Abstract
Using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry, we analyzed 1,404 UCBT patients [single (< 18 years) = 810, double (≥ 18 years) = 594] with acute leukemia to define the incidence of acute and chronic graft-vs.-host disease (GVHD), analyze clinical risk factors and investigate outcomes. After single UCBT, 100-day incidence of grades II–IV aGVHD was 39% (95% CI, 36–43%), grades III–IV aGVHD was 18% (95% CI, 15–20%), and 1-year cGVHD was 27% (95% CI, 24–30%). After double UCBT, 100-day incidence of grades II–IV aGVHD was 45% (95% CI, 41%–49%), grades III–IV aGVHD was 22% (95% CI, 19–26%), and 1-year cGVHD was 26% (95% CI, 22–29%). For single UCBT, multivariate analysis showed that absence of anti-thymocyte globulin (ATG) was associated with aGVHD, whereas prior aGVHD was associated with cGVHD. For double UCBT, absence of ATG and myeloablative conditioning were associated with aGVHD, while prior aGVHD predicted for cGVHD. Grades III–IV aGVHD led to worse survival whereas cGVHD had no significant effect on disease-free or overall survival. GVHD is prevalent after UCBT with severe aGVHD leading to higher mortality. Future research in UCBT should prioritize prevention of GVHD.
Keywords: GVHD, umbilical cord blood, allogeneic transplantation
INTRODUCTION
Acute and chronic graft-vs.-host disease (GVHD) are significant complications after allogeneic hematopoietic stem cell transplantation (HSCT). Many recent changes in practice have led to changing patterns of GVHD. These include the introduction of alternative donor sources, reduced intensity conditioning and novel prophylaxis for GVHD. Risk factors for and effects on outcomes from GVHD have been described for conventional adult donor HSCT.1
Umbilical cord blood (UCB) has emerged as an alternative donor source with the development of new protocols which have significantly improved outcomes.2 It is unclear if acute and chronic GVHD after UCBT has similar risk factors and effects on outcomes compared to conventional donor sources. In this study, we proposed to establish the incidence of clinically significant acute and chronic GVHD after UCBT, analyze the risk factors which are associated with its development and investigate the influence of acute and chronic GVHD on patient outcomes after UCBT.
METHODS
Data Source
The Center for International Blood and Marrow Transplant Research (CIBMTR) registry includes a voluntary working group of more than 450 centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantations to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; patients are followed longitudinally and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.3
Patient Selection
All patients who underwent UCBT for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) between 2003 and 2012 and reported to CIBMTR were included initially (n=2,663). Cases were then excluded for several reasons including: related UCB units (n=51), ex vivo expanded units (n=84), ex vivo TCD (n=7), lack of research consent (n=24), lack of conditioning (n=4), lack of GVHD prophylaxis (n=49), use of non-myeloablative conditioning (n=4), use of ≤ 3/6 HLA-matched UCB units (n=40), lack of calcineurin inhibitor (n=68), missing HLA-matching data (n=18) and use of alemtuzumab (n=8). Based on numbers of cases and standard practice, only recipients of double UCBT ≥ 18 years old (excluded 206 recipients < 18 years of age) who received myeloablative or reduced intensity conditioning, and only recipients of single UCBT < 18 years old (excluded 247 recipients ≥ 18 years of age) who received myeloablative conditioning were included. HLA-match status was based on intermediate resolution for HLA-A and B and high resolution for HLA-DRB1. In the context of double UCBT, data on specific cord unit dominance was not available. For purposes of analysis of double UCBT, HLA-matching (relative to the recipient) was analyzed using the following categories of double cord blood combinations: 1) 4/6 + 4/6, 2) 4/6 + ≥ 5/6, and 3) ≥ 5/6 + ≥ 5/6. Disease status at transplant was defined as early (first complete remission), intermediate (second, or greater, complete remission) and advanced (presence of active disease).
Study Endpoints
The diagnoses of acute and chronic GVHD were reported by the treating center. Acute GVHD was diagnosed and graded per previously published consensus guidelines.4 Chronic GVHD was diagnosed according to Seattle criteria5 as the National Institutes of Health (NIH) consensus criteria6 had not yet been implemented on CIBMTR forms during this time period. Overall survival considered death from any cause as the event, and surviving patients were censored at the date of last contact. Disease free survival was defined as survival without relapse or death from any cause, with patients who were alive and in complete remission censored at the time of last follow-up. Non-relapse mortality (NRM) was defined as death during a continuous complete remission. Relapse was defined as recurrence of the primary disease.
Statistical Analysis
Chi-square or Fisher’s exact tests were used to compare frequencies for categorical variables, and ANOVA (analysis of variance) was used to compare means for continuous variables in different subsets. Univariate probabilities for overall survival were calculated using the Kaplan-Meier estimator.7 Comparison of survival curves was made by the log-rank test. The cumulative incidences of acute GVHD and chronic GVHD were calculated by treating death as a competing risk.8 Multivariate analysis was performed using Cox proportional hazards models for OS, progression-free survival (PFS), relapse, NRM, acute GVHD and chronic GVHD. All the clinical variables were first tested for affirmation of the proportional hazards assumption. Factors that violate the proportional hazards assumption were adjusted through stratification. Then a stepwise model building procedure was used to develop models for each outcome with a threshold of 0.05 for both entry and retention in the model. We also assessed the effects of acute GVHD II–IV, acute GVHD III–IV and chronic GVHD on OS, progression-free survival, relapse and NRM by forcing acute GVHD II–IV, acute GVHD III–IV or chronic GVHD into the multivariable models as a time-dependent variable. Center effect was also adjusted in all of the multivariable models. SAS version9.3 (SAS Institute, Cary, NC) was used for all the analyses.
