2012;21:87–93. doi: 10.1111/j.1365-2869.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Ramos-Sepulveda A, Wohlgemuth W, Gardener H, et al. Snoring and insomnia are not associated with subclinical atherosclerosis in the Northern Manhattan Study. International journal of stroke: official journal of the International Stroke Society. 2010;5:264–8. doi: 10.1111/j.1747-4949.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta O, Carpanano GE, Lacedonia D, et al. Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA) Respiratory medicine. 2005;99:91–6. doi: 10.1016/j.rmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33:467–74. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]