Abstract
There is evidence that R-type Ca2+ channels contribute to synaptic transmission in the myenteric plexus. It is unknown if R-type Ca2+ channels contribute to neuromuscular transmission. We measured the effects of the nitric oxide synthase (NOS) inhibitor, nitro L-arginine (NLA), Ca2+ channel blockers and apamin (SK channel blocker) on neurogenic relaxations and contractions of the guinea pig ileum longitudinal muscle-myenteric plexus (LMMP) in vitro. We used intracellular recordings to measure inhibitory junction potentials (IJPs). Immunohistochemical and western blot techniques localized R-type Ca2+ channel protein in the LMMP and circular muscle. CdCl2 (pan Ca2+ channel blocker) blocked and NLA and NiCl2 (R-type Ca2+ channel blocker) reduced neurogenic relaxations in a non-additive manner. NiCl2 did not alter neurogenic cholinergic contractions but it potentiated neurogenic non-cholinergic contractions. Relaxations were inhibited by apamin, NiCl2 and NLA and were blocked by combined application of these drugs. Relaxations were reduced by NiCl2 or ω-conotoxin (ω-CTX, N-type Ca2+ channel blocker) and were blocked by combined application of these drugs. Longitudinal muscle IJPs were inhibited by NiCl2, but not MRS 2179 (P2Y1 receptor antagonist). Circular muscle IJPs were blocked by apamin, MRS 2179, ω-CTX and CdCl2 but not NiCl2. We conclude that neuronal R-type Ca2+ channels contribute to inhibitory neurotransmission to longitudinal muscle but less so or not all in the circular muscle of the guinea pig ileum.
Keywords: calcium channels, enteric nervous system, inhibitory neuromuscular transmission, nitric oxide synthase, purinergic signaling
Non-adrenergic non-cholinergic (NANC) myenteric neurons are responsible for inhibitory neuromuscular transmission and muscle relaxation in the gastrointestinal (GI) tract. Pharmacological and electrophysiological studies revealed that there are several inhibitory neurotransmitters released from NANC nerves and several postjunctional signaling mechanisms linked to smooth muscle relaxation. Inhibitory neuromuscular transmitters in the enteric nervous system (ENS) include nitric oxide (NO)(He et al., 1993; Osthaus and Galligan, 1992; Sanders et al., 1992; Smits and Lefebvre, 1996) and one or more purinergic neurotransmitters (Burnstock, 2008; Crist et al., 1992; Durin et al., 2014; Huang et al., 2001; Mutafova-Yambolieva and Durin, 2014; Mutafova-Yambolieva et al., 2007; Smits and Lefebvre, 1996). The purinergic neurotransmitter acts at P2Y1 receptors on GI smooth muscle (Benko et al., 2007; De Man et al., 2003; Gallego et al., 2006; Wang et al., 2007) and PDGF-α positive fibroblast-like cells (Kurashi et al., 2011, 2014). P2Y1 receptors link to Ca2+ release from intracellular stores and P2Y1 receptor activation causes fast inhibitory junction potentials (IJPs) and relaxation (Benko et al., 2007; Gallego et al., 2006; Wang et al., 2007; Zhang et al., 2010). IJPs result from Ca2+-induced activation of apamin-sensitive small-conductance Ca2+-dependent K+ channels (SK channels)(Crist et al., 1992; Pascuad et al., 1996; Wang et al., 2007; Zhang et al., 2010). NO production requires Ca2+-dependent activation of the neuronal isoform of nitric oxide synthase (nNOS)(Mashimo et al., 1996; Rao et al., 2008; Rekik et al., 1996; Sanders and Ward, 1992). NO diffuses from inhibitory nerve terminals to target smooth muscle cells and interstitial cells of Cajal (ICC)(Burns et al., 1996; Suzuki et al., 2003) where it activates soluble guanylate cyclase causing cyclic guanosine 5′-triphosphate (cGMP) formation. cGMP activates protein kinase G which links to smooth muscle relaxation (De Man et al., 2007; Ilino et al., 2009; Lies et al., 2014; Makhlouf and Murthy, 1997).
The current model of the nerve pathways and connections in the guinea pig ENS has two populations of inhibitory motorneuron supplying the circular muscle layer (Brookes, 2001). These neurons have short projecting axons and are identified by the co-expression of VIP, NOS, enkephalin and γ-amino butyric acid (GABA). The second class of inhibitory motorneuron supplying the circular muscle contains VIP, nNOS, neurofilament protein and gastrin releasing peptide. These neurons have long projecting axons. There is only one class of inhibitory motorneuron supplying the longitudinal muscle and these neurons contain VIP and nNOS (Brookes, 2001). Inhibitory motorneurons provide a small fraction of the innervation of the longitudinal muscle layer as >90% of the nerve fibers in the longitudinal muscle layer are cholinergic (Brookes et al., 1992). This is not surprising as inhibitory junction potentials (IJPs) are recorded rarely from the longitudinal muscle unlike the circular muscle in the guinea pig ileum (Bywater and Taylor, 1986). IJPs occur frequently in the longitudinal muscle layer of the guinea pig distal colon (Spencer and Smith, 2001; Spencer et al. 2003).
Neurotransmitter release requires activation of voltage-gated Ca2+ channels expressed by nerve terminals. R-type (CaV2.3)(Bian et al., 2004; Bian and Galligan, 2007), N-type (CaV2.2), P/Q type (CaV2.1), but not L-type (CaV1.2)(Cunningham et al., 1998; Kirchgessner and Liu, 1999; Lundy and Frew, 1994; Reis et al., 2000, 2002; Takahashi et al., 1992) voltage-gated Ca2+ channels contribute to neurotransmitter release from enteric neurons. R-type Ca2+ channels also couple to fast synaptic excitation in orally projecting pathways in the myenteric plexus where acetylcholine is the excitatory neurotransmitter (Naidoo et al., 2010). Fast synaptic excitation in descending pathways in the myenteric plexus uses acetylcholine and ATP as the excitatory neurotransmitters. These nerve terminals use N- and P/Q type Ca2+ channels to activate neurotransmitter release (Naidoo et al., 2007). These data suggest that R-type Ca2+ channels may be a marker for specific synapses in the myenteric plexus.
We tested the hypothesis that R-type Ca2+ channels may be a marker for subsets of motorneurons supplying the longitudinal muscle layer and that R-type Ca2+ channel blockers might selectively inhibit excitatory or inhibitory neuromuscular transmission in the guinea pig ileum.
MATERIALS AND METHODS
Ethical Approval
All animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Michigan State University (Animal use protocols: 12/12-215-00 and 07/16-121-00). Animal use protocols were aligned with guidelines established by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the United States Department of Agriculture (USDA). Adult male Hartley-strain guinea pigs weighing 250–350 g (n=40)(3 months of age) were purchased from Emergent BioSolutions, Lansing, Michigan. Guinea pigs and mice were anesthetized using isoflurane inhalation (Abbott Laboratories, Chicago, IL), followed by bilateral pneumothorax.
Longitudinal muscle-myenteric plexus (LMMP) preparation
The ileum was removed and placed in oxygenated (95% CO2, 5% CO2) Krebs solution of the following composition (millimolar): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. A glass rod was inserted into a 2 cm segment of the ileum and the longitudinal muscle attached with the myenteric plexus (LMMP) was then teased off with a cotton swab soaked in Krebs solution. One end of the LMMP preparation was mounted with silk ligatures to a stationary hook at the bottom of a tissue holder and the other end was connected to an isometric force transducer (Grass Instruments, FT03C, Quincy, MA, USA). The preparation was mounted in the longitudinal axis so that neurogenic contractions of the longitudinal muscle were recorded. The tissue holder also contained two platinum foil electrodes and the LMMP preparations were positioned between the electrodes. The tissue holder and attached LMMP were placed in a 20 ml jacketed organ bath containing oxygenated Krebs solution at 37 °C. A resting tension of 1 g was applied to each of four 2 cm segments of LMMP preparations. Tissues were allowed to equilibrate for 60 minutes, during which the Krebs solution was replaced in 15 minute intervals.
