Abstract
Background
Molecular and behavioral studies support a role for innate immune proinflammatory pathways in mediating the effects of alcohol. Increased levels of Toll-like receptors (TLRs) have been observed in animal models of alcohol consumption and in human alcoholics, and many of these TLRs signal via the MyD88-dependent pathway. We hypothesized that this pathway is involved in alcohol drinking and examined some of its key signaling components.
Methods
Different ethanol drinking paradigms were studied in male and female control C57BL/6J mice vs. mice lacking CD14, TLR2, TLR4 (C57BL/10ScN), or MyD88. We studied continuous and intermittent access two-bottle choice (2BC) and one-bottle and 2BC drinking-in-the-dark (DID) tests as well as preference for saccharin, quinine, and NaCl.
Results
In the 2BC continuous access test, ethanol intake decreased in male TLR2 knockout (KO) mice, and we previously reported reduced 2BC drinking in male and female CD14 KO mice. In the intermittent access 2BC test, ethanol intake decreased in CD14 KO male and female mice, whereas drinking increased in MyD88 KO male mice. In the 2BC-DID test, ethanol drinking decreased in male and female mice lacking TLR2, whereas drinking increased in MyD88 KO male mice. In the one-bottle DID test, ethanol intake decreased in female TLR2 KO mice. TLR2 KO and CD14 KO mice did not differ in saccharin preference but showed reduced preference for NaCl. MyD88 KO mice showed a slight reduction in preference for saccharin.
Conclusions
Deletion of key components of the MyD88-dependent pathway produced differential effects on ethanol intake by decreasing (TLR2 KO and CD14 KO) or increasing (MyD88 KO) drinking, while deletion of TLR4 had no effect. Some of the drinking effects depended on the sex of the mice and/or the ethanol-drinking model.
Keywords: two-bottle choice and drinking-in-the-dark, TLR4 deficient (C57BL/10ScN) mice, TLR2 KO, CD14 KO, MyD88 KO
Introduction
Neuroimmune and proinflammatory signaling mediate some of the acute and chronic effects of alcohol and support a neuroimmune hypothesis of alcohol addiction (Mayfield et al., 2013; Robinson et al., 2014; Crews and Vetreno, 2016). The immune activator, lipopolysaccharide (LPS), induced prolonged increases in ethanol consumption in mice (Blednov et al., 2011), and chronic intermittent ethanol intake or LPS treatment, produced overlapping changes in mouse brain transcriptomes (Osterndorff-Kahanek et al., 2013). In addition, deletion of neuroimmune-related genes that were associated with ethanol drinking based on meta-analyses in mice (Mulligan et al., 2006), rats (Kimpel et al., 2007), and humans (Liu et al., 2006; Liu et al., 2007; Flatscher-Bader et al., 2008) decreased ethanol intake in mice and provided behavioral validation of the genomic evidence (Blednov et al., 2012b). Research is currently focused on the neuroimmune pathways that are directly involved in regulating alcohol drinking and identifying drugs to target the predominant pathways.
Toll-like receptors (TLR) are a major component of innate immune signaling and have been hypothesized to mediate some effects of chronic alcohol in the brain in animal models and human alcoholics (Crews and Vetreno, 2016; Montesinos et al., 2016a). Increased levels of TLR mRNA and/or protein were reported in mouse brain following chronic and binge-like ethanol exposure as well as in human postmortem brain from alcoholics (Robinson et al., 2014). A few studies have suggested a role for TLR4 in ethanol drinking (Liu et al., 2011; Harris and Blednov, 2013; June et al., 2015), although this has not been consistently observed (Alfonso-Loeches et al., 2010; Pascual et al., 2011; Bajo et al., 2016; Harris et al., in press). The TLR family in mammals consists of 13 known members that are essential for defense against pathogens and are known as pathogen-associated molecular patterns (Beutler, 2009). Many TLRs (e.g., TLR2 and TLR4) utilize the co-receptor CD14 for signal recognition (Chun and Seong, 2010). Ligand recognition by TLRs leads to the recruitment of downstream adaptors such as MyD88 (Myeloid differentiation primary response protein MyD88), TIRAP [Toll/interleukin-1 receptor (TIR) domain-containing adapter protein], TRIF (TIR domain-containing adapter protein inducing interferon-β), and TRAM (TIR domain-containing adapter molecule); and TLR signaling occurs through MyD88-dependent and TRIF-dependent pathways (Akira et al., 2006; Beutler, 2009). The MyD88-dependent pathway is utilized by all TLRs except TLR3 (Miggin and O’Neill, 2006).
In order to evaluate specific components of the MyD88 pathway on ethanol consumption, we examined different tests of ethanol consumption that model chronic, intermittent, and binge-like drinking in male and female control mice vs. mice lacking CD14, TLR2, TLR4, or MyD88. We chose two tests of long-term access to ethanol [two-bottle choice (2BC) continuous and intermittent models] and two tests of limited access [drinking-in-the dark (DID) with and without free choice]. Compared to 2BC continuous drinking, 2BC intermittent (every-other-day) access can promote higher alcohol intake. DID was chosen as a binge-like model of consumption that produces acutely elevated blood alcohol levels. Different ethanol exposure paradigms can produce distinct changes in patterns of gene expression in mouse brain despite producing similar amounts of ethanol intake (Osterndorff-Kahanek et al., 2013). Also, drinking effects in a particular null mutant can depend on sex and the drinking protocol (Mayfield et al., 2016). These findings highlight the importance of examining genotype effects in male and female animal models using multiple tests of ethanol consumption. Based on our previous studies of mice lacking different immune-related genes (Blednov et al., 2012b), we predicted that ethanol consumption and preference would decrease in mice lacking components of the MyD88-dependent pathway.
Materials and Methods
Mice
Generation of Cd14 (B6.129S4-Cd14tm1Frm/J, stock #003726), Tlr2 (B6.129-Tlr2tm1Kir/J, stock #004650), and Myd88 (B6.129P2(SJL)-Myd88tm1.1Defr/J, stock #009088) knockout (KO) mice were described previously (Moore et al., 2000; Wooten et al., 2002; Hou et al., 2008). The C57BL/10ScN mouse was used as a TLR4 deficient model (B6.B10ScN-Tlr4lps-del/JthJ, stock #007227). This strain is homozygous for a deletion allele Tlr4lps-del that causes spontaneous deletion of TLR4, resulting in absence of TLR4 mRNA and protein. All mutant strains were purchased from Jackson Laboratories and were backcrossed on a C57BL/6J genetic background more than 6 generations, and thus the C57BL/6J inbred strain is an appropriate control for these studies (https://www.jax.org/jax-mice-and-services/customer-support/technical-support/breeding-and-husbandry-support/considerations-for-choosing-controls). C57BL/6J mice were taken from a colony maintained at The University of Texas at Austin (original breeders were purchased from Jackson Laboratories, Bar Harbor, ME). Mice were group-housed 4 to 5 to a cage based on genotype and sex. The humidity and temperature of the rooms were kept constant and they were maintained a 12/12 hour light /dark cycle with lights on at 7 AM. Food and water were available ad libitum. Behavioral testing began when the mice were at least 2 months old, and mice were weighed every 4–6 days. All experiments were conducted in isolated behavioral testing rooms in the Animal Resource Center at UT Austin with a reversed light/dark cycle to avoid external distractions. Before beginning experiments, mice were moved to their experimental room and remained there for 1–2 weeks to adapt to the new light/dark cycle. All experiments were approved by the university’s Institutional Animal Care and Use Committee.
Two-bottle choice (2BC)
The 24-h continuous 2BC protocol was carried out as previously described (Blednov et al., 2001), where mice had access to one bottle of ethanol and one bottle of water at all times. For this and other drinking tests described below, ethanol (Aaper Alcohol and Chemical, Shelbyville, KY) solutions were prepared fresh daily in tap water, and bottles were weighed before placement and after removal from the experimental cages. The location of the ethanol bottle was alternated daily to control for potential side preferences. Ethanol consumption (g/kg body weight/24h) was calculated for each mouse and values were averaged for each concentration of ethanol. Ethanol concentrations were increased from 3% to 21%, with each concentration being presented for 4 days.
Preference for non-ethanol tastants
Ethanol-naive control and mutant mice were tested for saccharin, quinine, and NaCl consumption using a 24-h 2BC protocol in which 1 bottle contained water and the other contained the tastant solution. Mice were offered a series of increasing concentrations of saccharin (0.00165, 0.0033, 0.0099, 0.0165, and 0.033%) and quinine hemisulfate (0.003, 0.009, 0.0165, 0.03, and 0.06 mM). Sodium chloride was presented in concentrations 75, 150, and 300 mM. For each tastant, the low concentration was always presented first. Each concentration was offered for 4 days, and bottle positions were changed daily.