RESULTS
Clinical Characteristics
A total of 810 recipients of single UCBT and 594 recipients of double UCBT were included. Patient and clinical characteristics are summarized in Table 1a. For the 810 recipients of single UCBT, median age was 6 (range, <1–18). 44% of patients had AML, 56% had ALL and all received myeloablative conditioning. 21% of patients received a 6/6 HLA-matched UCBT, 47% received a 5/6 HLA-matched UCBT and 32% received a 4/6 HLA-matched UCBT. All patients received calcineurin inhibitor-based GVHD prophylaxis. Anti-thymocyte globulin (ATG) was used in 64% of patients. Table 1a shows differences between patients who received ATG compared to those who did not.
Table 1a.
Clinical characteristics of patients undergoing single UCBT
| Characteristic | All | With ATG | Without ATG | p |
|---|---|---|---|---|
| N | 810 | 521 | 289 | |
| Number of centers | 105 | 88 | 68 | |
| Age, median (range), years | 6 (<1–18) | 5 (<1–18) | 7 (1–18) | <0.001 |
| Age at UCBT, years | <0.001 | |||
| 0–4 | 346 (43%) | 248 (48%) | 98 (34%) | |
| 5–9 | 275 (34%) | 164 (31%) | 111 (38%) | |
| 10–14 | 148 (18%) | 90 (17%) | 58 (20%) | |
| 15–17 | 41 (5%) | 19 (4%) | 22 (8%) | |
| Gender | 0.37 | |||
| Male | 451 (56%) | 284 (55%) | 167 (58%) | |
| Female | 359 (44%) | 237 (45%) | 122 (42%) | |
| Karnofsky/Lansky score | 0.66 | |||
| < 90 | 141 (17%) | 93 (18%) | 48 (17%) | |
| 90–100 | 669 (83%) | 428 (82%) | 241 (83%) | |
| Race of recipient | 0.03 | |||
| Caucasian | 469 (58%) | 320 (61%) | 149 (52%) | |
| African-American | 77 (10%) | 49 (9%) | 28 (10%) | |
| Asian/Pacific Islander | 45 (6%) | 28 (5%) | 17 (6%) | |
| Hispanic | 176 (22%) | 97 (19%) | 79 (27%) | |
| Native American | 11 (1%) | 9 (2%) | 2 (< 1%) | |
| Missing | 32 (4%) | 18 (3%) | 14 (5%) | |
| CMV status of recipient | 0.16 | |||
| Negative | 412 (51%) | 278 (53%) | 134 (46%) | |
| Positive | 391 (48%) | 239 (46%) | 152 (53%) | |
| Missing | 7 (< 1%) | 4 (< 1%) | 3 (1%) | |
| Disease | <0.001 | |||
| AML | 356 (44%) | 255 (49%) | 101 (35%) | |
| ALL | 454 (56%) | 266 (51%) | 188 (65%) | |
| Disease status at UCBT | 0.07 | |||
| Early | 282 (35%) | 182 (35%) | 100 (35%) | |
| Intermediate | 412 (51%) | 253 (49%) | 159 (55%) | |
| Advanced | 114 (14%) | 84 (16%) | 30 (10%) | |
| Missing | 2 (<1%) | 2 (<1%) | 0 | |
| Donor-Recipient sex match | 0.71 | |||
| F-M | 215 (26%) | 141 (27%) | 74 (26%) | |
| F-F | 175 (22%) | 116 (22%) | 59 (20%) | |
| M-M | 234 (29%) | 142 (27%) | 92 (32%) | |
| M-F | 184 (23%) | 121 (23%) | 63 (22%) | |
| Missing | 2 (<1%) | 1 (<1%) | 1 (<1%) | |
| HLA-matching | 0.05 | |||
| 6/6 | 169 (21%) | 111 (21%) | 58 (20%) | |
| 5/6 | 382 (47%) | 230 (44%) | 152 (53%) | |
| 4/6 | 259 (32%) | 180 (34%) | 79 (27%) | |
| Total nucleated cell dose, pre-cryo, median (range) × 107/kg | 7 (3–56) | 7 (3–50) | 6 (3–56) | 0.03 |
| Total nucleated cell dose, pre-cryo, × 107/kg | <0.001 | |||
| 3–5 | 195 (24%) | 107 (21%) | 88 (30%) | |
| 5–8 | 223 (28%) | 133 (26%) | 90 (31%) | |
| ≥ 8 | 289 (36%) | 205 (39%) | 82 (39%) | |
| Missing | 103 (13%) | 76 (15%) | 27 (9%) | |
| Conditioning regimen | ||||
| Myeloablative | 810 (100%) | 521 (100%) | 289 (100%) | – |
| Reduced Intensity | 0 | 0 | 0 | |
| TBI used | 573 (71%) | 314 (60%) | 259 (90%) | <0.001 |
| GVHD prophylaxis | <0.001 | |||
| CNI + SIRO | 34 (4%) | 6 (1%) | 28 (10%) | |
| CNI + MMF | 327 (40%) | 151 (29%) | 176 (61%) | |
| CNI + MTX | 102 (13%) | 56 (11%) | 46 (16%) | |
| CNI + COR | 247 (30%) | 220 (42%) | 27 (9%) | |
| CNI ± other | 100 (12%) | 88 (17%) | 12 (4%) | |
| Year of UCBT | <0.001 | |||
| 2003–2005 | 202 (25%) | 184 (35%) | 18 (6%) | |
| 2006–2008 | 288 (36%) | 182 (35%) | 106 (37%) | |
| 2009–2012 | 320 (39%) | 155 (30%) | 165 (57%) |
Abbreviations: UCBT = Umbilical Cord Blood Transplant; GVHD = graft-vs.-host disease; CNI = Calcineurin Inhibitor (either Cyclosporine or Tacrolimus); SIRO = Sirolimus; MMF = Mycophenolate mofetil; MTX = Methotrexate; COR = Corticosteroids (systemic).