Transmural electrical field stimulation (EFS)
To study inhibitory mechanisms in the LMMP, each tissue was first pre-contracted with histamine (1 μM) to induce sustained baseline resting tension. Relaxation responses were expressed as a percentage decrease in histamine-induced tone. Neurogenic relaxations (scopolamine, 1 μM present) were induced by brief trains of transmural electrical stimuli (20 Hz, 0.5 ms pulse duration, 1 s train duration, 0.25 Hz train rate, 45 mA) using a constant current unit (Stimu-Splitter II Med-Lab, Inc. Loveland, CO USA). To determine the action of antagonists on relaxations, non-cumulative concentration response curves were created with a 15 minute interval between successive doses.
Single electrical stimuli (0.1 Hz, 0.5 ms, 45 mA) were used to evoke neurogenic cholinergic contractions (scopolamine free Krebs solution). Trains of stimuli (20 Hz, 1 s, 0.5 ms pulse duration, 45 mA, 0.25 Hz train rate) were used to evoke non-cholinergic contractions (scopolamine, 1 μM present). To test the effect of antagonists on both kinds of contraction, non-cumulative concentration response curves were created with a 15 minute interval between addition of successive antagonist concentrations.
Transmural electrical stimuli were provided by a Grass S48 stimulator and mechanical activity of the LMMP was recorded using Labscribe software (iWorx, Dover, NH, USA) and a personal computer.
Intracellular electrophysiological recordings
A 1.5 cm section of the ileum was cut open along the mesenteric border and pinned flat in a silicone elastomer-lined (Sylgard, Dow Corning, Midland, MI) petri dish containing Krebs solution. The Krebs solution contained scopolamine (1 μM) and nifedipine (1 μM) to block muscarinic receptors and L-type Ca2+ channels on smooth muscle cells, respectively. The submucosa was removed to expose the LMMP. A 5 mm2 section of the LMMP was then transferred to a smaller silicone elastomer-lined recording chamber (2 ml) with the circular muscle layer facing up. When recording from circular muscle cells, the circular muscle layer was left intact. When longitudinal muscle cells were targeted, the circular muscle was removed using fine forceps. The chamber was mounted on a stage of an inverted microscope (Olympus CK-2, Tokyo, Japan). The LMMP was pinned tautly and bathed with oxygenated (95% O2, 5% O2) Krebs’ solution at a flow rate of 4 ml/min at 37 °C. The preparation was equilibrated for 40 min before commencing intracellular recordings. Single smooth muscle cells were impaled with glass microelectrodes (1 mm diameter, FHC Inc., Bowdoin, ME USA) filled with 2 M KCl (tip resistance 80–120 MΩ). Membrane potential was recorded using an Axoclamp 2A amplifier (Molecular Devices Corp., Sunnyvale, CA) in bridge mode. IJPs were evoked focally using microelectrode capillary glass (World Precision Instruments, Sarasota, FL USA) pulled to a tip diameter of ~60 μm using a horizontal microelectrode puller (PN-31 Narishige Instruments, E. Meadow, NY USA). The stimulating electrode (filled with Krebs solution) was positioned near the recording site and single stimuli (4 ms duration) and short trains of stimulation (1, 5 and 10 Hz, 1 s, 45 mA), provided by a pulse generator (Master 8, AMPI, Jerusalem, Israel) and a constant current stimulation unit (Grass Technologies, West Warwick, RI) were used to evoke IJPs. The resting membrane potential (RMP) of smooth muscle cells was allowed to stabilize for 10 minutes. Cells with RMPs less than −40 mV were not included in the analysis. Signals were recorded using an A/D converter (Digidata 1322A, Molecular Devices), Axoscope 10 software (Molecular Devices), and a desktop computer. Amplified signals were sampled at 2 kHz and filtered at 1 kHz. We measured IJP amplitude (longitudinal muscle) and the area under the curve (AUC)(circular muscle) as this is an integration of both IJP amplitude and duration. This allowed us to study the changes in responses induced by drugs. AUCs were measured under control conditions and during drug treatment.
Drugs were dissolved in oxygenated Krebs solution and were applied from an array of 6 quartz glass tubes (300 μm tip diameter, Polymicro Technologies, Phoenix, AZ USA) using gravity driven flow from a 10 cc syringe reservoir. The flow tube array was mounted on a plexiglass platform with a flexible heating foil (Minco Inc. www.minco.com) glued to the surface. Solutions flowing (0.1 ml/minute) through the tubes were warmed to 36 °C before flowing into the tissue chamber. The flow tube was positioned over the recording site using a micromanipulator so that the recording site was bathed in drug-containing buffer. This technique permitted use of small volumes of toxin containing solutions while allowing construction of steady state concentration response curves.
Immunohistochemical studies
Segments of guinea pig and mouse (WT and CaV2.3 knockout) ileum were cut open along the mesenteric border, stretched and pinned flat in a silicone elastomer-lined Petri dish, which was then filled with Zamboni’s fixative (2% [v/v] formaldehyde and 0.2% [v/v] saturated picric acid in PBS). Tissues were fixed overnight (4 °C) and then washed three times with DMSO at 10-min intervals, followed by three washes in PBS at 10-min intervals. Whole-mount LMMP preparations were dissected and then preincubated in 4% normal donkey serum in PBS for 30 min at room temperature. All primary antiserum were diluted in PBS. Tissues were incubated with primary antibodies overnight at room temperature. NOS immunoreactivity was localized using a sheep polyclonal antiserum (1:200 dilution, protein concentration not available from the manufacturer)(Millipore, USA, Cat. No: AB1529). A rabbit polyclonal α1E antiserum (final dilution = 1.5 μg/ml) was used to localize CaV2.3 (Alomone laboratories, Jerusalem, Cat. No. ACC-006). The antibody was raised against the intracellular loop connecting domains II and III of the rat CaV2.3 protein. After overnight incubation, unbound antibody was rinsed away with 3 washes of PBS at 10 min intervals. The preparations were then incubated in indocarbocyanine (Cy3)(7.5 μg/ml in PBS) or fluorescein isothiocyanate (FITC)(37.5 μg/ml in PBS) conjugated secondary antibodies (Jackson Immunochemicals, West Grove, PA, USA) reconstituted in PBS for 2 h at room temperature. The tissue was washed in PBS 3 times at 10 min intervals and then mounted in buffered glycerol (pH = 8.6) for fluorescence microscopy. Staining was viewed using a Nikon fluorescence microscope (model TE 2000-U; Nikon Corporation, Tokyo, Japan) and METAMORPH software (Molecular Devices, Sunnyvale, CA, USA) or a Leica TCS SL laser scanning confocal microscope (40 X oil immersion 1.3 n.a. objective) using a Leica DMLFSA microscope body (Leica Microsystems Inc., Buffalo Grove, IL USA). Specificity of the CaV2.3 antibody was verified using whole mounts of LMMP preparations from WT and α1E KO mice (Wilson et al., 2000).
Drugs
All drugs were obtained from Sigma-Aldrich (St Louis, MO), except ω-conotoxin GVIA (ω-CTX)(Alomone Labs, Jerusalem, Israel). Drugs were dissolved in deionized water with the exception of nitro-L-arginine (NLA), which was dissolved in HCl (0.1 N) and nifedipine which was dissolved in 95% ethanol.
Data Analysis
Data are presented as the mean ± S.D. “n” refers to the number of animals from which tissues were obtained. Data were analyzed using a paired t-test for comparison of control and single treatment effects or a one way analysis of variance followed by Bonferoni’s post hoc test for control and multiple treatment effects. Drug concentration response curves were fit using a 4 parameter (max, min, slope, EC50) non-linear logistic function in GraphPad Prism 6.0 (GraphPad Software, Inc., LaJolla, CA). P < 0.05 was considered statistically significant.