Two-bottle choice-every-other-day (2BC-EOD)
Intermittent (EOD) access to ethanol increases voluntary drinking in rats (Wise, 1973; Simms et al., 2008) and mice (Melendez, 2011; Rosenwasser et al., 2013). Mice were given EOD access to ethanol (15 or 20%) and water for 24-h sessions, and water only was offered on off days (Osterndorff-Kahanek et al., 2013). The side placement of the ethanol bottles was alternated with each drinking session. The quantity of ethanol consumed was calculated as g/kg body weight/24h.
One-bottle drinking-in-the-dark (1B-DID)
Consumption of ethanol (20%) under binge-like drinking conditions achieves pharmacologically significant blood ethanol levels (Rhodes et al., 2005). Beginning 3 h after lights off, the water bottle in each cage was replaced with a bottle containing 20% ethanol. The ethanol bottle remained in place for either 2 h (days 1–3) or 4 h (day 4) and was then replaced with a water bottle. Bottle positions were changed daily. Except for this short period of ethanol exposure, mice had unlimited access to water. The quantity of ethanol consumed was calculated as g/kg body weight every 2 or 4 h.
Two-bottle choice drinking-in-the-dark (2BC-DID)
This was similar to the 1B-DID test described above by Rhodes et al. (2005) except that 2 bottles containing either 20% ethanol or water were used beginning 3 h after lights off (Blednov and Harris, 2008). The ethanol and water bottles remained in place for 3 h. After their removal, mice had unlimited access to 1 bottle of water. Bottle positions were changed daily. The quantity of ethanol consumed was calculated as g/kg body weight/3h.
Statistical Analysis
Data are reported as the mean ± S.E.M. The statistics software program GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) was used to perform one or two-way ANOVAs and Bonferroni or Student’s t-tests. Data from male and female mice were analyzed separately.
Results
Two-bottle choice (2BC) drinking
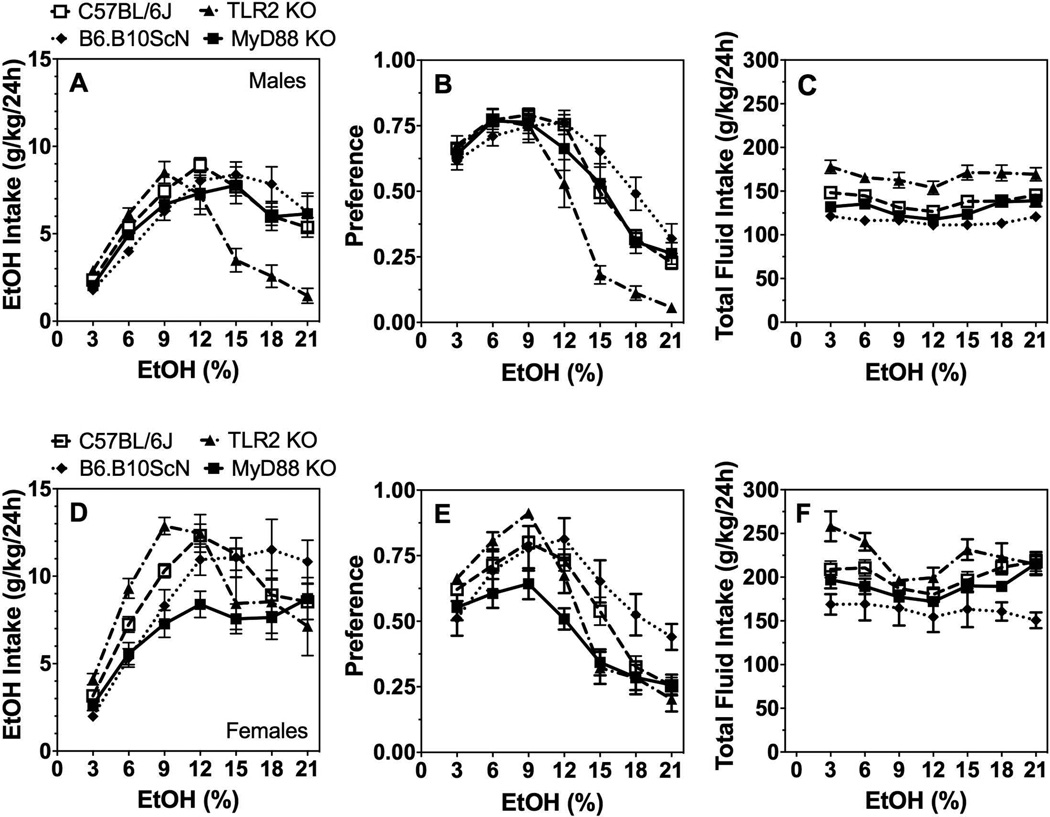
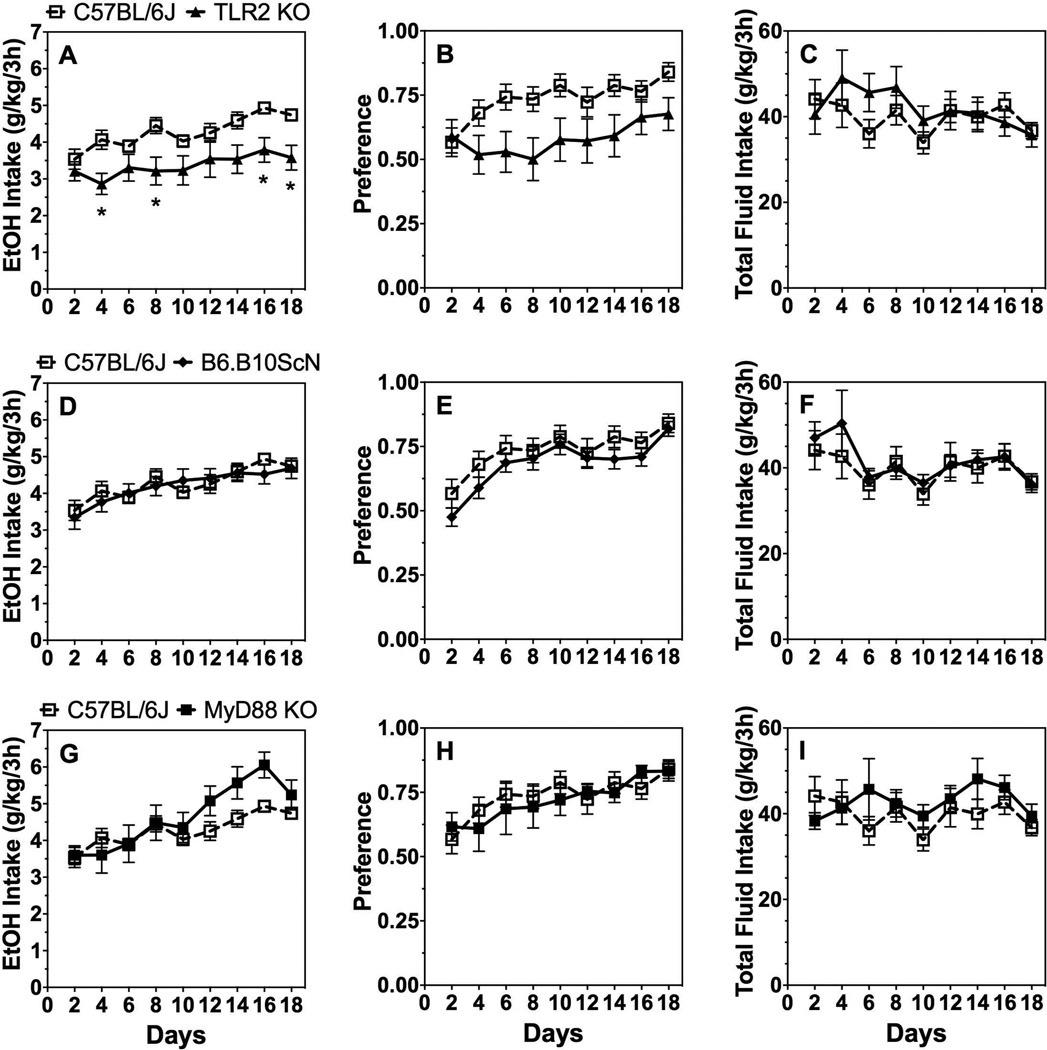
In the 2BC paradigm in which mice could drink either water or a series of increasing ethanol (3–21%) concentrations, ethanol intake [effect of genotype: F(1,36)=9.7;P < 0.01] and preference [effect of genotype: F(1,36)=11.3;P < 0.01] decreased while total fluid intake increased [effect of genotype: F(1,36)=14.2;P < 0.001] in TLR2 KO male mice compared with C57BL/6J control mice (Fig. 1A–C). Lack of TLR4 or MyD88 did not alter ethanol consumption or preference in male mice (Supplemental Table 1), but there was a significant genotype x concentration interaction effect on ethanol intake [F(6,240)=4.1;P < 0.001] and preference [F(6,240)=5.5;P < 0.001] in TLR4 deficient male mice (Fig. 1A,B). TLR4 male and female mutant mice had slightly reduced total fluid intake compared to controls [effect of genotype: F(1,40)=16.6;P < 0.001 for males and F(1,34)=7.1;P < 0.05 for females] (Fig. 1C,F). No significant differences in ethanol intake, preference, or total fluid intake were found in female mutant vs. control mice (Fig. 1D–F; Supplemental Table 1). There was a significant genotype x concentration interaction effect for ethanol intake and preference in female mutant mice as well as a genotype x concentration interaction for total fluid intake in female TLR2 and TLR4 mutants (Supplemental Table 1).