For the 594 recipients of double UCBT, median age was 42 (range, 18–79). 72% of recipients had AML and 28% had ALL. 59% of patients received myeloablative conditioning while 41% received reduced intensity conditioning. 26% received a 5/6 + 5/6-matched combination, 21% received a 5/6 + 4/6 combination, 42% of recipients received a 4/6 + 4/6 combination, and 10% received combinations including a 6/6 UCB unit. All patients received calcineurin inhibitor-based GVHD prophylaxis. ATG was employed in 21% of patients. Table 1b shows differences between patients who received ATG compared to those who did not.
Table 1b.
Clinical characteristics of patients undergoing double UCBT
| Characteristic | All | With ATG | Without ATG | p |
|---|---|---|---|---|
| N | 594 | 122 | 472 | |
| Number of centers | 87 | 38 | 75 | |
| Age, median (range), years | 42 (18–79) | 49 (18–74) | 41 (18–79) | 0.005 |
| Gender | 0.16 | |||
| Male | 297 (50%) | 54 (44%) | 253 (51%) | |
| Female | 297 (50%) | 68 (56%) | 229 (49%) | |
| Karnofsky/Lansky score | <0.001 | |||
| < 90 | 171 (29%) | 50 (41%) | 121 (26%) | |
| 90–100 | 423 (71%) | 72 (59%) | 351 (74%) | |
| Race | 0.02 | |||
| Caucasian | 371 (62%) | 86 (70%) | 285 (60%) | |
| African-American | 69 (12%) | 19 (16%) | 50 (11%) | |
| Asian/Pacific Islander | 49 (8%) | 6 (5%) | 43 (9%) | |
| Hispanic | 84 (14%) | 8 (7%) | 76 (16%) | |
| Native American | 4 (< 1%) | 0 | 4 (< 1%) | |
| Missing | 17 (3%) | 3 (2%) | 14 (3%) | |
| CMV status of recipient | 0.01 | |||
| Negative | 183 (31%) | 25 (20%) | 158 (33%) | |
| Positive | 397 (67%) | 95 (78%) | 302 (64%) | |
| Missing | 17 (3%) | 2 (2%) | 12 (3%) | |
| Disease | 0.006 | |||
| AML | 428 (72%) | 100 (82%) | 328 (69%) | |
| ALL | 166 (28%) | 22 (18%) | 144 (31%) | |
| Disease status at UCBT | <0.001 | |||
| Early | 278 (47%) | 46 (38%) | 232 (49%) | |
| Intermediate | 225 (38%) | 43 (35%) | 182 (39%) | |
| Advanced | 91 (15%) | 33 (27%) | 58 (12%) | |
| Donor-Recipient gender | 0.52 | |||
| (F,F)-M or (F,M)-M | 207 (35%) | 38 (31%) | 169 (36%) | |
| All other combinations | 343 (58%) | 76 (62%) | 267 (57%) | |
| Missing | 44 (7%) | 8 (7%) | 36 (8%) | |
| HLA-matching | 0.77 | |||
| 4/6 + 4/6 | 251 (42%) | 48 (39%) | 203 (43%) | |
| One 4/6 UCB unit | 134 (23%) | 29 (24%) | 105 (22%) | |
| No 4/6 UCB units | 209 (35%) | 45 (37%) | 164 (35%) | |
| Total nucleated cell dose, pre-cryo, median (range) × 107/kg | 5 (3–55) | 4 (3–31) | 5 (3–55) | 0.02 |
| Total nucleated cell dose, pre-cryo, × 107/kg | 0.17 | |||
| 3–5 | 268 (45) | 64 (52%) | 204 (43%) | |
| 5–8 | 190 (32) | 31 (25%) | 159 (34%) | |
| ≥ 8 | 47 (8) | 7 (6%) | 40 (8%) | |
| Missing | 89 (15) | 20 (16%) | 69 (15%) | |
| Conditioning Regimen | 0.002 | |||
| Myeloablative | 351 (59%) | 57 (47%) | 294 (62%) | |
| Reduced Intensity | 243 (41%) | 65 (53%) | 178 (38%) | |
| TBI used | 497 (83%) | 60 (49%) | 437 (93%) | <0.001 |
| GVHD prophylaxis | <0.001 | |||
| CNI + SIRO | 27 (5%) | 14 (11%) | 13 (3%) | |
| CNI + MMF | 535 (90%) | 96 (79%) | 439 (93%) | |
| CNI + MTX | 15 (3%) | 4 (3%) | 11 (2%) | |
| CNI ± other | 17 (4%) | 8 (6%) | 9 (2%) | |
| Year of UCBT | <0.001 | |||
| 2003–2008 | 188 (32%) | 58 (48%) | 130 (28%) | |
| 2009–2012 | 406 (68%) | 64 (52%) | 342 (72%) |
Abbreviations: UCBT = Umbilical Cord Blood Transplant; GVHD = graft-vs.-host disease; CNI = Calcineurin Inhibitor (either Cyclosporine or Tacrolimus); SIRO = Sirolimus; MMF = Mycophenolate mofetil; MTX = Methotrexate; COR = Corticosteroids (systemic).