Results
Effect of nerve stimulation
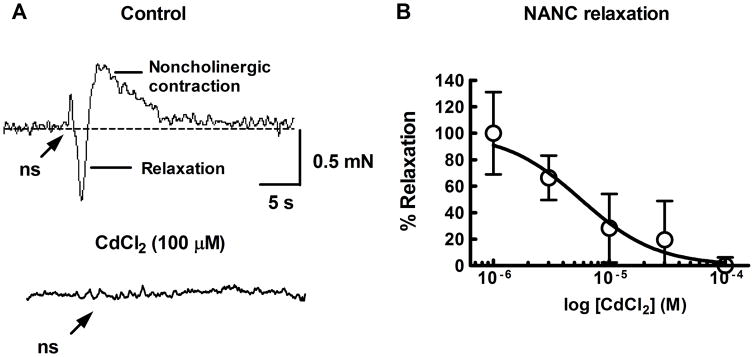
Transmural nerve stimulation (20 Hz, 1s train duration) in histamine (1 μM) pre-contracted LMMP preparations (scopolamine 1 μM, present) produced a relaxation followed by a slowly developing noncholinergic contraction in most preparations (Fig. 1A). The relaxation and noncholinergic contraction were blocked by tetrodotoxin (TTX, 0.3 μM)(not shown). The non-selective Ca2+ channel blocker CdCl2 produced a concentration-dependent and complete inhibition of the relaxation (Fig. 1A) and noncholinergic contraction (Fig. 1B) with an IC50 value of 5.6 ± 2.9 μM (n=6).
Fig. 1.
Representative traces showing neurogenic responses after transmural electrical field stimulation of the guinea pig LMMP in vitro. A top, Relaxations were induced in the presence of histamine (1 μM) to increase tone and scopolamine (1 μM) to block muscarinic receptors. Nerve stimulation (ns, 20 Hz, 1 s) caused a relaxation followed by a noncholinergic contraction. A, bottom. The relaxation and contraction were blocked by the non-selective Ca2+ channel blocker CdCl2 (100 μM). B, Inhibition of the relaxation by CdCl2 was concentration dependent (n=6). The curve was fit to the data points using a 4-parameter (max, min, slope, EC50) non-linear logistic function.
NiCl2 and nitro-L-arginine (NLA) inhibit neurogenic relaxations
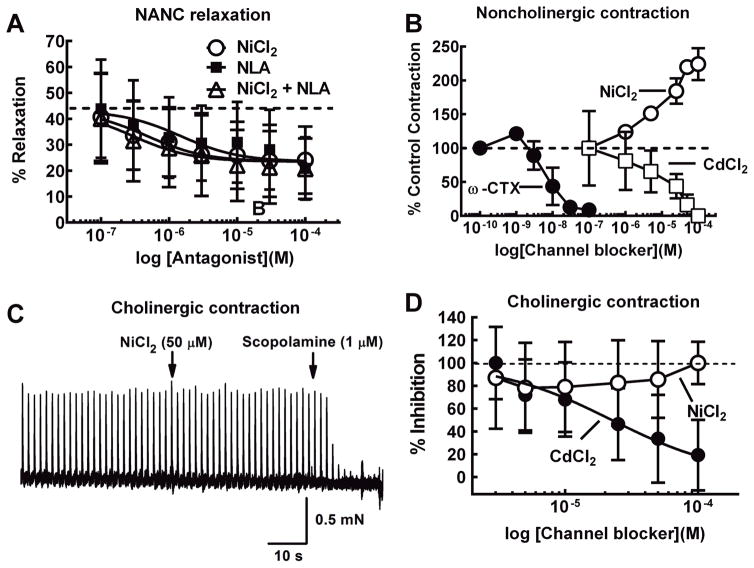
At concentrations ≤50 μM, NiCl2 can selectively block R-type Ca2+ channels (Gasparini et al, 2001; Tottene et al, 2000; Wang et al, 1999; Wu et al, 1998). We found that NiCl2 (0.1–100 μM), caused a concentration-dependent inhibition of neurogenic LMMP relaxations (Fig. 2A). The NOS inhibitor, NLA (0.1–100 μM), also reduced the peak relaxation with a maximum inhibition of 20 ± 12%. Co-application of NiCl2 with NLA inhibited the relaxation amplitude by 21 ± 12%. There were no differences in the concentration response curves for NiCl2, NLA or NiCl2 with NLA for inhibition of the NANC relaxations (P>0.05, n = 8 for all groups)(Fig. 2A).
Fig. 2.
NiCl2 inhibits neurogenic relaxations but not neurogenic cholinergic or noncholinergic contractions of the LMMP. A, NiCl2 and NLA produced a concentration-dependent inhibition of the relaxation evoked by nerve stimulation (20 Hz, 1 s). The control relaxation was approximately 40% of histamine-induced tone. This relaxation was reduced by ~50% by NLA or NiCl2. Combined application of NiCl2 and NLA did not produce a greater inhibition then either drug alone (n = 8 for all groups). B, The N-type Ca2+ channel blocker ω-conotoxin GVIA (ω-CTX, n = 3) and CdCl2 (n = 6) blocked non-cholinergic contractions of the LMMP (20 Hz 1 s, scopolamine 1 μM present). NiCl2 produced a concentration dependent increase in the amplitude of the NANC contraction (n = 3). C, Representative recording of contractions of the LMMP evoked by single electrical stimuli. Addition of NiCl2 did not affect contraction amplitude while subsequent addition of the muscarinic receptor antagonist, scopolamine blocked these contractions completely confirming that they were mediated by nerve released acetylcholine. D, NiCl2 did not inhibit LMMP contractions caused by single shocks (0.1 Hz, n = 6). These contractions were blocked completely by the muscarinic antagonist scopolamine (not shown). CdCl2 produced a concentration- dependent inhibition of the neurogenic cholinergic contraction (n = 6).
While NiCl2 blocks R-type Ca2+ channels, it could also block other Ca2+-dependent mechanisms to alter smooth muscle tone. Therefore, we tested the actions of NiCl2 on cholinergic contractions of the LMMP caused by single electrical stimuli applied at 0.1 Hz (Galligan, 1993). These responses were blocked by scopolamine (1 μM) indicating that they are mediated by acetylcholine acting at muscarinic receptors on the longitudinal muscle (Fig. 2C)(Galligan, 1993). NiCl2 (1–100 μM, n=6) did not alter cholinergic contractions while CdCl2 (1–100 μM) produced a concentration dependent inhibition of the same responses (Fig. 2D); the IC50 was 8.2 ± 3.4 μM (n=6). We further verified the specificity of NiCl2 for NANC relaxations by testing its effects on non-cholinergic contractions evoked by short trains of stimulation (10 Hz, 1 s) in the presence of scopolamine (1 μM) to block muscarinic receptors. These non-cholinergic contractions are mediated predominately by substance P acting at NK-1 type receptors (Lippi et al., 1998). NiCl2 produced a concentration-dependent (EC50 = 24 ± 1.9 μM, n=3) increase in the amplitude of the non-cholinergic contractions (Fig. 2C). CdCl2 (IC50 = 35 ± 23.3 μM, n=6) and the N-type Ca2+ channel blocker ω-conotoxin GVIA (ω-CTX)(IC50 = 6.9 ± 4.1 nM, n=3) both inhibited the noncholinergic contraction (Fig. 2C).