Figure 1. 2BC continuous access drinking in mice lacking TLR2, TLR4, or MyD88.
Ethanol intake (g/kg/24h) (A), preference for ethanol (B), and total fluid intake (g/kg/24h) (C) in male control (C57BL/6J, n= 26) vs. mutant mice (n= 7, 12, and 16 for MyD88 KO, TLR2 KO, and TLR4 mutant, respectively). Ethanol intake (g/kg/24h) (D), preference for ethanol (E), and total fluid intake (g/kg/24h) (F) in female control (n= 26) vs. mutant mice (n= 7 for MyD88 KO; n= 10 for TLR2 KO and TLR4 mutant). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests.
Two-bottle choice-every-other-day (2BC-EOD) drinking
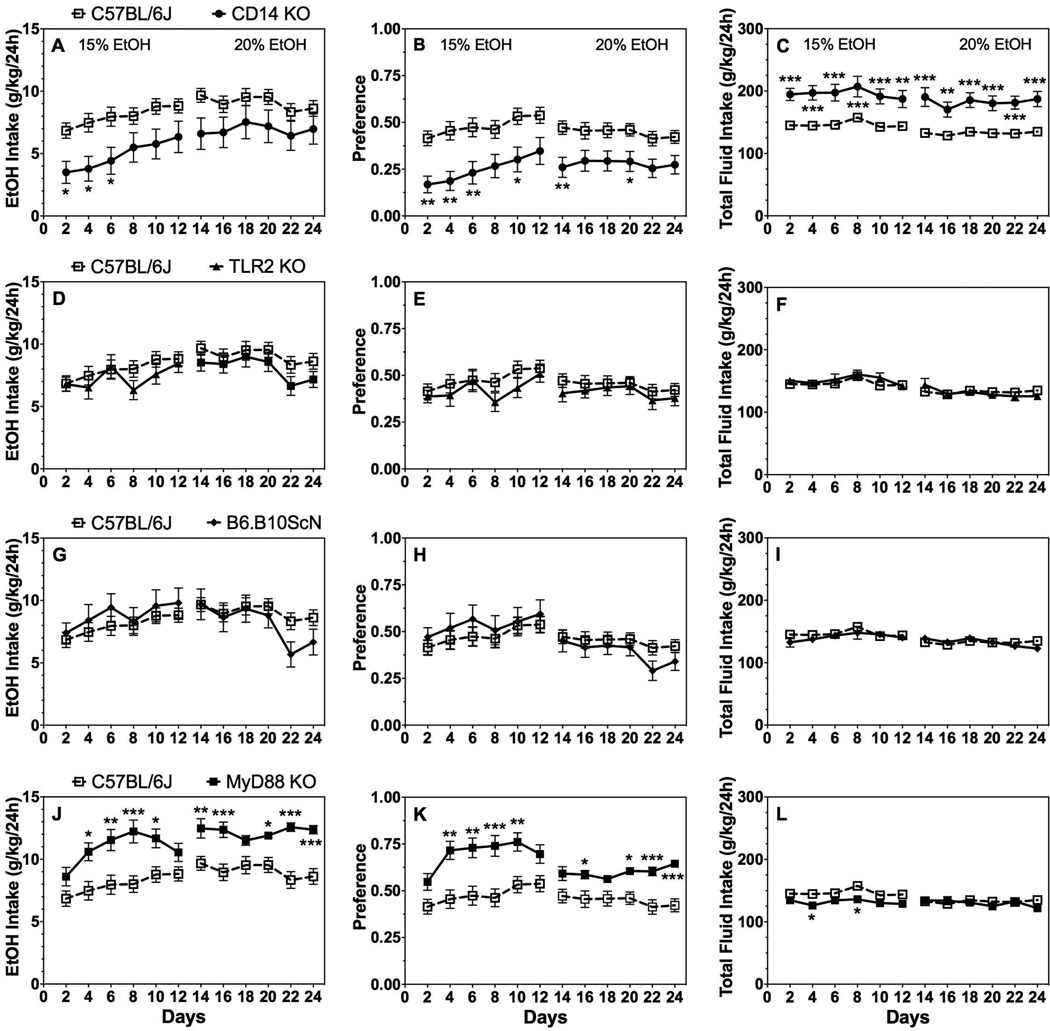
Over 24 total days of intermittent (EOD) consumption of 15% followed by 20% ethanol, intake and preference decreased in CD14 KO male mice while total fluid intake increased compared to control mice. There was an effect of genotype on 15% [F(1,40)=7.7;P < 0.01] but not 20% ethanol intake; a genotype effect on preference for 15% and 20% ethanol, respectively [F(1,40)=11.8;P < 0.01 and F(1,40)=7.9;P < 0.01]; and genotype effect on total fluid intake in the presence of 15% and 20% ethanol, respectively [F(1,40)=17.9;P < 0.001 and F(1,40)=22.3;P < 0.001] (Fig. 2A–C; Supplemental Table 2). No significant differences in ethanol intake, preference, or total fluid intake were found in male mice lacking TLR2 (Fig. 2D–F) or TLR4 (Fig. 2G–I) compared to control mice (Supplemental Table 2). Surprisingly, MyD88 KO male mice showed significantly increased ethanol intake and preference for both 15% and 20% ethanol solutions [genotype effect on intake for 15% and 20% ethanol, respectively: F(1,40)=10.0;P < 0.01 and F(1,40)=17.9;P < 0.001 and genotype effect on preference for 15% and 20% ethanol, respectively: F(1,40)=12.8;P < 0.001 and F(1,40)=12.7;P < 0.001] (Fig. 2J,K; Supplemental Table 2). Total fluid intake was slightly reduced in the presence of 15% [effect of genotype: F(1,40)=7.2;P < 0.05], but not 20% ethanol (Fig. 2L; Supplemental Table 2).
Figure 2. 2BC-EOD drinking in male mice lacking CD14, TLR2, TLR4, or MyD88.
Ethanol intake (g/kg/24h) (A, D, G, J), preference for ethanol (B, E, H, K), and total fluid intake (C, F, I, L) in control (C57BL/6J, n=26) vs. mutant male mice (n= 18 for MyD88 KO, TLR2 KO, and CD14 KO; n= 12 for TLR4 mutant). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, **P < 0.01, ***P < 0.001 compared to control).