Single UCBT - GVHD
After single UCBT, the cumulative incidence at of grades II–IV and grades III–IV acute GVHD at 100 days was 39% (95% CI, 36%–43%) and 18% (95% CI, 15%–20%), respectively (Table 2). Median time to acute GVHD was 25 days (range, 3–211). Multivariate analysis showed that absence of ATG was the only significant factor associated with grades II–IV (HR 1.56, 95% CI 1.21–2.01, p = 0.0006) (Figure 1). No significant factors were associated with grades III–IV acute GVHD. Notably, the following were not associated with acute GVHD: age, race, gender, CMV serostatus, HLA-matching, TNC dose, year of transplant, underlying disease, total body irradiation (TBI) and GVHD prophylaxis regimen. Given the clinical differences between recipients of ATG and those who did not, we performed this analysis in patients who did not receive ATG. In this subset, no factors were significantly associated with the development of acute GVHD.
Table 2.
Cumulative incidences of acute and chronic GVHD
| All (95% CI) | With ATG (95% CI) | Without ATG (95%CI) | |
|---|---|---|---|
| Single UCBT | 810 | 521 (64%) | 289 (36%) |
| Cumulative incidence of grades II–IV acute GVHD1 | 39% (36%–43%) | 33% (29%–37%) | 50% (44%–56%) |
| Cumulative incidence of grades III–IV acute GVHD1 | 18% (15%–20%) | 15% (12%–19%) | 21% (17%–26%) |
| Cumulative incidence of chronic GVHD2 | 27% (24%–30%) | 22% (19%–26%) | 35% (29%–41%) |
| Double UCBT | 594 | 122 (21%) | 472 (79%) |
| Cumulative incidence of grades II–IV acute GVHD1 | 45% (41%–49%) | 26% (18–34%) | 50% (46%–55%) |
| Cumulative incidence of grades III–IV acute GVHD1 | 22% (19%–26%) | 16% (10%–23%) | 24% (20%–28%) |
| Cumulative incidence of chronic GVHD2 | 26% (22%–29%) | 21% (15%–29%) | 27% (23%–31%) |
Cumulative incidence of acute GVHD calculated through day +100 after UCBT
Cumulative incidence of chronic GVHD calculated through 1 year after UCBT
Abbreviations: UCBT = umbilical cord blood transplant; GVHD = graft-versus-host disease; ATG = anti-thymocyte globulin
Figure 1.
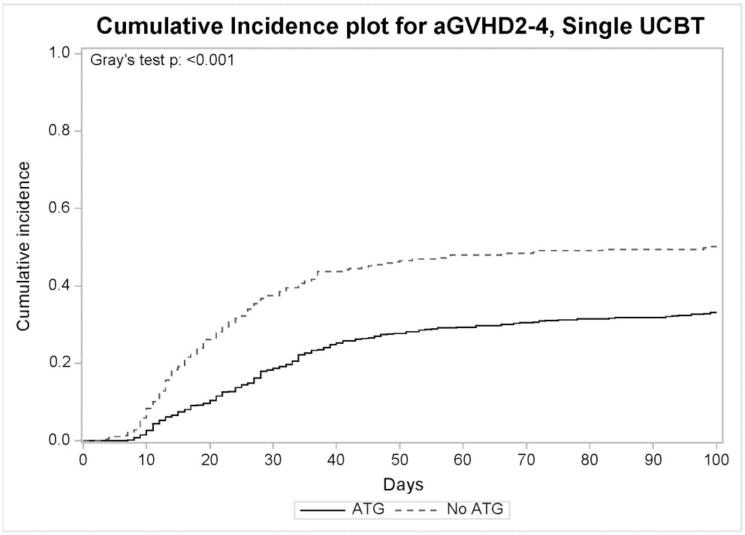
Cumulative incidence of grades 2–4 acute GVHD in recipients of single UCBT who received ATG (n=521) and those who did not (n=289).
After single UCBT, the cumulative incidence of chronic GVHD was 27% (95% CI, 24%–30%) at one year and median time to chronic GVHD was 5.3 months. Multivariate analysis showed that prior acute GVHD (HR 2.02, 95% CI, 1.51–2.70, p<0.0001) was the only significant factor associated with chronic GVHD. Notably, absence of ATG was not significantly associated with the development of chronic GVHD (HR 1.05, 95% CI, 0.76–1.44, p=0.78).
Double UCBT – GVHD
For recipients of double UCBT, the cumulative incidence at day 100 of grades II–IV and grades III–IV acute GVHD was 45% and 22%, respectively. Median onset to acute GVHD was 26 days (range 6–380). Multivariate analysis demonstrated that absence of ATG was associated with grades II–IV acute GVHD (HR 2.33, 95% CI 1.54–3.52, p = 0.0001) (Figure 2), but not with grades III–IV acute GVHD (HR 1.28, 0.89–1.84, p = 0.17). In addition, reduced intensity conditioning protected from both II–IV (HR 0.73, 95% CI 0.56–0.95, p = 0.019) and III–IV (HR 0.63, 95% CI 0.44–0.92, p = 0.016) acute GVHD. Notably, the following factors were not associated with acute GVHD: age, gender, CMV serostatus, HLA-matching, TNC dose, underlying disease, TBI, year of transplant and GVHD prophylaxis regimen. In patients who did not receive ATG, no factors were shown to be significantly associated with acute GVHD.
Figure 2.