NiCl2, NLA and apamin increase noncholinergic contractions
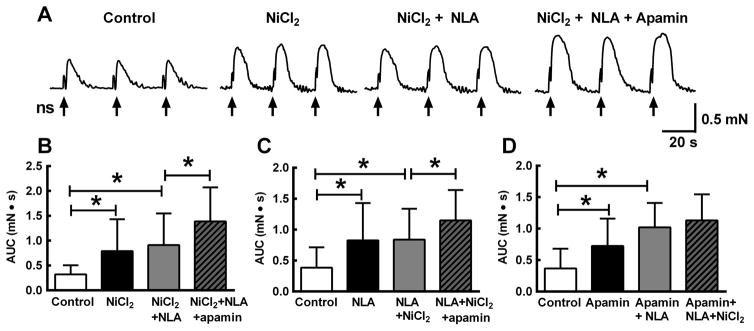
We next tested the effect of NiCl2 (50 μM), NLA (100 μM) and apamin (0.1 μM) on the noncholinergic contraction (scopolamine 1 μM present) as this response was more stable than the neurogenic relaxation and permitted sequential application of drugs over a long time course experiment. In these experiments, the area under the contraction curve (AUC) was measured because drug treatments increased the amplitude and duration of the contraction. NiCl2 significantly increased the AUC Fig. 3A,B; P<0.05). Subsequent addition of NLA did not further increase the contraction while addition of apamin further increased the AUC. The magnitude of this effect was statistically greater than the effect of NiCl2 or NiCl2 + NLA (Fig. 3A,B; n = 10, P<0.05). In a second set of experiments, NLA applied first increased contraction AUC (Fig. 6C, P<0.05) while addition of NiCl2 did not produce a further increase in the contraction (P>0.05). However, subsequent addition of apamin caused further increase in the AUC which was greater than the combined effect of NiCl2 and NLA (Fig. 3C, P<0.05). Finally, apamin alone increased the contraction AUC (Fig. 3D, P<0.05) and addition of.NLA in the presence of apamin further increased the AUC (P<0.05). NiCl2 did not further increase the contraction in the presence of apamin and NLA (Fig. 3D).
Fig. 3.
Potentiation of LMMP non-cholinergic contractions. A, Non-cholinergic contractions (scopolamine 1 μM present) were evoked by a train of nerve stimulation (ns, 20 Hz, 1 s). NiCl2 (50 μM) increased the peak amplitude and duration and subsequent addition of NLA (100 μM) did not produce any further increase. However, addition of the SK channel blocker apamin (0.1 μM) further increased contraction amplitude and duration. B, Quantitative data for experiment illustrated in “A”. C, Data from experiment similar to that shown in “A” except the sequence of drug application was altered with NLA application preceding NiCl2 followed by apamin. NLA increased the contraction while addition of NiCl2 did not increase the contraction further. Apamin did produce an addition increased in contraction amplitude and duration. D, Sequence of drug application was apamin followed by addition of NLA followed by addition of NiCl2. Apamin and then NLA produced sequential increased in the contraction while NiCl2 did not produce a further increase. For all figures * indicates P < 0.05, n=10 for each experiment. All data analyzed by one way ANOVA and Tukey’s post hoc test.
Combined effect of NiCl2/NLA and apamin on neurogenic relaxations
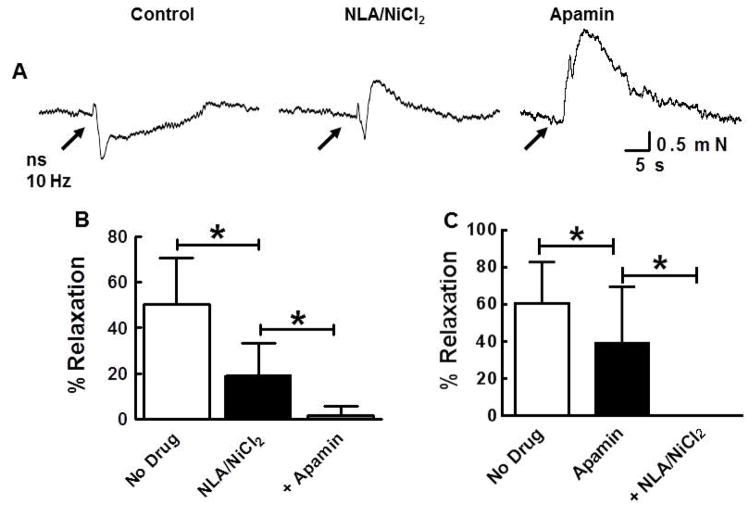
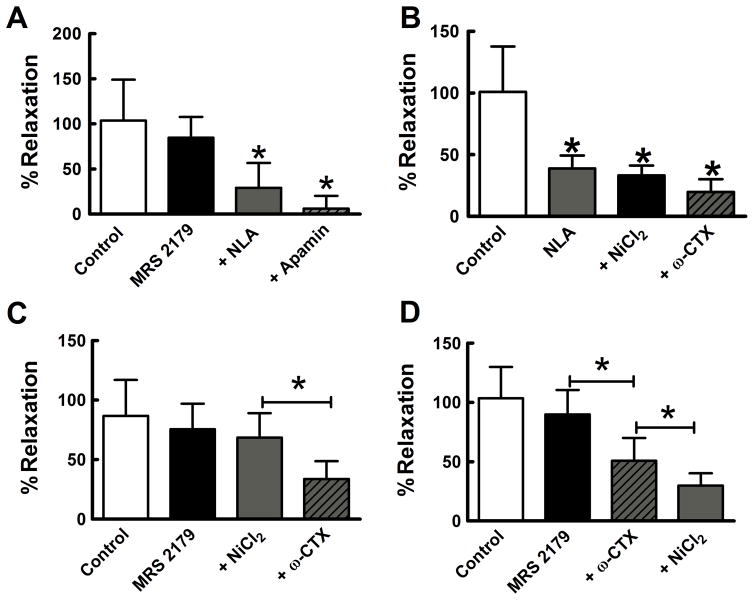
The contribution of SK channels to neurogenic relaxation was examined using apamin (0.1 μM). Combined application of NiCl2 (50 μM) and NLA (100 μM) reduced the neurogenic relaxation by 62% (Fig. 4A,B). Cumulative addition of apamin blocked completely the neurogenic relaxation (Fig. 4A,B). Apamin alone reduced the relaxation by 36% and cumulative addition of NLA with NiCl2 completely blocked the relaxation (Fig. 4C).
Fig. 4.
Nitrergic and SK channel mediated components of the neurogenic relaxation. A, Representative experiment showing the effect of NLA/NiCl2 (100 μM/50 μM) and apamin (0.1 μM) and on the neurogenic relaxation. B, Quantification of the data for experiment illustrated in “A”. Control relaxation was approximately 60% of histamine-induced tone. Addition of NLA/NiCl2 caused approximately 30% reduction of the peak relaxation amplitude and cumulative addition of apamin abolished the remaining response (n = 7; *P <0.05). C, Similar experiment as shown in A and B but apamin was applied first. Apamin produced 21% reduction of peak relaxation amplitude and this effect was significant when compare to control (n=12; *P<0.05). Cumulative application of NLA/NiCl2 abolished the apamin-resistant relaxation (*P<0.05). All data analyzed by one way ANOVA and Tukey’s post hoc test.
Actions of Ca2+ channel blockers and a P2Y1 receptor antagonist on neurogenic relaxations
The data above show that the neurogenic relaxation is apamin-sensitive and previous work has shown that purinergic receptors link to the Ca2+-activated SK channel activation in gut smooth muscle (De Man et al, 2003; Gallego et al, 2006; Zhang et al, 2010). In the next experiment (scopolamine 1 μM present), we used MRS 2179 to block P2Y1 purinergic receptors (Baurand et al, 2001; Boyer et al, 1998) but, in contrast to our expectations, we found that MRS 2179 had no effect on the amplitude of the neurogenic relaxation (Fig. 5A). Cumulative addition of NLA reduced the neurogenic relaxation and subsequent addition of apamin further reduced the relaxation although this was not statistically different from the effect of NLA (P>0.05). NLA inhibited the neurogenic relaxation by 61% while cumulative addition of NiCl2 (50 μM) and ω-CTX (0.1 μM) did not produce any further inhibition (Fig. 5B). In another set of experiments, MRS 2179 applied first did not affect the neurogenic relaxation and cumulative addition of NiCl2 also did not reduce the relaxation. However, further addition of ω-CTX inhibited the relaxation by 62% (Fig. 5C). In the last set of these experiments, MRS 2179 did not affect the neurogenic relaxation but further addition of ω-CTX reduced the relaxation by 52% (P<0.05) and further addition of NiCl2 reduced the relaxation by 40% (P<0.05) (Fig. 5D).
Fig. 5.