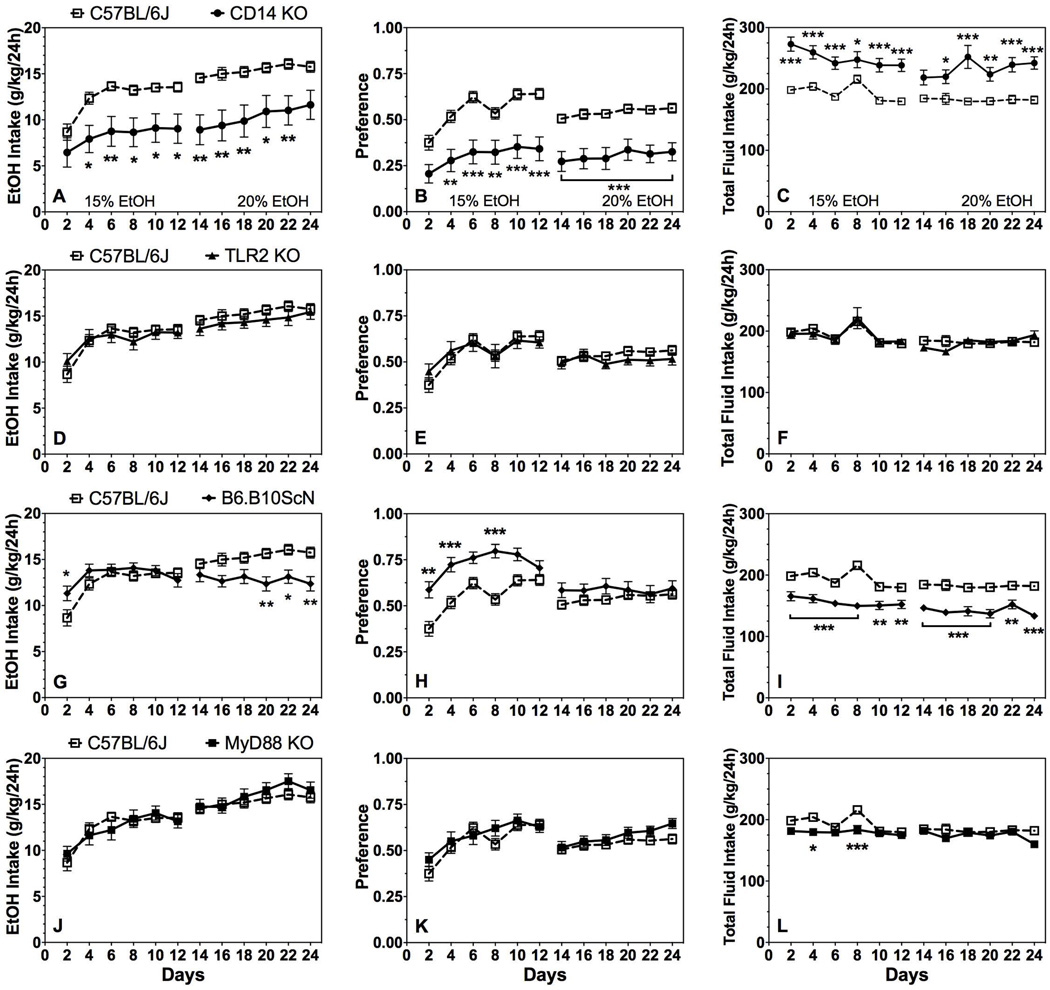
Similar to male mice, CD14 KO female mice showed a large reduction in 15% and 20% ethanol intake and preference and an increase in total fluid intake compared to control mice (Fig. 3A–C; Supplemental Table 3). There was an effect of genotype on ethanol intake for 15% and 20% ethanol, respectively [F(1,36)=10.1;P < 0.01 and F(1,36)=12.7;P < 0.01]; a genotype effect on preference for both concentrations [F(1,36)=20.6;P < 0.001 and F(1,36)=23.4;P < 0.001]; and a genotype effect on total fluid intake for both concentrations [F(1,36)=38.9;P < 0.001 and F(1,36)=20.6;P < 0.001]. There was no difference between control and TLR2 KO female mice in any of the parameters (Fig. 3D–F; Supplemental Table 3). TLR4 mutant female mice showed no difference in 15% ethanol intake, increased preference for this concentration [effect of genotype: F(1,35)=21.1;P < 0.001], but decreased total fluid intake [F(1,35)=38.3;P < 0.001] (Fig. 3G–I; Supplemental Table 3). Consumption of 20% ethanol [effect of genotype: F(1,35)=11.3;P < 0.01] and total fluid [F(1,35)=26.9;P < 0.001] intake decreased in TLR4 mutant females with no change in preference for ethanol. No difference in ethanol intake or preference was found between control and MyD88 KO female mice (Fig. 3J–L; Supplemental Table 3). In the presence of 15% ethanol only, total fluid intake decreased slightly in MyD88 KO female mice [effect of genotype: F(1,36)=7.2;P < 0.05].
Figure 3. 2BC-EOD drinking in female mice lacking CD14, TLR2, TLR4, or MyD88.
Ethanol intake (g/kg/24h) (A, D, G, J), preference for ethanol (B, E, H, K), and total fluid intake (C, F, I, L) in control (C57BL/6J, n=22) vs. mutant female mice (n= 15 for TLR4 mutant; n= 16 for MyD88 KO, TLR2 KO, and CD14 KO). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, **P < 0.01, ***P < 0.001 compared to control). Data above the horizontal lines indicate that each point was different from its corresponding control measure at the designated P-level.
Two-bottle choice drinking-in-the-dark (2BC-DID)
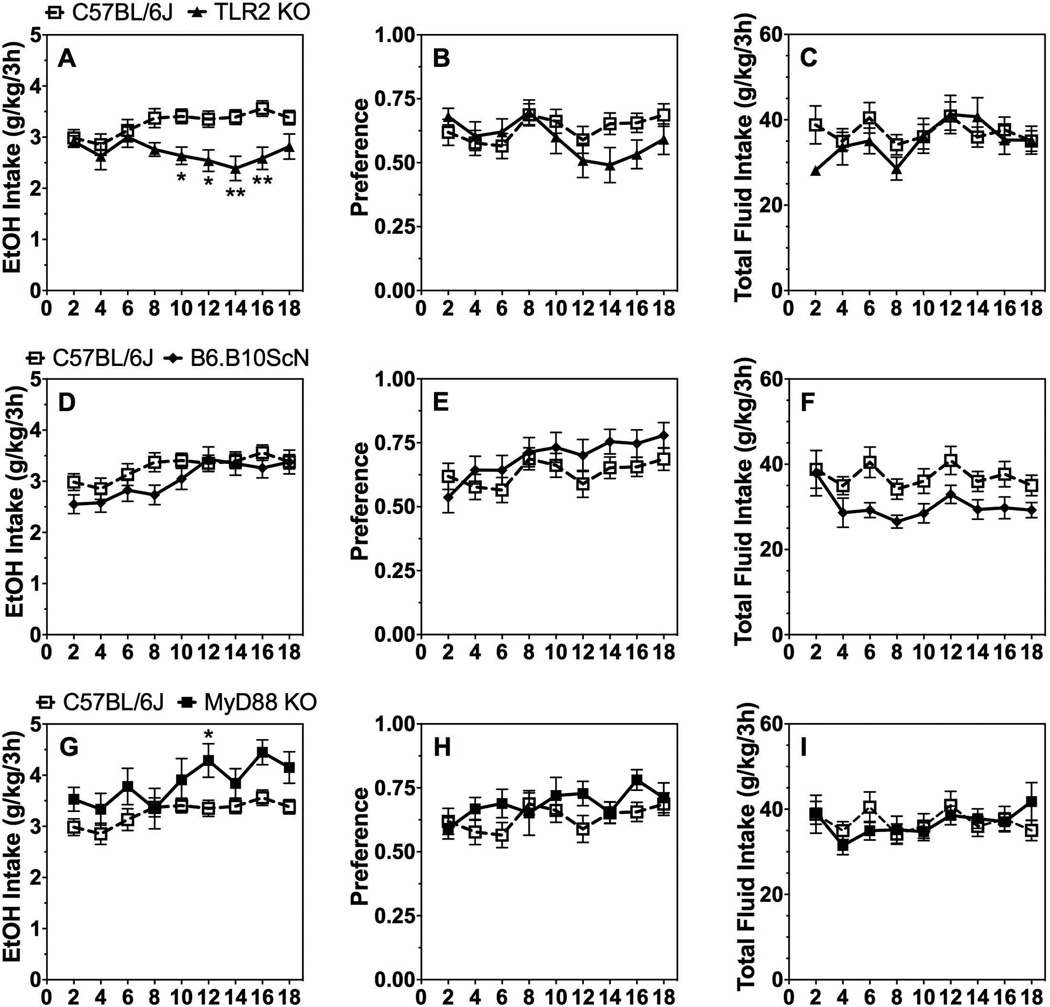
As shown in Fig. 4A, TLR2 KO male mice drank less ethanol (20%) compared to control mice in the 2BC-DID test [effect of genotype: F(1,40)=10.0;P < 0.01]. Although preference for ethanol was not significantly different, there was a significant genotype x time interaction effect [F(8,320)=2.3; P < 0.05; Fig. 4B]. Total fluid intake was not different in control and TLR2 KO mice (Fig. 4C; Supplemental Table 4). There were no differences in ethanol intake and preference in TLR4 mutant and control male mice (Fig. 4D,E), but total fluid consumption decreased in TLR4 mutants [effect of genotype: F(1,37)=4.8;P < 0.05; Fig. 4F). MyD88 KO male mice consumed slightly more ethanol than control mice in the 2BC-DID test [effect of genotype: F(1,30)=6.0;P < 0.05], but there was no change in ethanol preference or total fluid intake (Fig. 4G–I; Supplemental Table 4).
Figure 4. 2BC-DID in male mice lacking TLR2, TLR4, or MyD88.
Ethanol intake (g/kg/24h) (A, D, G), preference for ethanol (B, E, H), and total fluid intake (C, F, I) in control (C57BL/6J, n=22) vs. mutant male mice (n= 10, 17, and 20 for MyD88 KO, TLR4 mutant, and TLR2 KO, respectively). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, **P < 0.01 compared to control).
Similar to TLR2 KO male mice, TLR2 KO female mice showed reduced ethanol intake and preference compared to control mice [genotype effect on intake and preference, respectively: F(1,26)=8.8;P < 0.01 and F(1,26)=5.0;P < 0.05], and no change in total fluid consumption (Fig. 5A–C; Supplemental Table 4). No differences between control and TLR4 or MyD88 mutant female mice were found for any drinking parameters (Fig. 5D–I; Supplemental Table 4). However, there was a significant genotype x time interaction effect [F(8,192)=2.8;P < 0.01] for the amount of ethanol consumed in female MyD88 KO mice (Fig. 5G; Supplemental Table 4).
Figure 5. 2BC-DID in female mice lacking TLR2, TlR4, or MyD88.
Ethanol intake (g/kg/24h) (A, D, G), preference for ethanol (B, E, H), and total fluid intake (C, F, I) in control (C57BL/6J, n=16) vs. mutant female mice (n= 10, 12, and 16 for MyD88 KO, TLR2 KO, and TLR4 mutant, respectively). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05 compared to control).