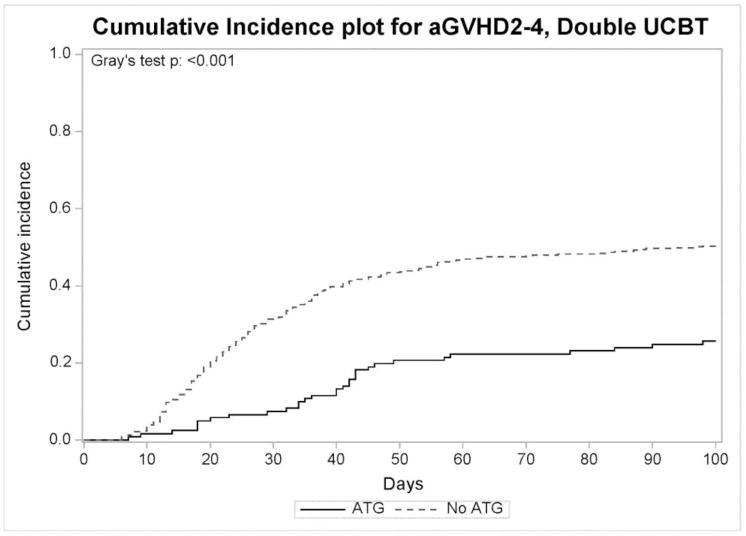
Cumulative incidence of grades 2–4 acute GVHD in recipients of double UCBT who received ATG (n=122) and those who did not (n=472).
For all recipients of double UCBT, the cumulative incidence of chronic GVHD was 26% (95% CI, 22%–29%) at one year. Median time to chronic GVHD was 5.3 months. Multivariate analysis showed that prior acute GVHD (HR 2.12, 95% CI, 1.52–2.95, p <0.0001) was associated with chronic GVHD, while ATG had no significant effect.
Single UCBT – Effect of GVHD on NRM, Relapse, DFS, OS
For all recipients of single UCBT, the development of grades II–IV acute GVHD (HR 2.06, 95% CI 1.47–2.88, p < 0.0001) and grades III–IV acute GVHD (HR 2.75, 95% CI 1.92–3.93, p < 0.0001) were associated with increased NRM. For disease relapse, grades II–IV acute GVHD was protective (HR 0.69, 95% CI 0.51–0.93, p = 0.014) while grades III–IV disease had no significant effect (HR 0.78, 95% CI 0.53–1.15, p = 0.22). For DFS, the development of grades II–IV acute GVHD has no effect (HR 1.06, 95% CI 0.86–1.32, p = 0.58) while grades III–IV disease was associated with shorter DFS (HR 1.38, 95% CI 1.07–1.79, p = 0.014). Similarly, for OS, grades II–IV acute GVHD had no effect (HR 1.08, 95% CI 0.87–1.34, p = 0.50) while grades III–IV disease was associated with shorter survival (HR 1.51, 95% CI 1.17–1.95, p = 0.0017) (see Table 3a). When analyzed independently, grade II acute GVHD did not have a significant effect on DFS or OS compared to those without acute GVHD (HR 0.74, 95% CI 0.55–0.99, P=0.045 for OS; HR 0.77, 95% CI 0.57–1.03, p=0.08 for DFS). Compared to patients with grade III–IV acute GVHD, patients with grade II disease had similar rates of relapse, but less NRM and improved DFS and OS (data not shown). After single UCBT, chronic GVHD led to a higher risk of NRM (HR 1.53, 95% CI 0.97–1.90, p = 0.022) but no effect on relapse (HR 1.10, 95% CI 0.74–1.63, p = 0.63), DFS (HR 1.23, 95% CI 0.91–1.65, p = 0.18) and OS (HR 0.96, 95% CI 0.72–1.29, p = 0.82) (see Table 3a). Table 3b shows full results of the multivariate modeling.
Table 3a.
Effect of acute and chronic GVHD on outcomes after single and double UCBT
| Single UCBT | Relapse | Non-Relapse Mortality | Disease-Free Survival | Overall Survival |
|---|---|---|---|---|
| Gr. 2–4 Acute GVHD | HR 0.69 (0.51–0.93) p = 0.014 |
HR 2.06 (1.47–2.88) p < 0.0001 |
HR 1.06 (0.86–1.32) p = 0.58 |
HR 1.08 (0.87–1.34) p = 0.50 |
| Gr. 3–4 Acute GVHD | HR 0.78 (0.53–1.15) p = 0.22 |
HR 2.75 (1.92–3.93) p < 0.0001 |
HR 1.38 (1.07–1.79) p = 0.014 |
HR 1.51 (1.17–1.95) p = 0.0017 |
| Chronic GVHD | HR 1.10 (0.74–1.63) p = 0.63 |
HR 1.53 (0.97–2.42) p = 0.068 |
HR 1.23 (0.91–1.65) p = 0.18 |
HR 0.96 (0.72–1.29) p = 0.82 |
| Double UCBT | Relapse | Non-Relapse Mortality | Disease-Free Survival | Overall Survival |
| Gr. 2–4 Acute GVHD | HR 0.87 (0.63–1.20) p = 0.39 |
HR 1.41 (1.05–1.90) p = 0.022 |
HR 1.11 (0.90–1.37) p = 0.34 |
HR 1.05 (0.86–1.30) p = 0.60 |
| Gr. 3–4 Acute GVHD | HR 0.68 (0.44–1.06) p = 0.084 |
HR 2.24 (1.66–3.04) p < 0.0001 |
HR 1.41 (1.11–1.79) p = 0.005 |
HR 1.48 (1.17–1.86) p = 0.001 |
| Chronic GVHD | HR 0.59 (0.36–0.96) p = 0.033 |
HR 1.49 (0.98–2.29) p = 0.065 |
HR 0.98 (0.71–1.33) p = 0.87 |
HR 0.95 (0.71–1.27) p = 0.70 |
Abbreviations: UCBT=Umbilical Cord Blood Transplant; GVHD = Graft-vs.-Host Disease; Gr. = Grade
Table 3b.