Contributions of P2Y1 receptors, nitric oxide, N-type and R-type Ca2+ channels to neurogenic relaxation of the longitudinal muscle. A, The P2Y1 receptor antagonist, MRS2179 (10 μM) did not affect the neurogenic relaxation while NLA (100 μM) inhibited the relaxation (*P < 0.05 vs Control and MRS 2179). Apamin (0.1 μM) produced a further reduction in relaxation amplitude although this was not different from NLA alone (*P < 0.05 vs Control and MRS 2179, n=7). B, NLA alone inhibited the neurogenic relaxation while cumulative application of NiCl2 and ω-CTX (0.1 μM) did not produce any further inhibition of the relaxation (*P<0.05 vs. Control). C, Cumulative addition of MRS 2179 followed by NiCl2 did not alter the neurogenic relaxation while subsequent addition of ω-CTX reduced the relaxation by ~52% (*P<0.05 vs. NiCl2, n=7). D, MRS 2179 did not inhibit the neurogenic relaxation while subsequent cumulative application of ω-CTX reduced the relaxation by 56% while cumulative addition of NiCl2 reduced the relaxation by an additional 40% (*P< 0.05, n=7). All data analyzed by one way ANOVA and Tukey’s post hoc test. In these experiments, the Y axis represents % of the control (maximal) relaxation (in the absence of antagonist drugs) as the % relaxation normalized to histamine- induced tone was greater than 100% in some tissues.
Longitudinal muscle Inhibitory junction potentials (IJPs) are NiCl2 sensitive
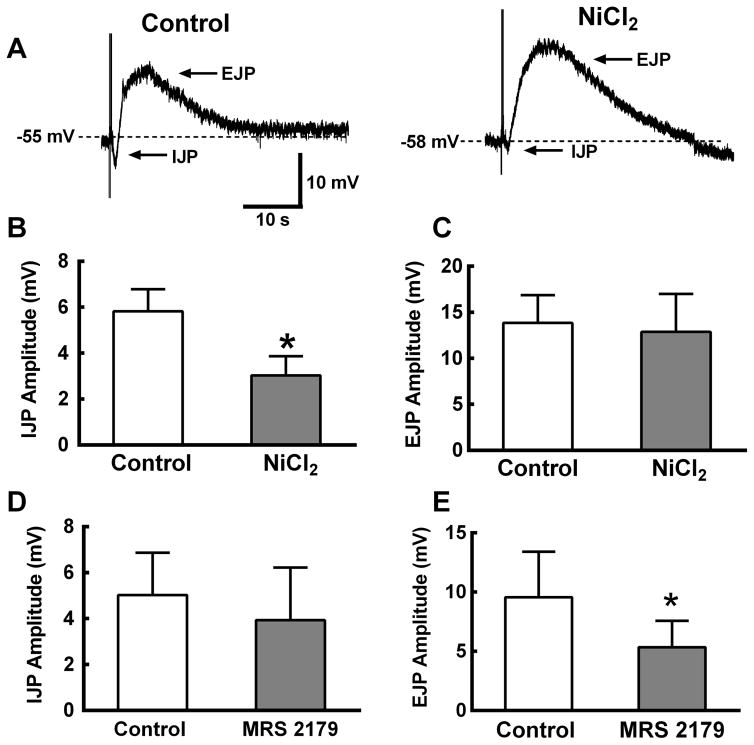
Focal stimulation (10 Hz, 1s) was used to evoke IJPs in longitudinal smooth muscle cells. The IJP was followed by a noncholinergic (scopolamine 1 μM present) EJP (Fig. 6A). NiCl2 (50 μM) inhibited the IJP by ~50% but it did not affect the EJP (Fig. 6A,B,C). MRS 2179 (10 μM) did not affect the IJP but it reduced EJP amplitude by ~45% (Fig. 6 D,E).
Fig. 6.
NiCl2 inhibits inhibitory (IJP) but not excitatory (EJP) junction potentials recorded from longitudinal smooth muscle cells. A, Representative recordings of an IJP and EJP from a longitudinal smooth muscle cell. NiCl2 (50 μM) inhibits the IJP but not the EJP. B, Summary data showing NiCl2 inhibits the IJP (n = 4). C, NiCl2 does not inhibit the EJP (n=5). D, The P2Y1 receptor antagonist, MRS 2179, did not alter the IJP but reduced the EJP by ~50% (n=6). All data in this figure were analyzed using a paired t-test (*P < 0.05 vs. control).
Circular muscle IJPs are NiCl2 insensitive
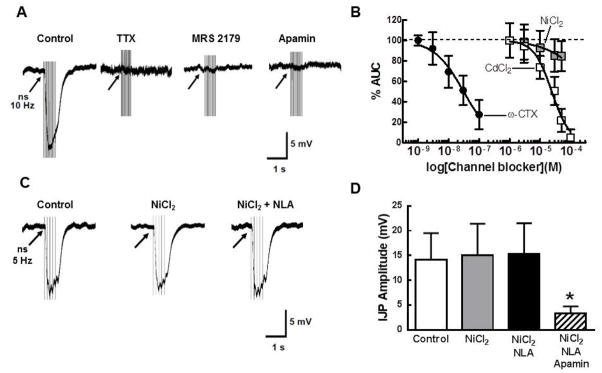
Focal stimulation (10 Hz, 1 s) was used to evoke circular muscle IJPs which were blocked by TTX (0.3 μM), MRS 2179 (10 μM) and apamin (0.1 μM)(Fig. 7A). CdCl2 produced a concentration-dependent inhibition of circular muscle IJPs (IC50 = 24 ± 2.2 μM) as did the N-type Ca2+ channel blocker ω-CTX (IC50 = 0.03 ± .02 μM). NiCl2 (up to 50 μM) did not alter the circular muscle IJP (Fig. 7B). We also used 5 Hz stimulation to evoke slower developing IJPs that may reveal a nitrergic component (Fig. 7C). However, neither NiCl2 alone or in combination with NLA changed the amplitude or time course of the IJP. The 5 Hz IJPs were inhibited significantly by apamin (Fig. 7D).
Fig. 7.
CdCl2, ω-CTX but not NiCl2 inhibit IJPs recorded from circular smooth muscle cells. A, IJPs were evoked by a 10 Hz, 1 s stimulus train. These IJPs were blocked by tetrodotoxin (TTX, 0.3 μM) the P2Y1 receptor antagonist, MRS2179 (10 μM) and by the SK channel blocker, apamin (0.1 μM). The stimulus artifacts have been truncated for clarity. B, Concentration inhibition curves for the N-type Ca2+ blocker ω-CTX, and CdCl2 and NiCl2. NiCl2 (up to 50 μM) did not inhibit the IJP (n=4 for each drug). C. Circular muscle IJPs evoked by 5 Hz stimulation are not affected by NiCl2 (50 μM) or NLA (100 μM). D, NiCl2 or NiCl2 plus NLA did not change the amplitude of the single stimuli IJP (n=9). Subsequent addition of apamin (0.1 μM) inhibited the IJP (n=4, *P<0.05 vs. Control).