One-bottle drinking-in-the-dark (1B-DID)
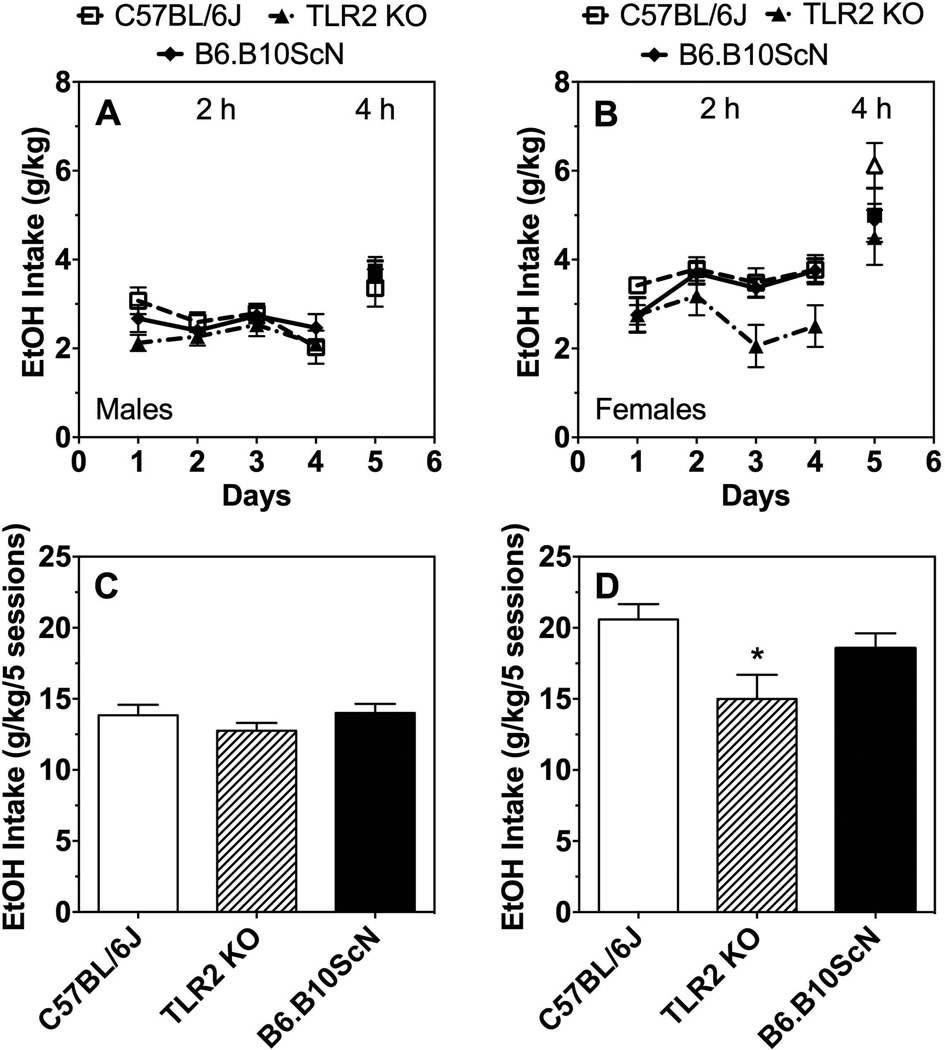
Only TLR2 KO female mice showed reduced ethanol (20%) intake compared to control mice during 2-h access in the 1B-DID test (Fig. 6A,B; Supplemental Table 5). Ethanol consumption decreased in female TLR2 KO mice over five drinking sessions (four 2-h and one 4-h sessions) [one-way ANOVA; F(14,16) = 4.7; P < 0.05] (Fig. 6C,D). Ethanol consumption did not differ in TLR4 mutant male or female mice compared to controls (Supplemental Table 5). Because only 1 ethanol bottle is offered, ethanol preference cannot be measured in this test. Due to limited numbers of MyD88 KO mice, we could not perform the 1B-DID test using these mice.
Figure 6. 1B-DID in male and female mice lacking TLR2 or TLR4.
Ethanol intake (g/kg) after 2 and 4 h in male (A) and female (B) control (C57BL/6J) vs. mutant mice. Total ethanol intake (g/kg) over 5 drinking sessions in male (C) and female (D) control (C57BL/6J) vs. mutant mice. Data in panels A and B were analyzed by Student’s t-test or two-way ANOVA, and data in panels C and D were analyzed by one-way ANOVA (*P < 0.05); males: n= 9 for C57BL/6J; n= 10 for TLR2 KO and TLR4 mutant; females: n= 5 for TLR4 mutant, n= 6 for C57BL/6J and TLR2 KO.
Preference for non-ethanol tastants
Because changes in taste perception can alter ethanol consumption in mice (Belknap et al., 1993; Blednov et al., 2008; Blednov et al., 2010), we also studied consumption of saccharin, quinine, and NaCl using a 24-h 2BC test to determine if altered taste could account for changes in ethanol consumption in the mutants.
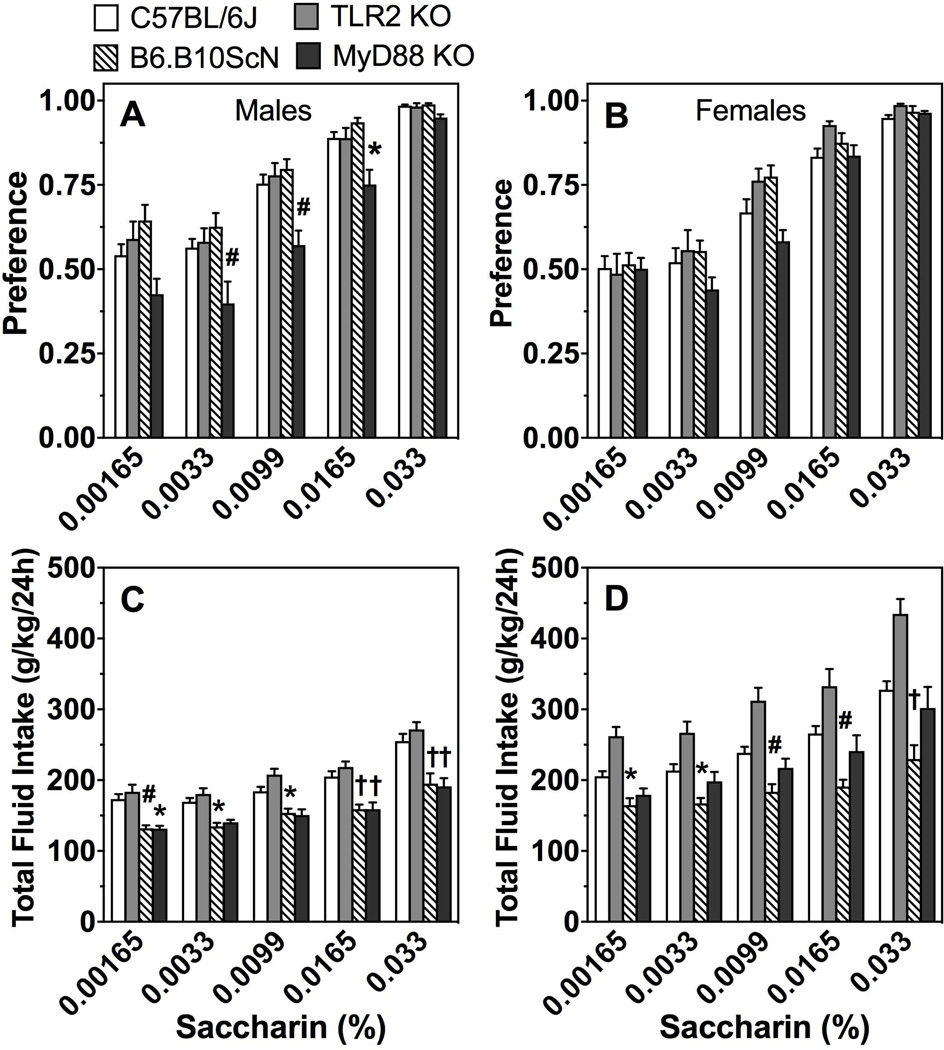
As shown in Fig. 7A,B, only MyD88 KO male mice showed a change (reduction) in preference for saccharin compared to control mice [effect of genotype: F(1,29)=11.5;P < 0.01; Supplemental Table 6]. Total fluid intake decreased in MyD88 and TLR4 mutant male mice (Fig. 7C; Supplemental Table 6). Total fluid intake also decreased in TLR4 mutant female mice, but increased in TLR2 KO female mice (Fig. 7D; Supplemental Table 6).
Figure 7. Saccharin consumption in mice lacking TLR2, TLR4, or MyD88.
Preference for saccharin in male (A) and female (B) control (C57BL/6J) vs. mutant mice. Total fluid intake (g/kg/24h) in male (C) and female (D) mice. Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, #P < 0.01, †P < 0.001); males: n= 7, 12, 16, and 24 for MyD88 KO, TLR2 KO, TLR4 mutant, and C57BL/6J, respectively; females: n= 7 for MyD88 KO; n= 10 for TLR2 KO and TLR4 mutant; n= 20 for C57BL/6J.