Hazard ratios for clinically significant predictors of major outcomes after single and double UCBT
| Single UCBT | |||||
|---|---|---|---|---|---|
| Overall Survival | Disease-Free Survival | Relapse | Non-Relapse Mortality | Chronic GVHD | |
| Gr. II–IV Acute GVHD | 0.69 (0.51–0.93) p=0.014 |
2.06 (1.47–2.88) p<0.0001 |
2.02 (1.51–2.70) p<0.0001 |
||
| Primary Disease1 | 0.73 (0.58 – 0.91) p=0.0054 |
0.71 (0.57–0.89) p=0.0024 |
|||
| Disease Status at Transplant2 | 1.37 (1.06 – 1.76) 2.89 (2.14–3.91) p<0.0001 |
1.32 (1.03–1.68) 2.58 (1.90–3.50) p<0.0001 |
1.57 (1.12–2.21) 4.88 (3.28–7.27) p<0.0001 |
||
| CMV Positivity | 1.47 (1.19–1.82) p=0.0003 |
1.45 (1.17–1.79) p=0.0006 |
1.77 (1.27–2.46) p=0.0008 |
||
| Age3 | 0.77 (0.56–1.07) 0.61 (0.41–0.89) p=0.011 |
1.40 (0.95–2.07) 2.03 (1.37–3.01) p=0.0018 |
|||
| Absence of ATG | 1.49 (1.08–2.07) p=0.016 |
0.44 (0.30–0.66) p<0.0001 |
|||
| TBI Use | 0.59 (0.43–0.81) p=0.0013 |
||||
| Double UCB | |||||
| Overall Survival | Disease-Free Survival | Relapse | Treatment-Related Mortality | Chronic GVHD | |
| Gr. II–IV Acute GVHD | 2.12 (1.52–2.95) p<0.0001 |
||||
| Primary Disease1 | 1.02 (0.81–1.28) 1.86 (1.39–2.48) p<0.0001 |
||||
| Disease Status at Transplant2 | 1.02 (0.81–1.28) 1.56 (1.12–2.18) p=0.022 |
1.42 (1.01–2.00) 3.56 (2.27–5.57) p<0.0001 |
|||
| TBI Use | 0.73 (0.55–0.97) p=0.032 |
0.67 (0.45–1.00) p=0.049 |
|||
| Karnofsky Performance Status4 | 0.71 (0.57–0.89) p=0.0028 |
0.77 (0.61–0.97) p=0.024 |
0.66 (0.48–0.90) p=0.0095 |
||
| Conditioning Regimen5 | 1.36 (1.10–1.69) p=0.0048 |
2.53 (1.84–3.47) p<0.0001 |
0.59 (0.40–0.87) p=0.0083 |
||
| Age3 | 1.28 (0.88–1.87) 1.83 (1.17–2.86) p=0.029 |
||||
Hazards for each relationship presented, p values for entire trend presented
ALL vs AML
Intermediate vs. Early, Advanced vs. Early
Age 5–9 vs. 0–4 and 10–17 vs. 0–4 for single UCBT; Age 18–29 vs. 30–49 vs. 50+ for double UCBT
Karnofsky performance status < 90 vs 90–100
Redcued Intensity vs. Myeloablative
Double UCBT – Effect of GVHD on NRM, Relapse, PFS, OS
For all recipients of double UCBT, the development of grades II–IV acute GVHD (HR 1.41, 95% CI 1.05–1.90, p = 0.022) and grades III–IV acute GVHD (HR 2.24, 95% CI 1.66–3.04, p < 0.0001) were associated with increased NRM. In terms of disease relapse, neither grades II–IV acute GVHD (HR 0.87, 95% CI 0.63–1.20, p = 0.39) nor grades III–IV disease (HR 0.68, 95% CI 0.44–1.06, p = 0.084) had any significant impact. Concerning DFS, the development of grades II–IV acute GVHD had no effect (HR 1.11, 95% CI 0.90–1.37, p = 0.34) while grades III–IV disease was associated with shorter DFS (HR 1.41, 95% CI 1.11–1.79, p = 0.005). Similarly, for OS, grades II–IV acute GVHD had no effect (HR 1.05, 95% CI, 0.86–1.30, p = 0.60) while grades III–IV disease was associated with shorter survival (HR 1.48, 95% CI, 1.17–1.86, p = 0.001) (see Table 3a). When analyzed independently, grade II acute GVHD did not have a significant effect on DFS (HR 0.80, 95% CI, 0.60–1.06, p = 0.12) but did lead to improved OS (HR 0.69, 95% CI, 0.52–091, p = 0.0084) when compared to those without acute GVHD. Compared to patients with grades III–IV disease, patients with grade II acute GVHD had similar rates of relapse, but had significantly less NRM and improved DFS and OS (data not shown). For all recipients of double UCBT, chronic GVHD showed a borderline significant association with a higher risk of NRM (HR 1.49, 95% CI, 0.98–2.29, p = 0.065) and a significantly lower relapse (HR 0.59, 95% CI 0.36–0.96, p= 0.033), but had no significant effect on DFS (HR 0.98, 95% CI 0.71–1.33, p = 0.87) or OS (HR 0.95, 95% CI 0.71–1.27, p = 0.70) (see Table 3a). Table 3b shows full results of the multivariate modeling.