Localization of the α1E subunit of CaV2.3 in guinea pig ileum
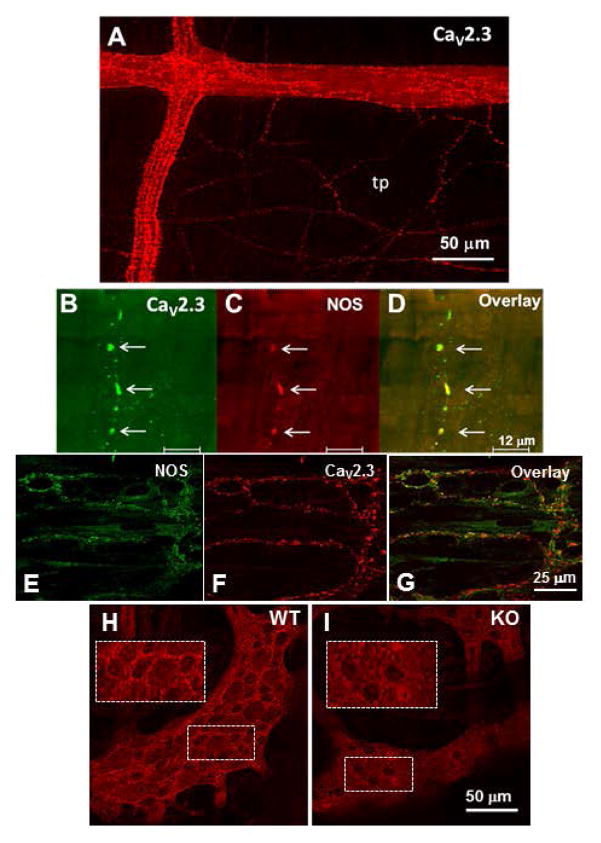
Immunohistochemical studies revealed α1E immunoreactivity in varicose nerve fibers in myenteric ganglia and in the tertiary plexus that supplies the longitudinal muscle layer (Fig. 8A) Preparations were co-stained with the α1E antibody and a nNOS antibody. α1E- and nNOS-immunoreactivity were co-localized in nerve fibers in the tertiary plexus (Fig. 8B,C,D). α1E- and nNOS-immunorectivity were expressed in separate populations of nerve fibers in the deep muscular plexus supplying the circular muscle layer (Fig. 8E,F,G). We tested the specificity of the antibody for the α1E protein in whole mounts of WT and α1E KO mouse colon. The antibody revealed bright varicose nerve fibers that appeared to encircle nerve cell bodies in myenteric ganglia in tissues from WT mice (Fig. 8H). We did not detect prominent α1E positive nerve fibers in the longitudinal muscle in preparations from WT mice. We also did not detect varicose nerve fibers in myenteric ganglia in tissues from α1E KO mice (Fig. 8I).
Fig. 8.
Immunohistochemical localization of CaV2.3 and neuronal NOS. A, Image shows distribution of CaV2.3-immunoreactive varicose nerve fibers in myenteric ganglia and in the tertiary plexus (tp) that supplies the longitudinal muscle. B,C,D, High magnification confocal images of a single nerve fiber in the tertiary plexus that co-expresses the α1E subunit of CaV2.3 and nNOS-immunoreactivity. E,F,G, Localization of NOS-immunoreactivity and α1E subunit of CaV2.3 immunoreactivity in the deep muscular plexus of the circular muscle. NOS and Cav2.3 α1E immunoreactivity are localized to different nerve fibers in the deep muscular plexus. H, CaV2.3 subunit in the myenteric plexus of the mouse colon. There are brightly fluorescent varicose nerve fibers that appear to encircle nerve cell bodies not labelled by the CaV2.3 antibody. I, CaV2.3 positive nerve fibers are not detected in myenteric ganglia in tissues from CaV2.3 KO mice. Insets show higher magnification images of α1E positive varicose nerve fibers in the ganglia from the WT but not CaV2.3 KO mouse.
DISCUSSION
R-type Ca2+ channels couple to NOS activation
N-type Ca2+ channels contribute to release of excitatory and inhibitory neuromuscular transmitters throughout the gastrointestinal tract (Wilson et al., 2000). However, we have identified selective contributions of R-type Ca2+ channels to NO-mediated inhibitory neuromuscular transmission to the longitudinal muscle layer in the guinea pig ileum. We used NiCl2 as a tool to test the function of R-type Ca2+ channels because it blocks these channels selectively at concentrations ≤50 μM (Gasparini et al., 2001; Tottene et al., 2000; Wang et al., 1999; Wilson, et al., 2000; Wu et al., 1998). In histamine pre-contracted and scopolamine-treated tissues, electrical field stimulation caused transient relaxations followed by a noncholinergic contraction. Both responses were blocked by TTX indicating they were neurogenic and by the non-selective Ca2+ channel subtype blocker, CdCl2. As shown previously in the guinea pig ileum LMMP the NOS inhibitor, NLA, produces a concentration-dependent but incomplete inhibition of the neurogenic relaxation (Osthaus and Galligan, 1992). NiCl2 produced a similar inhibitory effect on the neurogenic relaxation while combined application of NiCl2 and NLA did not produce an effect that differed from either blocker alone. These data suggest that NiCl2 and NLA act on a common mechanism to inhibit neurogenic relaxations of the longitudinal muscle.
Although NiCl2 is a potent inhibitor of R-type Ca2+ channels, it is possible that NiCl2 could directly alter muscle contractility by blocking other cation channels or that it blocks other Ca2+ permeable channels. However, Talukder and Harrison (1995) found that NiCl2 at concentrations up to 200 μM did not alter K+ currents recorded from hippocampal neurons. There are also studies demonstrating that R-type calcium channels are expressed by vascular smooth muscle cells (Link et al., 2008; Bkaily et al., 2015) so NiCl2 could directly affect longitudinal muscle contractility. We addressed this issue by studying the actions of NiCl2 on neurogenic contractions caused by single electrical stimuli of the LMMP in the absence of scopolamine. These contractions are mediated by acetylcholine acting at muscarinic receptors on the longitudinal muscle (Osthaus and Galligan, 1992). NiCl2 did not alter the cholinergic contraction while CdCl2 blocked this response. In addition, NiCl2 enhanced non-cholinergic neurogenic contractions of the longitudinal muscle. These data indicate that NiCl2 does not have general inhibitory effects on neurotransmitter release or muscle contractility. It is possible that NiCl2 could interact directly with NOS to inhibit its function. Studies in brain homogenates revealed that NiCl2 can directly inhibit nNOS activity (Mittal et al., 1995). However, in these studies, nNOS inhibition only occurred at very high NiCl2 concentrations as the Ki value for NiCl2 acting directly on nNOS was 360 μM. This is >7-fold higher than the concentrations of NiCl2 used in our studies to inhibit neurogenic relaxations and therefore direct inhibition of nNOS is unlikely to account for NiCl2 induced inhibition of neurogenic relaxations in our studies.
We found that the non-cholinergic contraction increased in amplitude and duration in the presence of NiCl2 and NLA. As NiCl2 and NLA reduced inhibitory neuromuscular transmission, simultaneous excitatory responses would be enhanced. We used the enhanced non-cholinergic contraction as an additional test for coincident sites of action of NiCl2 and NLA on neuromuscular transmission. We also varied the sequence of drug application. NiCl2 and NLA alone both increased the NANC contraction but the effects of these blockers were not additive. However, subsequent addition of apamin caused a further increase in the NANC contraction. Similarly, apamin alone increased the NANC contraction with either NiCl2 or NLA producing a further increase. These data indicate that NiCl2 and NLA act at a shared target (nNOS) while apamin further increased the NANC contraction by blocking SK channels on the muscle. These are non-overlapping pathways causing smooth muscle relaxation. Our data show that ω-CTX partly inhibits the neurogenic relaxation of longitudinal muscle as has also been shown for the guinea pig tenia coli preparation (Lundy and Frew, 1994) and canine ileocolonic circular muscle (Boeckxstaens et al., 1993). We also found that addition of NiCl2 further reduces this response indicating that release of transmitters from longitudinal muscle inhibitory motorneurons is under dual control by N- and R-type Ca2+ channels. Localization of R-type Ca2+ channels to longitudinal muscle inhibitory motorneurons is further supported by our immunohistochemical data showing that immunoreactivity to the α1E Ca2+ channel subunit (R-type Ca2+ channel protein subunit) is co-localized with nNOS-ir in the tertiary plexus that provides the innervation of the longitudinal muscle layer. These data also show expression of the α1E subunit in varicose nerve fibers in myenteric ganglia. This observation is consistent with our previous results showing that R-type Ca2+ channels contribute to fast synaptic transmission in the myenteric plexus (Naidoo et al., 2010). These data all support a role for R-type Ca2+ channels in synaptic and neuromuscular transmission in the guinea pig small intestine.