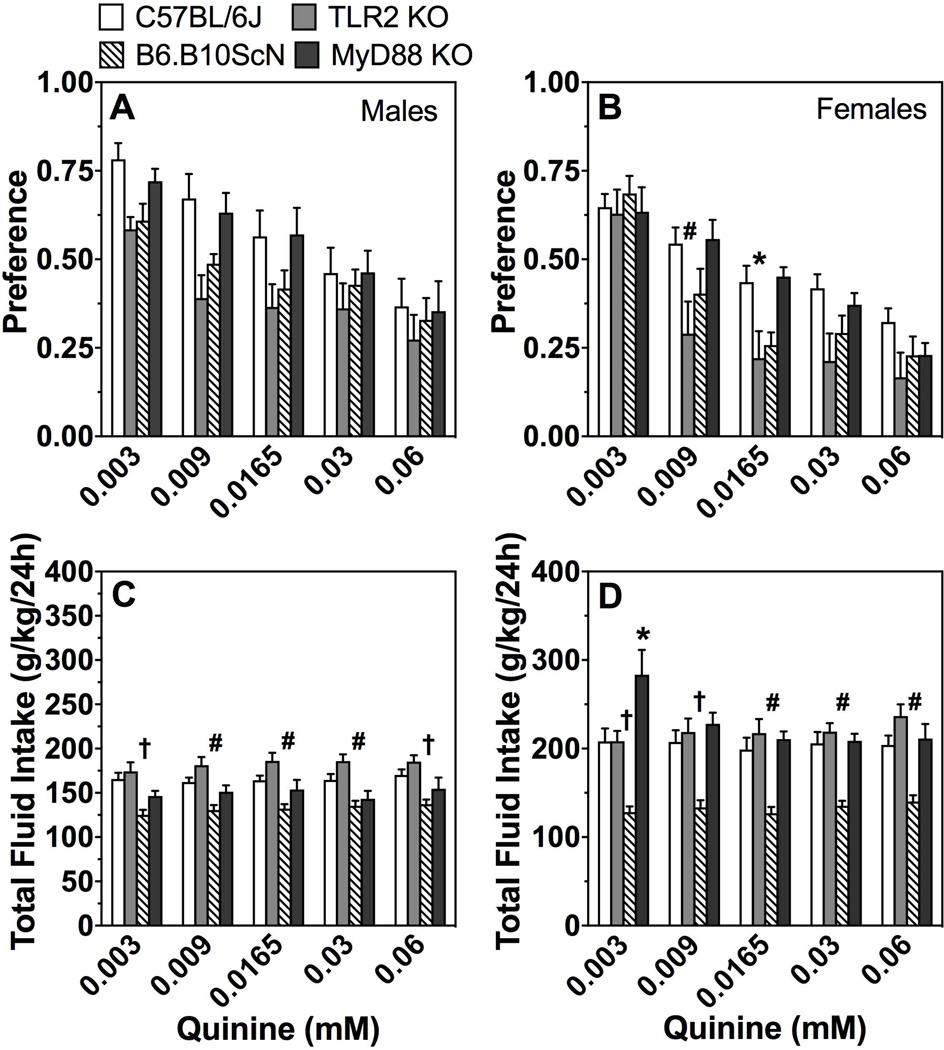
As shown in Fig. 8A,B, there was a significant effect of concentration on preference for quinine in all groups. Preference for quinine was reduced in TLR2 KO male and female mice compared to control mice [effect of genotype in males and females, respectively: F(1,26)=4.4;P < 0.05 and F(1,28)=5.6;P < 0.05]. TLR2 and TLR4 mutant female mice demonstrated a significant interaction between genotype and concentration of quinine (Supplemental Table 7). Compared to control mice, total fluid intake was significantly reduced in both male and female mice lacking TLR4 (Fig. 8C,D; Supplemental Table 7).
Figure 8. Quinine consumption in mice lacking TLR2, TLR4, or MyD88.
Preference for quinine in male (A) and female (B) control (C57BL/6J) vs. mutant mice. Total fluid intake (g/kg/24h) in male (C) and female (D) mice. Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, #P < 0.01, †P < 0.001 compared to control); males: n= 7 for MyD88 KO; n= 12 for TLR2 KO and TLR4 mutant; n= 16 for C57BL/6J; females: n= 7 for MyD88 KO; n= 10 for TLR2 KO and TLR4 mutant; n= 20 for C57BL/6J.
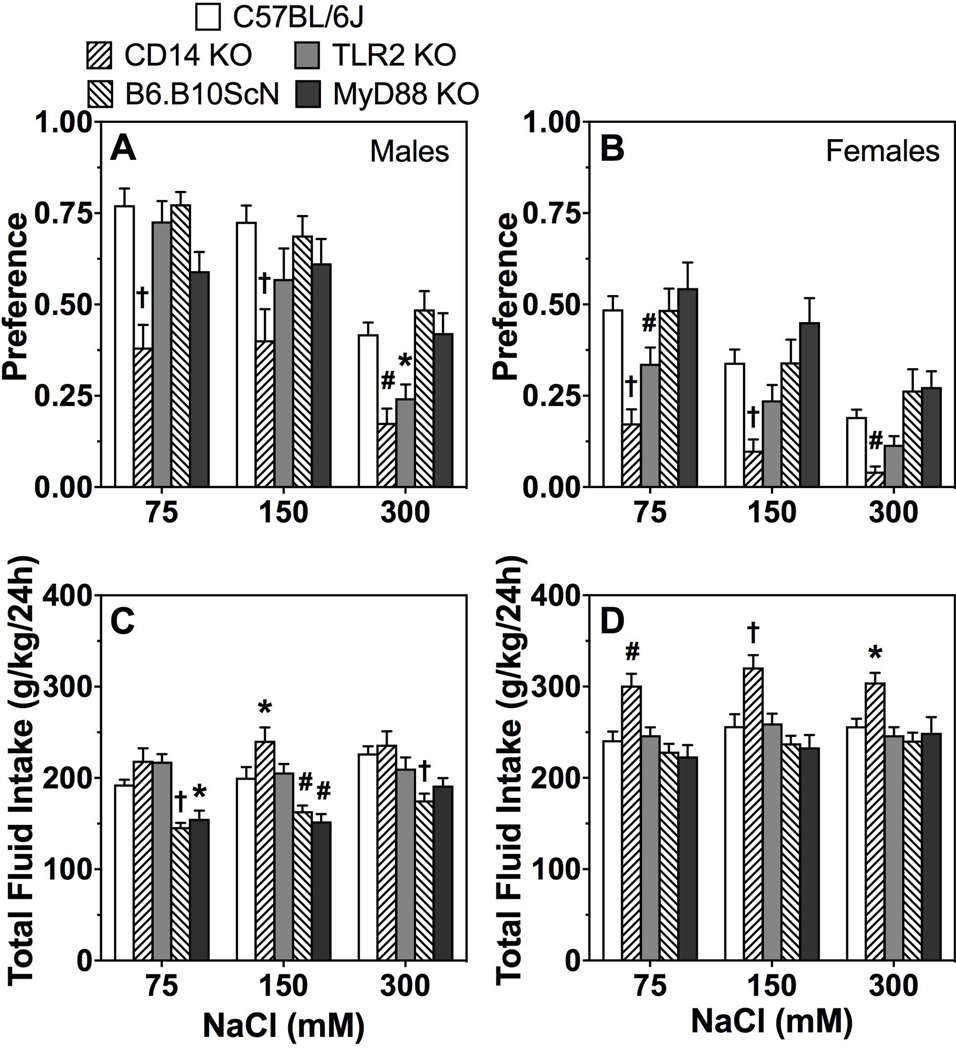
As seen in Fig. 9A,B, there was a significant effect of concentration on preference for NaCl in all groups. Male and female CD14 and TLR2 KO mice had reduced preference for NaCl compared to control mice [genotype effect in CD14 males and females, respectively: F(1,26)=25;P < 0.001 and F(1,27)=35.6;P < 0.001 and in TLR2 KO males and females, respectively: F(1,27)=4.4;P < 0.05 and F(1,30)=5.9;P < 0.05]. Male MyD88 KO mice showed a significant genotype x concentration interaction effect (Fig. 9A,B; Supplemental Table 8). There were significant decreases in total fluid intake in TLR4 and MyD88 mutant male mice and significant increases in CD14 KO female mice (Fig. 9C,D; Supplemental Table 8). CD14 and TLR2 KO male mice showed a significant genotype x concentration interaction effect (Supplemental Table 8).
Figure 9. NaCl consumption in mice lacking CD14, TLR2, TLR4, or MyD88.