DISCUSSION
We performed a large registry analysis using the CIBMTR database to define the incidence of acute and chronic GVHD, the clinical factors associated with their development and the effect of GVHD on outcomes after pediatric single and adult double UCBT. Our results confirm that the incidence of acute GVHD after UCBT is comparable to that observed with conventional donor sources7, but the incidence of chronic GVHD appears to be less, which has been reported previously.8,9
In our study, for pediatric recipients of single UCBT, the absence of ATG was significantly associated with the development of grades II–IV acute GVHD. Prior acute GVHD was associated with chronic GVHD. In the setting of adult double UCBT, the absence of ATG and myeloablative conditioning were associated with grades II–IV acute GVHD while prior acute GVHD was associated with chronic GVHD. As expected, severe acute GVHD resulted in increased NRM and decreased DFS and OS after both single and double UCBT. After single UCBT, the development of chronic GVHD appeared to increase NRM; whereas after double UCBT, chronic GVHD was associated with less disease relapse. Yet, chronic GVHD clearly had no significant effect on DFS and OS.
Several retrospective analyses have previously attempted to define risk factors for acute and chronic GVHD after UCBT and these are summarized in Tables 4a and 4b, respectively. Similar to our analysis, lack of ATG and myeloablative conditioning have been associated with acute GVHD in other studies,10–12 yet unlike our study, degree of HLA-matching has also been shown to be influential.10,12,13 For chronic GVHD, as observed in our analysis, prior acute GVHD has been shown to be the most important factor,10–12,14 and several groups have also reported the association of higher HLA-mismatch with chronic GVHD.10,12,14
Table 4a.
Summary of studies analyzing risk factors for acute GVHD after UCBT
| Study | Population | Incidence of grades II–IV acute GVHD | Incidence of grades III–IV acute GVHD | Risk Factors |
|---|---|---|---|---|
| Macmillan et al.11 | Single UCBT (n=80) |
39% | 18% | Use of 2 UCB units NMA conditioning regimen No ATG |
| Double UCBT (n=185) |
58% | 19% | ||
| Lazaryan et al.10 | Single UCBT (n=295) |
26% | 7% | Age ≥ 18 Higher HLA-mismatcha |
| Double UCBT (n=416) |
56% | 21% | No ATGa Year of UCBT 2006 or latera Higher HLA-mismatchb Myeloablative conditioningb |
|
| Ponce et al.13 | Double UCBT (n=115) |
53% | 23% | Higher HLA-mismatchb |
| Xavier et al.12 | Double UCBT (n=921) |
36% | 15% | Myeloablative conditioninga Higher HLA-mismatcha No ATG Advanced disease stageb |
| Chen et al. | Single UCBT (n=810) |
39% | 18% | No ATG |
| Double UCBT (n=594) |
45% | 22% | No ATGa Myeloablative conditioning |
Abbreviations: GVHD = graft-vs.-host disease; UCBT = umbilical cord blood transplantation; UCB = umbilical cord blood; NMA = non-myeloablative; ATG = anti-thymocyte globulin; HLA = human leukocyte antigen; iv-TCD = in vivo T-cell depletion
Only for grades II–IV acute GVHD
Only for grades III–IV acute GVHD
Table 4b.
Summary of studies analyzing risk factors for chronic GVHD after UCBT
| Study | Population | Incidence of chronic GVHD | Risk Factors |
|---|---|---|---|
| Macmillan et al.11 | Single UCBT (n=80) |
18% | Prior grades II–IV acute GVHD |
| Double UCBT (n=185) |
17% | ||
| Lazaryan et al.10 | Single UCBT (n=295) |
7% | Age ≥ 18 Non-use of cyclosporine/MMF for GVHD prophylaxis |
| Double UCBT (n=416) |
26% | Higher HLA-mismatch Myeloablative conditioning Prior grades II–IV acute GVHD |
|
| Ponce et al.13 | Double UCBT (n=115) |
23% | Not reported |
| Narimatsu et al.14 | Single UCBT (n=1072) |
28% | Higher recipient body weight Higher HLA-mismatch Myeloablative conditioning Use of mycophenolate mofetil Prior grades II–IV acute GVHD |
| Xavier et al.12 | Double UCBT (n=921) |
25% | Higher HLA-mismatch Prior grades II–IV acute GVHD |
| Chen et al. | Single UCBT (n=810) |
27% | Prior grades II–IV acute GVHD |
| Double UCBT (n=594) |
26% |
Abbreviations: GVHD = graft-vs.-host disease; UCBT = umbilical cord blood transplantation; UCB = umbilical cord blood; MMF = mycophenolate mofetil; ATG = anti-thymocyte globulin; HLA = human leukocyte antigen; iv-TCD = in vivo T-cell depletion
Our study represents the largest study of UCBT patients investigating risk factors for acute and chronic GVHD, as well as the effect that acute and chronic GVHD has on long-term outcomes. The use of ATG appeared to be the most significant factor associated with acute GVHD after both single UCBT and double UCBT. It is important to note that our analysis did not distinguish different types of ATG, incorporate information on the dose or schedule employed as well as accompanying systemic corticosteroids given or report the rationale behind why treating physicians chose to use ATG. It is interesting that use of ATG was not significantly associated with chronic GVHD after single or double UCBT and this may reflect a significant difference between UCBT and other donor sources. After multivariate analysis, ATG had no significant effect on PFS or OS after single or double UCBT. While the overall effect of ATG was not a primary objective of this study, we do believe that future analyses are warranted with a focus on other important endpoints not collected here such as graft failure, specific infections and post-transplant lymphoproliferative disease. The use of ATG in UCBT is controversial as recent studies have suggested a benefit in terms of protection from GVHD,15,16 while other studies have shown that ATG is associated with an increase in overall mortality.17–19
Interestingly, the degree of HLA-mismatch was not a significant factor in our multivariate analysis for single UCBT, and we could not analyze this factor accurately in double UCBT due to missing information on specific cord unit dominance. Other limitations of our analysis include those inherent to any large registry analysis including heterogeneity of practice. Specifically, for studies involving GVHD, the diagnosis does not undergo rigorous central review. This point is emphasized by a recent analysis describing characteristics of chronic GVHD in 87 patients undergoing UCBT at a single center. After review of medical records for the 54 patients who were reported to have chronic GVHD, only 7 had classic chronic GVHD.20 In our analysis, the severity of chronic GVHD was not able to be analyzed as the modern NIH classification and grading system for chronic GVHD was developed in the midst of the era of transplantation for this group.6 We also did not review or analyze any information on treatment for GVHD or response, but this should certainly be studied, especially as GVHD after UCBT may respond differently compared to GVHD after transplantation from other donor sources.21
In conclusion, acute and chronic GVHD remain significant complications after UCBT with severe acute GVHD clearly impacting long-term survival. Rates of acute GVHD appear comparable to what is observed with conventional matched donor sources, yet the incidence of chronic GVHD appears to be significantly less. In our study, omission of ATG was the most important risk factor associated with the development of acute GVHD, and prior acute GVHD was the most significant risk factor for the development of chronic GVHD. Preventing acute GVHD for patients after UCBT should be a priority and possible avenues include formally defining the role of ATG, enhanced or novel GVHD prophylaxis regimens22,23 and improving methods of UCB expansion or activation to use better HLA-matched units.24 While chronic GVHD appears to be less prevalent after UCBT, a future analysis should be performed when a large number of patients have been classified according to a standard grading scheme where severity of disease can be taken into account to truly assess its impact.