R-type Ca2+ channels link to release of the mediator of IJPs recorded from longitudinal but not circular smooth muscle
The data discussed above indicate that R-type Ca2+ channels provide the Ca2+ needed for nNOS activation in longitudinal muscle inhibitory motorneurons but not for purine release which is required for apamin-sensitive SK channel activation. This issue was addressed more directly by studying IJPs recorded from smooth muscle cells. NiCl2 partly inhibited IJPs recorded from longitudinal smooth muscle cells while the P2Y1 receptor antagonist, MRS 2179, had no effect on these IJPs. This result is not consistent with a model where there are dual mediators of neurogenic relaxation, the NiCl2/LNA sensitive NO-mediated component and the apamin sensitive, presumably purine mediated component. However, there are likely multiple purinergic inhibitory neuromuscular transmitters and their actions may be differentially sensitive to P2Y1 receptor antagonists (Mutafova-Yambolieva et al., 2007). For example, hyperpolarization of murine, human and monkey colonic smooth muscle caused by β-NAD and nerve stimulation is blocked by a P2Y1 receptor antagonist, while hyperpoloarization caused by ATP is not (Hwang et al., 2001; 3 Mutafova-Yambolieva et al., 2007). The specific purine responsible for the fast IJP in guinea pig ileum longitudinal muscle may be a purine whose actions are not altered by MRS 2179. Others have shown that ATP acting at purinergic receptors may be an excitatory neurotransmitter in the guinea pig longitudinal muscle layer (Ivancheva et al., 2000). Our data support this hypothesis as we show that the EJP recorded from longitudinal muscle cells is inhibited by MRS 2179. Interestingly, in the longitudinal muscle of the guinea-pig distal colon, stretch- and distention-evoked IJPs are blocked by NOS inhibitors suggesting that fast IJPs do not occur in the longitudinal muscle layer of the guinea-pig distal colon (Spencer and Smith, 2001; Spencer et al., 2003).
IJPs recorded from circular muscle were blocked completely by MRS 2179 and apamin. The circular muscle IJPs were also blocked by ω-CTX indicating that N-type Ca2+ channels are responsible for release of the mediator(s) of the IJP as also occurs in the circular muscle of the canine ileum (Cayabyab et al., 1997), porcine ileum (Borderies et al., 1997) and rat colon (Borderies et al., 1996). Zagorodnyuk and Maggi (1994) found apamin-resistant and apamin sensitive IJPs in guinea pig colon circular muscle cells. The apamin-sensitive but not apamin-insensitive IJPs were blocked by ω-CTX and the apamin-resistant IJP was attributed to the actions of NO. We did not detect apamin-resistant nitergic IJPs however we did find that the apamin-sensitive IJPs were blocked by ω-CTX and by MRS 2179 indicating that release of the purinergic transmitter is dependent only on N-type Ca2+ channels. NO causes smooth muscle relaxation by K+ channel-dependent and independent-mechanisms. K+ channel independent mechanisms include protein kinase G-mediated suppression of Ca2+ release from the endoplasmic reticulum and inhibition of smooth muscle phosphodiesterases (De Man et al., 2007; Makhlouf and Murthy, 1997). These non-K+ channel mechanisms could mediate the apamin-resistant IJPs. We did detect α1E immunoreactivity in nerve fibers supplying the circular muscle layer and we did not detect NOS-ir in these nerve fibers. In addition the circular muscle IJPs were not affected by NiCl2. It is possible that the α1E positive nerve fibers are excitatory motorneurons. This issue requires further study.
Functional significance of R-type Ca2+ channels in neuromuscular transmission
Our data show that R-type Ca2+ channels couple to NOS activation and NO release while N-type Ca2+ channels control the release of a purine transmitter to cause GI smooth muscle relaxation in the guinea pig ileum. The current model of the nerve supply of the longitudinal muscle layer indicates that there is only one class of inhibitory neuron identified by NOS immunostaining (Brookes et al., 2001). Therefore, longitudinal muscle inhibitory motorneurons should release NO and a purine to cause muscle relaxation. However, our data suggest that there might be two classes of longitudinal muscle inhibitory motorneurons in the guinea pig where one class uses NO as the transmitter while the second class is purinergic. R-type Ca2+ channels are localized to nitrergic nerve terminals while N-type channels are localized to purinergic nerve terminals. We localized α1E immunoreactivity (R-type Ca2+ channel subunit) in myenteric ganglia and α1E immunoreactivity co-localized with NOS immunoreactivity in individual nerve varicosities in tertiary plexus that supplies the longitudinal muscle. α1E positive nerve fibers were detected in the myenteric plexus of the mouse colon but not in the muscle layers. There are likely species differences in the localization and function of R-type calcium channels in the enteric nervous system. We used the colon from an α1E KO mouse (Wilson et al., 2000) to check the specificity of the α1E antibody and α1E positive nerve fibers were not detected in colon preparations from these mice.
Unfortunately, there are no reliable markers available for purinergic nerve fibers in the ENS, although the vesicular nucleotide transporter (VNUT, SLC17A9) may prove to be the needed marker (Sawada et al., 2008). Immunohistochemical studies using a VNUT antibody would provide a direct test of the hypothesis that there are separate classes of inhibitory neurons supplying the longitudinal muscle. We also found that R-type Ca2+ channels do not contribute to IJPs in the circular muscle. However, R-type Ca2+ channels may contribute to nitrergic and/or excitatory neurotransmission to the circular muscle which was not addressed in our study.
Summary and conclusions
Our data support a model in which R-type Ca2+ channels couple to NOS activation and NO release from nerve fibers supplying the longitudinal muscle layer. N-type Ca2+ channels contribute to non-nitrergic, purinergic inhibitory neuromuscular transmission. Separate control of the release of inhibitory neuromuscular transmitters provides an additional level of modulation of muscle tone and also provides a safety factor that ensures inhibitory neurotransmission is maintained if one mechanism is compromised.
What is the central question of this study?
Subtypes of enteric neurons are coded by the neurotransmitters they synthesize but it is not known if enteric neuron subtypes might also be coded by other proteins including calcium chanele subtypes controlling neurotransmitter release.
What is the main finding and its importance?
Our data indicate that guinea pig ileum myenteric neuron subtypes may be coded by calcium channel subtypes. We found that R-type calcium channels are expressed by inhibitory but not excitatory longitudinal muscle motorneurons. R-type calcium channels are also not expressed by circular muscle inhibitory motorneurons. Calcium channel subtype selective antagonists could be used to target subtypes of neurons to treat gastrointestinal motility disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK57039; DK094932).
Footnotes
disclosures
The authors have no financial conflicts to disclose.
VN, ERT and MD performed all of the studies of neuromuscular transmission.
ERT performed the immunohistochemical and electrophysiological studies and prepared a first draft of the manuscript.
APM performed immunohistochemical studies.
JJG provided research funding, contributed to experimental design and data analysis and prepared the final draft the manuscript.