Preference for sodium chloride in males (A) and females (B). Total fluid intake (g/kg/24h) in males (C) and females (D). Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni post hoc tests (*P < 0.05, #P < 0.01, †P < 0.001 compared to control); males: n= 7, 10, 11, 16, and 18 for MyD88 KO, CD14 KO, TLR2 KO, TLR4 mutant, and C57BL/6J; females: n= 11 for TLR2 and TLR4 mutants; n= 12, 13, and 16 for MyD88 KO, CD14 KO, and C57BL/6J.
Discussion
Deletion of certain immune-related genes decreases ethanol consumption in knockout mouse models (reviewed in Mayfield et al., 2016). Some studies have implicated the TLR4 pathway in ethanol drinking or other behavioral effects of ethanol, but a role for the individual components of TLR signaling had not been given due attention thus far. Here we evaluated TLRs implicated in ethanol’s neuroinflammatory and/or behavioral effects (TLR2, TLR4) as well as proteins known to signal through multiple TLRs (CD14, MyD88). We tested male and female mutant and control mice using different tests of voluntary ethanol consumption that model drinking patterns found in alcohol-dependent human subjects (Table 1). Different ethanol exposure paradigms produce distinct changes in gene expression in mouse prefrontal cortex, and the transcriptome profile following chronic intermittent drinking overlaps with changes induced by immune activation (Osterndorff-Kahanek et al., 2013), highlighting the importance of comparing the mutant mice in different behavioral models.
Table 1.
Summary of ethanol intake and preference in different drinking models in mutant mice.
| 2BC | 2BC-EOD | 2BC-DID | 1B- DID |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant | EtOH intake (3- 21%) |
Pref. | Total fluid intake |
EtOH intake (15, 20%) |
Pref. | Total fluid intake |
EtOH intake (20%) |
Pref. | Total fluid intake |
EtOH intake (20%) |
||||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| CD14 | = | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | = | = | = | = | = | = | = | = |
| TLR2 | ↓ | = | ↓ | = | ↑ | = | = | = | = | = | = | = | ↓ | ↓ | = | ↓ | = | = | = | ↓ |
| TLR4 | = | = | = | = | ↓ | ↓ | = | ↓ | = | = | = | ↓ | = | = | = | = | ↓ | = | = | = |
| MyD88 | = | = | = | = | = | = | ↑ | = | ↑ | = | = | = | ↑ | = | = | = | = | = | ||
Ethanol (EtOH) intake, ethanol preference (pref.), and total fluid intake in male (M) and female (F) mutant vs. control C57BL/6J mice. The concentrations of the EtOH solutions used are listed in parenthesis. Effects in CD14 KO mice in the 2BC, 2BC-DID, and 1B-DID tests were taken from our previous publication (Blednov et al., 2012b). 2BC, 24-h continuous access two-bottle choice; 2BC-EOD, 24-h two-bottle choice with every-other-day access to ethanol; 2BC-DID, drinking-in-the-dark with two-bottle choice; 1B-DID, drinking-in-the-dark with access to 1 bottle of ethanol. ↓ indicates reduced response compared with corresponding parameter in control mice; ↑ indicates increased response compared with corresponding parameter in control mice; = indicates no significant difference from control mice.
Based on our previous findings in mice lacking immune-related genes (Blednov et al., 2012b), we predicted that ethanol consumption and preference would also decrease in the mutant mice tested here. However, this was only observed in TLR2 KO and CD14 KO mice. In contrast, deletion of MyD88, the key downstream mediator for most TLRs, had no effect in female mice but increased ethanol intake in limited access tests in male mice. Drinking effects in some mutants depended on the sex of the mice or the ethanol exposure protocol (Table 1). A review of many different mouse knockout models provides evidence that drinking effects in a given genotype may depend on sex, ethanol concentration and time of exposure, as well as the drinking protocol (Mayfield et al., 2016).
We previously showed that injection of LPS produced prolonged increases in ethanol consumption while lack of CD14 reduced consumption in mice (Blednov et al., 2011), and initially hypothesized that TLR4 inflammatory signaling was responsible for the increased drinking. Treatment with LPS or chronic intermittent ethanol produced similar changes in mouse brain transcriptomes (Osterndorff-Kahanek et al., 2013), suggesting that chronic ethanol also alters expression of proinflammatory genes. Although TLR4 is a primary immune target, LPS can activate other immune mediators, such as TLR2, via co-receptors (van Bergenhenegouwen et al., 2013). LPS-mediated effects via alternate (non-TLR4) or interacting (TLR4/TLR2) immmune pathways may thus explain the increased ethanol drinking that we reported previously.
Our findings in C57BL/10ScN mice are consistent with reports in TLR4 KO mice (Alfonso-Loeches et al., 2010; Pascual et al., 2011), which also did not support a role for TLR4 in ethanol consumption. These studies agree with a comprehensive INIA-Neuroimmune study in TLR4 KO rats, C57BL/6J mice treated with the specific TLR4 inhibitor (+)-naloxone, and TLR4 knockdown in mouse nucleus accumbens, showing that none of these pharmacologic or genetic manipulations consistently reduced ethanol consumption in any drinking model (Harris et al., in press). Although the TLR4 antagonist T5342126 decreased drinking in ethanol-dependent and non-dependent mice, this was attributed to non-specific effects (Bajo et al., 2016). Inconsistent effects of high dose (+)-naloxone in mice (Harris et al., in press) may also have been due to non-specific effects as reported in (Tanda et al., 2016). In contrast to these negative findings, TLR4 knockdown in the CeA or ventral tegmental area reduced binge-like drinking in alcohol preferring (P) rats (Liu et al., 2011; June et al., 2015). The decreased drinking was associated with reduced expression of the GABAA α2 subunit in the CeA (Liu et al., 2011), indicating that TLR4 may interact with neurotransmitter receptors (or other targets) to regulate ethanol consumption in some animal models of alcohol consumption. Although there is ample evidence that TLR4 mediates alcohol-neuroimmune signaling (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010; Zou and Crews, 2014; Bajo et al., 2016), a direct causal link between TLR4 and alcohol drinking behavior has not been substantiated. The TLR4 pathway is associated with increased anxiety-related behavior during ethanol withdrawal and release of brain inflammatory mediators (Breese et al., 2008; Pascual et al., 2015) that could potentially play a role in relapse drinking. A study of TLR4 KO adolescent mice showed slightly reduced ethanol preference following intermittent i.p. ethanol treatment (Montesinos et al., 2016b). Despite the limited evidence for its role in voluntary ethanol drinking, there is consistent evidence that TLR4 mediates the sedative effects of ethanol in mice (Wu et al., 2012; Corrigan et al., 2015) (also see our companion study Blednov et al., submitted) and rats (Harris et al., in press), as well as a role for the Toll innate immune pathway in ethanol-induced resistance to sedation in Drosophila (Troutwine et al., 2016).
The most surprising finding of the current study was the increased ethanol drinking seen in MyD88 KO male mice in limited access tests (2BC-EOD and 2BC-DID). Inhibition of MyD88 signaling would be expected to block signaling through all TLRs except TLR3 (Miggin and O'Neill, 2006). The increased ethanol consumption in MyD88 KO male mice suggests that another signaling branch, mediated by the TRIF-dependent pathway, may be important in binge-like drinking. In support of this, we observed increased expression of several components of TRIF-dependent signaling (e.g., TLR3, TRIF, IRF3) in the prefrontal cortex of C57BL/6J mice after chronic 2BC-EOD drinking, and mice treated with poly(I:C), a TLR3 agonist, also showed increased ethanol consumption in the 2BC-EOD test (Y.A. Blednov, unpublished observations). In addition, ethanol potentiated poly(I:C)-induced blood and brain proinflammatory responses, and this was associated with increased TLR3 expression in mouse brain (Qin and Crews, 2012). MyD88 can function as a negative regulator of TLR3/TRIF signaling (Johnson et al., 2008; Siednienko et al., 2011), and this inhibitory mechanism may be impaired in MyD88 KO mice. These findings support further study of the TLR3/TRIF pathway as a potential target for regulating ethanol consumption.
CD14 is a co-receptor for TLRs 1–4, 6, 7, and 9 (Zanoni and Granucci, 2013). Deletion of CD14 would thus be expected to interfere with signaling through these TLRs, but not TLRs 5 and 8. The decreased drinking in CD14 KO mice was not likely due to impaired signaling through TLR4 because deletion of TLR4 itself had no overall effect. The role for TLR2 signaling in CD14 KO mice is, however, less clear. Although decreased ethanol drinking was observed in TLR2 KO and CD14 KO mice in the 2BC test, consistent phenotypes for both of these mutants were not found in the other tests. While TLRs 4, 5, or 8 are not likely involved in the decreased drinking in CD14 KO mice, there is some support for TLR2 in these mice. And unlike TLR4, TLR2 was implicated in ethanol drinking behavior in different models in male and female mice.