Supplementary Material
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
AUTHORSHIP CONTRIBUTIONS
YC, CSC, TW, SRS, MA drafted the research plan; YC, CSC, TW, SRS, MA, MTH, DRC, AA, JP, AU, SWC, TN, TT, YI, BW, DIM, HA, LL, LY, MB, MSC, MQ, RS, RPG, RM, SJ, AB, BS, HF, IDL, JS, MA, MAK, MA, OR, RR, RFO, SH, SS, TRS, MLM, AL critically revised research plan; TW and MTH performed statistics; YC, CSC, TW, SRS, MA analyzed and interpreted data; YC, CSC, TW, SRS, MA drafted the paper; YC, CSC, TW, SRS, MA, MTH, DRC, AA, JP, AU, SWC, TN, TT, YI, BW, DIM, HA, LL, LY, MB, MSC, MQ, RS, RPG, RM, SJ, AB, BS, HF, IDL, JS, MA, MAK, MA, OR, RR, RFO, SH, SS, TRS, MLM, AL critically revised the paper.
Disclosure of Conflicts of Interest: The authors have no relevant conflicts to declare.
References
- 1.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transp. 2010;I:87–105. [PubMed] [Google Scholar]
- 4.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 6.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunstein CG, Eapen M, Ahn KW, Ballen KK, Champlin RE, Cutler C, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazaryan A, Weisdorf DJ, DeFor T, Brunstein CG, MacMillan ML, Behanyan N, et al. Risk Factors for Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation with Umbilical Cord Blood and Matched Related Donors. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xavier E, Ruggeri A, Labopin M, Blaise D, Chevallier P, Fegeux N, et al. Double cord blood transplantation: incidence, organ involvement and risk factors of acute graft versus host disease. A retrospective analysis on behalf of Eurocord, ALWP and Cqwp-EBMT. Blood. 2014;124:187. (abstract) [Google Scholar]
- 13.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant. 2013;19:904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narimatsu H, Miyakoshi S, Yamaguchi T, Kami M, Matsumura T, Yuji K, et al. Chronic graft-versus-host disease following umbilical cord blood transplantation: retrospective survey involving 1072 patients in Japan. Blood. 2008;112:2579–2582. doi: 10.1182/blood-2007-11-118893. [DOI] [PubMed] [Google Scholar]
- 15.Ponce DM, Eapen M, Sparapani R, O’Brien TA, Chan KW, Chen J, et al. In Vivo T Cell Depletion with Myeloablative Regimens on Outcomes after Cord Blood Transplantation for Acute Lymphoblastic Leukemia in Children. Biol Blood Marrow Transplant. 2015;12:2173–2179. doi: 10.1016/j.bbmt.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–32. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 17.Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milipied N, Chevallier P, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50:45–50. doi: 10.1038/bmt.2014.216. [DOI] [PubMed] [Google Scholar]
- 18.Pascal L, Tucunduva L, Ruggeri A, Blaise D, Ceballos P, Chevallier P, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126:1027–1032. doi: 10.1182/blood-2014-09-599241. [DOI] [PubMed] [Google Scholar]
- 19.Zheng C, Luan Z, Fang J, Sun X, Chen J, Li CK, et al. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high-risk or advanced hematological malignancies. Biol Blood Marrow Transplant. 2015;21:707–712. doi: 10.1016/j.bbmt.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Newell LF, Flowers ME, Gooley TA, Milano F, Carpenter PA, Martin PJ, et al. Characteristics of chronic GVHD after cord blood transplantation. Bone Marrow Transplant. 2013;48:1285–90. doi: 10.1038/bmt.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murata M, Nakasone H, Kanda J, Nakane T, Fukuda T, Mori T, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19:1183–1189. doi: 10.1016/j.bbmt.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Bejanyan N, Rogosheske J, DeFor T, Lazaryan A, Esbaum K, Holtan S, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;5:926–933. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation-from niche to bedside. Nat Rev Clin Oncol. 2015;12:163–174. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