References
- Baurand A, Raboisson P, Freund M, Léon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- Benko R, Undi S, Wolf M, Vereczkei A, Illenyi L, Kassai M, Cseke L, Kelemen D, Horvath OP, Antal A, Magyar K, Bartho L. P2 purinoceptor antagonists inhibit the non-adrenergic, non-cholinergic relaxation of the human colon in vitro. Neuroscience. 2007;147:146–152. doi: 10.1016/j.neuroscience.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Bian X, Zhou X, Galligan JJ. R-type calcium channels in myenteric neurons of guinea pig small intestine. Am J Physiol. 2004;287:G134–142. doi: 10.1152/ajpgi.00532.2003. [DOI] [PubMed] [Google Scholar]
- Bian X, Galligan JJ. Alpha2-adrenoceptors couple to inhibition of R-type calcium currents in myenteric neurons. Neurogastroenterol Motil. 2007;19:845–855. doi: 10.1111/j.1365-2982.2007.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G, Avedanian L, Al-Khoury J, Chamoun M, Semaan R, Jubinville-Leblanc C, D’Orléans-Juste P, Jacques D. Nuclear membrane R-type calcium channels mediate cytosolic ET-1-induced increase of nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol. 2015;93:291–297. doi: 10.1139/cjpp-2014-0519. [DOI] [PubMed] [Google Scholar]
- Borderies JR, Goñalons E, Angel F, Vergara P, Jiménez M. Effect of different calcium channel blockers on inhibitory junction potentials and slow waves in porcine ileum. Life Sci. 1997;60:883–892. doi: 10.1016/s0024-3205(96)00670-4. [DOI] [PubMed] [Google Scholar]
- Borderies JR, Jiménez M, Angel F. Non-adrenergic, non-cholinergic inhibitory junction potential in rat colonic circular muscle is partly sensitive to omega-conotoxin GVIA and resistant to L-, P- or Q-type calcium channel blockers. Neurosci Lett. 1996;210:91–94. doi: 10.1016/0304-3940(96)12671-9. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, De Man JG, Pelckmans PA, Cromheeke KM, Herman AG, Van Maercke YM. Ca2+ dependency of the release of nitric oxide from non-adrenergic non-cholinergic nerves. Br J Pharmacol. 1993;110:1329–1334. doi: 10.1111/j.1476-5381.1993.tb13964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Song ZM, Steele PA, Costa M. Identification of motor neurons to the longitudinal muscle of the guinea pig ileum. Gastroenterology. 1992;103:961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- Bywater RA, Taylor GS. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab FS, Jiménez M, Vergara P, deBruin H, Daniel EE. Influence of nitric oxide and vasoactive intestinal peptide on the spontaneous and triggered electrical and mechanical activities of the canine ileum. Can J Physiol Pharmacol. 1997;75:383–397. [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SM, Mihara S, Higashi H. Presynaptic calcium channels mediating synaptic transmission in submucosal neurones of the guinea-pig caecum. J Physiol. 1998;509:425–435. doi: 10.1111/j.1469-7793.1998.425bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, De Winter BY, Herman AG, Pelckmans PA. Study on the cyclic GMP-dependency of relaxations to endogenous and exogenous nitric oxide in the mouse gastrointestinal tract. Br J Pharmacol. 2007;150:88–96. doi: 10.1038/sj.bjp.0706964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, De Winter BY, Seerden TC, De Schepper HU, Herman AG, Pelckmans PA. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol. 2003;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Kurahashi M, Drumm BT, Ward SM, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci USA. 2014;111:15821–15826. doi: 10.1073/pnas.1409078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol. 2006;291:G584–594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Differential inhibition of cholinergic and noncholinergic neurogenic contractions by mu opioid and alpha-2 adrenergic agonists in guinea pig ileum. J Pharmacol Exp Ther. 1993;264:375–383. [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Rivier JR. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurons of the gut: evidence from the use of selective VIP antagonists and VIP antiserum. J Pharmacol Exp Ther. 1990;253:738–742. [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2001;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil. 2009;21:542–550. e12–13. doi: 10.1111/j.1365-2982.2008.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancheva C, Itzev D, Radomirov R. Functional antagonism between nitric oxide and ATP in the motor responses of guinea-pig ileum. J Auton Pharmacol. 2000;20:147–156. doi: 10.1046/j.1365-2680.2000.00175.x. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Differential localization of Ca 2+ channel alpha1 subunits in the enteric nervous system: presence of alpha1B channel-like immunoreactivity in intrinsic primary afferent neurons. J Comp Neurol. 1999;409:85–104. doi: 10.1002/(sici)1096-9861(19990621)409:1<85::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol. 2014;307:C561–570. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Groneberg D, Friebe A. Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastroenterol Motil. 2014;26:901–912. doi: 10.1111/nmo.12367. [DOI] [PubMed] [Google Scholar]
- Link TE, Murakami K, Beem-Miller M, Tranmer BI, Wellman GC. Oxyhemoglobin-induced expression of R-type Ca2+ channels in cerebral arteries. Stroke. 2008;39:2122–2128. doi: 10.1161/STROKEAHA.107.508754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi A, Santicioli P, Criscuoli M, Maggi CA. Depolarization evoked co-release of tachykinins from enteric nerves in the guinea-pig proximal colon. Naunyn-Schmiedeberg’s Arch Pharmacol. 1998;357:245–251. doi: 10.1007/pl00005164. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Frew R. Effect of omega-agatoxin-IVA on autonomic neurotransmission. Eur J Pharmacol. 1994;261:79–84. doi: 10.1016/0014-2999(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Makhlouf GM, Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–276. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal CK, Harrell WB, Mehta CS. Interaction of heavy metal toxicants with brain constitutive nitric oxide synthase. Mol Cell Biochem. 1995;149:263–265. doi: 10.1007/BF01076586. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Durnin L. The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol Ther. 2014;144:162–191. doi: 10.1016/j.pharmthera.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V, Dai X, Galligan JJ. R-type Ca2+ channels contribute to fast synaptic excitation and action potentials in subsets of myenteric neurons in the guinea pig intestine. Neurogastroenterol Motil. 2010;22:e353–63. doi: 10.1111/j.1365-2982.2010.01596.x. [DOI] [PubMed] [Google Scholar]
- Osthaus LE, Galligan JJ. Antagonists of nitric oxide synthesis inhibit nerve-mediated relaxations of longitudinal muscle in guinea pig ileum. J Pharmacol Exp Ther. 1992;260:140–145. [PubMed] [Google Scholar]
- Pacaud P, Feolde E, Frelin C, Loirand G. Characterization of the P2Y-purinoceptor involved in the ATP-induced rise in cytosolic Ca2+ concentration in rat ileal myocytes. Br J Pharmacol. 1996;118:2213–2219. doi: 10.1111/j.1476-5381.1996.tb15665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao YM, Chaudhury A, Goyal RK. Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. Am J Physiol. 2008;294:G627–634. doi: 10.1152/ajpgi.00519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekik M, Delvaux M, Tack I, Frexinos J, Bueno L. VIP-induced relaxation of guinea-pig intestinal smooth muscle cells: sequential involvement of cyclic AMP and nitric oxide. Br J Pharmacol. 1996;118:477–484. doi: 10.1111/j.1476-5381.1996.tb15428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HJ, Bíscaro FV, Gomez MV, Romano-Silva MA. Depolarization-evoked GABA release from myenteric plexus is partially coupled to L-, N-, and P/Q-type calcium channels. Cell Mol Neurobiol. 2002;22:805–811. doi: 10.1023/A:1021821427540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HJ, Massensini AR, Prado MA, Gomez RS, Gomez MV, Romano-Silva MA. Calcium channels coupled to depolarization-evoked glutamate release in the myenteric plexus of guinea-pig ileum. Neuroscience 2000. 2000;101:237–242. doi: 10.1016/s0306-4522(00)00354-7. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992;262:G379–392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits GJ, Lefebvre RA. ATP and nitric oxide: inhibitory NANC neurotransmitters in the longitudinal muscle-myenteric plexus preparation of the rat ileum. Br J Pharmacol. 1996;118:695–703. doi: 10.1111/j.1476-5381.1996.tb15456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol. 2003;284:G231–241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GDS. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tsunoda Y, Lu Y, Wiley J, Owyang C. Nicotinic receptor-evoked release of acetylcholine and somatostatin in the myenteric plexus is coupled to calcium influx via N-type calcium channels. J Pharmacol Exp Ther. 1992;263:1–5. [PubMed] [Google Scholar]
- Talukder G, Harrison NL. On the mechanism of modulation of transient outward current in cultured rat hippocampal neurons by di- and trivalent cations. J Neurophysiol. 1995;73:73–79. doi: 10.1152/jn.1995.73.1.73. [DOI] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. alpha1E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Dayanithi G, Newcomb R, Lemos JR. An R-type Ca2+ current in neurohypophysial terminals preferentially regulates oxytocin secretion. J Neurosci 1999. 1999;19:9235–9241. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol. 2007;292:G1483–1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Voltage-gated calcium channels in autonomic neuroeffector transmission. Prog Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Maggi CA. Electrophysiological evidence for different release mechanism of ATP and NO as inhibitory NANC transmitters in guinea-pig colon. Br J Pharmacol. 1994;112:1077–1082. doi: 10.1111/j.1476-5381.1994.tb13193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther. 2010;333:602–611. doi: 10.1124/jpet.109.160978. [DOI] [PubMed] [Google Scholar]