The dependence of voluntary ethanol intake on sensitivity to sweet taste in mice is well documented (Belknap et al., 1993; Bachmanov et al., 1996; Yoneyama et al., 2008; Blednov et al., 2012a), and deletion of different types of taste receptors reduced ethanol intake and preference in mice (Blednov et al., 2008). Most of the mammalian TLRs are also expressed in taste bud cells (Wang et al., 2009). Furthermore, mice lacking MyD88 (which causes impaired TLR signaling) showed significantly reduced preference for saccharin, while lack of the upstream receptors (TLR2, TLR4) did not alter preference (Table 2). However, decreased preference for saccharin would not account for the increased ethanol consumption in MyD88 KO mice. Preference for ethanol (especially high concentrations) is also positively correlated with preference for NaCl (Blednov et al., 2010). TLR2 KO and CD14 KO mice showed reduced preference for NaCl, which might suggest that altered taste perception could contribute to the reduced ethanol intake and preference observed in these mutants. However, TLR2 and CD14 KO mice also showed increased total fluid (water) intake in several drinking tests. Changes in NaCl and water intake in these mutants warrant consideration of a potential role for angiotensin in mediating these effects, and there is some evidence for an association between TLR2 and AGTR1 following ethanol exposure (Wang et al., 2014). With global KO models, we do not know if the resulting phenotypes are due to central or peripheral deletion of immune-related proteins or if any compensatory changes in other molecular targets in the periphery and/or the brain may account for these findings.
Table 2.
Summary of preference for non-ethanol tastants in mutant mice.
| Saccharin | Quinine | NaCl | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant |
Preference | Total fluid intake |
Preference | Total fluid intake |
Preference | Total fluid intake |
||||||
| M | F | M | F | M | F | M | F | M | F | M | F | |
| CD14 | = | = | ↑ | ↑ | = | = | ↑ | ↑ | ↓ | ↓ | = | ↑ |
| TLR2 | = | = | = | ↑ | ↓ | ↓ | = | = | ↓ | ↓ | = | = |
| TLR4 | = | = | ↓ | ↓ | = | = | ↓ | ↓ | = | = | ↓ | = |
| MyD88 | ↓ | = | ↓ | = | = | = | = | = | = | = | ↓ | = |
Total fluid intake and preference for saccharin, quinine, and NaCl in 24-h two-bottle choice tests in male (M) and female (F) mutant vs. control C57BL/6J mice. Effects in CD14 KO mice were taken from our previous publication (Blednov et al., 2012b). ↓ indicates reduced response compared with corresponding parameter in control mice; ↑ indicates increased response compared with corresponding parameter in control mice; = indicates no significant difference from control mice.
Although overall evidence does not support a role for TLR4 in mediating ethanol consumption, we show that other signaling molecules (e.g., TLR2, CD14) may be involved. We previously reported decreased voluntary ethanol drinking in CD14 KO mice (Blednov et al., 2012b), and our current findings further support the involvement of CD14 (and/or TLRs that are associated with this co-receptor) in other drinking models in both male and female mice. TLR2 has been implicated in ethanol responses in animal models and human alcoholics (reviewed in Robinson et al., 2014), and we provide initial evidence that it may be important in regulating ethanol consumption in different drinking tests. In addition, the MyD88 KO model implicates TLR3 in ethanol drinking and supports future studies investigating this immune mediator. We show that inhibiting different components of the MyD88 pathway can produce opposite effects on ethanol drinking, and these effects depend on sex and/or the ethanol exposure procedure. Other studies also support the importance of examining different drinking models (Osterndorff-Kahanek et al., 2013) and including both male and female subjects (Pascual et al., 2016). The review by Mayfield et al. (2016) further demonstrates the potential impact of sex and the ethanol exposure conditions on ethanol consumption in different knockout mouse models.
In summary, specific MyD88 signaling components differentially regulated ethanol consumption in mice. In general, deletion of CD14 or TLR2 reduced ethanol drinking while deletion of MyD88 surprisingly increased drinking. Deletion of TLR4 did not significantly alter ethanol consumption in any test. The effects in some of the null mutants depended on the ethanol exposure protocol, suggesting that specific patterns of brain gene expression induced by the pattern of drinking may underlie some of the alcohol-related phenotypes.
Supplementary Material
Acknowledgments
Funding: This research was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA013520/INIA-Neuroimmune and AA006399).
Footnotes
Author Contributions
YAB designed and performed experiments, analyzed data, prepared graphs/tables, and wrote the manuscript; MB, JC, and AD performed experiments; JM prepared graphs/tables, wrote, and edited the manuscript; RAH designed experiments and edited the manuscript.
The authors declare no conflicts of interest.
REFERENCES
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Montgomery SE, Cates LN, Nadav T, Delucchi AM, Cheng K, Yin H, Crawford EF, Roberts AJ, Roberto M. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol Alcohol. 2016a;51:541–548. doi: 10.1093/alcalc/agw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- Blednov YA, Mayfield RD, Belknap J, Harris RA. Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav. 2012a;11:424–435. doi: 10.1111/j.1601-183X.2012.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, Dacosta A, Harris RA. (submitted) The MyD88-dependent pathway regulates the sedative effects of ethanol: II. Loss of righting reflex and motor incoordination in mice lacking TLR2, TLR4, CD14, or MyD88. Alc Clin Exp Res. doi: 10.1111/acer.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alcohol consumption. Behav Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25 Suppl. 2011;1:S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012b;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–876. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2010;10:98–106. doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Wu Y, Tuke J, Coller JK, Rice KC, Diener KR, Hayball JD, Watkins LR, Somogyi AA, Hutchinson MR. Alcohol-induced sedation and synergistic interactions between alcohol and morphine: a key mechanistic role for Toll-like receptors and MyD88-dependent signaling. Brain Behav Immun. 2015;45:245–252. doi: 10.1016/j.bbi.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2016;233:1543–1557. doi: 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Zuvela N, Landis N, Wilce PA. Smoking and alcoholism target genes associated with plasticity and glutamate transmission in the human ventral tegmental area. Hum Mol Genet. 2008;17:38–51. doi: 10.1093/hmg/ddm283. [DOI] [PubMed] [Google Scholar]
- Harris RA, Blednov YA. In: Neuroimmune genes and alcohol drinking behavior. Cui C, Grandison L, Noronha A, editors. New York: Neural-immune interactions in brain function and alcohol related disorders Springer; 2013. pp. 425–440. [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt J, de Guglielmo G, Lasek AW, Logrip ML, Vendruscolo LF, Roberts AJ, Roberts E, George O, Mayfield J, Billiar TR, Hackam DJ, Mayfield RD, Koob GF, Roberto M, Homanics GE. Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J Neurosci. doi: 10.1523/JNEUROSCI.2002-16.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40:1549–1559. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31:1460–1466. doi: 10.1111/j.1530-0277.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr., Aurelian L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23:513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Arends MA, Harris RA, Blednov YA. Genes and Alcohol Consumption: Studies with Mutant Mice. Int Rev Neurobiol. 2016;126:293–355. doi: 10.1016/bs.irn.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miggin SM, O’Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006;80:220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol Clin Exp Res. 2016a;40:2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2016b;53:159–171. doi: 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Andersson LP, Ingalls RR, Monks BG, Li R, Arnaout MA, Golenbock DT, Freeman MW. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J Immunol. 2000;165:4272–4280. doi: 10.4049/jimmunol.165.8.4272. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA. Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One. 2013;8:e59870. doi: 10.1371/journal.pone.0059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25 Suppl. 2011;1:S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ, Guerri C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol Oct 4. 2016 doi: 10.1111/adb.12461. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA. Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. Int Rev Neurobiol. 2014;118:13–39. doi: 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol. 2013;18:496–507. doi: 10.1111/j.1369-1600.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siednienko J, Gajanayake T, Fitzgerald KA, Moynagh P, Miggin SM. Absence of MyD88 results in enhanced TLR3-dependent phosphorylation of IRF3 and increased IFN-beta and RANTES production. J Immunol. 2011;186:2514–2522. doi: 10.4049/jimmunol.1003093. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, clinical and experimental research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman J, Coggiano M, Katz JL. Lack of Specific Involvement of (+)-Naloxone and (+)-Naltrexone on the Reinforcing and Neurochemical Effects of Cocaine and Opioids. Neuropsychopharmacology. 2016 Jun 14; doi: 10.1038/npp.2016.91. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutwine BR, Ghezzi A, Pietrzykowski AZ, Atkinson NS. Alcohol resistance in Drosophila is modulated by the Toll innate immune pathway. Genes Brain Behav. 2016;15:382–394. doi: 10.1111/gbb.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergenhenegouwen J, Plantinga TS, Joosten LA, Netea MG, Folkerts G, Kraneveld AD, Garssen J, Vos AP. TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J Leukoc Biol. 2013;94:885–902. doi: 10.1189/jlb.0113003. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 2009;1170:596–603. doi: 10.1111/j.1749-6632.2009.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Shen Q, Li X, Lu J, Li X, Yin D, Peng Y. Valsartan blocked alcohol-induced, Toll-like receptor 2 signaling-mediated inflammation in human vascular endothelial cells. Alcohol Clin Exp Res. 2014;38:2529–2540. doi: 10.1111/acer.12532. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, Watkins LR, Somogyi AA, Hutchinson MR. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. British journal of pharmacology. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9:e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.